Abstract
AIM: To explore the etiology and pathogenesis of human primary intrahepatic cholangiocarcinoma, the expression of HBV genes and HBV-antigens was detected in the cancerous tissue and its surrounding hepatic tissues.
METHODS: HBV-antigens were detected by immunohistochemical technique and HBV genes were examined with in situ hybridization.
RESULTS: In 20 cases of cholangiocarcinoma, the positive detection rate of HBxAg, pre-S1, pre-S2, HBsAg and HBcAg was 75%, 40%, 40%, 10% and 0%, respectively, and in the surrounding hepatic tissues of 19 cases the positive rates were 84.2%, 47.9%, 47.9%, 31.6% and 31.6%. Among 40 cases of cholangiocarcinoma, the positive rate of HBVDNA, x-gene, pre-s gene, s gene and s gene fell on 77.5%, 70.0%, 47.5%, 40% and 42.5%, respectively, and of the surrounding hepatic tissues in 33 cases, 87.9%, 84.8%, 63.6%, 69.7% and 66.7%.
CONCLUSION: The development of human primary intrahepatic cholangiocarcinoma bears a close relationship with chronic persistent HBV infection. Particularly, the x gene of HBV and its protein (HBxAg) might play an important role in pathogenesis of hepatic carcinoma.
Keywords: hepatitis B virus; gene, viral; antigens, viral; in situ hybridization; immunohistochemistry; cholangiocarcinoma
INTRODUCTION
A large number of studies indicate a close relationship between human primary hepatocellular carcinoma and hepatitis B virus (HBV) infection, which is considered generally as an important factor in the development of hepatic carcinoma[1,2]. In human primary hepatic carcinoma, hepatocellular carcinoma is more frequently encountered, while intrahepatic cholangiocarcinoma (ChC), including hepatocholangiocarcinoma (HChC), is relatively less, being 8%-10%[3]. For a long time, the etiology and pathogenesis of intrahepatic cholangiocarcinoma have been unclear. A few reports considered it to be related to infestation with clonorchiasis sinensis[4,5], but never involved with HBV infection. We used immunohistochemical technique and in situ hybridization methods to detect HBV genes and their-related antigens in the tissues of intrahepatic cholangiocarcinoma and its surrounding hepatic tissues for the purpose of exploring the etiology and pathogenesis of intrahepatic cholangiocarcinoma.
MATERIAL AND METHODS
All the 40 cases of surgically resected specimens of intrahepatic cholangiocarcinoma and hepato cholangiocarcinoma were from the Pathologic Laboratory of the Affiliated Hospital of Fourth Military Medical University. The specimens were fixed in 10% formalin, paraffin embedded and serially sectioned 5 μm in thickness. The HBV antigens (HBxAg, HBsAg, HBcAg, pre-S1 and pre S2) were detected by immunohistochemical techniques (ABC and PAP). In situ hybridization method was adopted for detection of HBV genes (HBV-DNA, x gene, s gene, pre s gene and c gene). Of the main reagents of immunohistochemistry, the antibodies of anti-HBx, S1 and S2 were gained from Fox Chase Cancer Center, Philadelphia, USA, and ABC kit and PAP kit (including anti HBs and anti-HBc) were purchased from BioGenes Lab, USA. The ABC and PAP methods were in accordance with the instructions of the kits. For in situ hybridization, Bio-11-dUTP was purchased from the Institute of Pharmacology of Beijing Medical University. The gene primers of s, pre s and c of HBV were acquired from Fox Chase Cancer Center, Philadelphia, USA. The colony of E. Coli- C600-HBV (containing PCP 10) was supplied by the Research Institute of Liver Diseases of Beijing Medical University. The colony of PMM15-HBV was furnished by the Basic Medical Institute of Military Medical Academy, Beijing. E. Coli, DNA polymerase I, restriction endonuclease EcoRI, Bam HI and Bgl II were purchased from Hua Mei Biotechnology, Co. As for the preparation of probes, the plasmids of PMM15-HBV and PCP-HBV were extracted through lysis by alkali. PCP 10-HBV was cleaved by EcoRI and various sized gene fragments of 3.2 kb were harvested by freeze-squeezing method, and then, cleaved with Bam HI and Bgl II. 584 X gene fragments of nucleotide were obtained by PMM15-HBV. The probes of HBV-DNA and X gene were labeled through nicking translation, and the fragments of C gene, S gene and pre S gene of HBV were labeled with PCR. Control groups were set up including positivity, negativity, blank and replacement.
RESULTS
Pathological characteristics
In the specimens of 40 cases, 30 cases were confirmed by pathological examination as intrahepatic cholangiocarcinoma and 10 as hepato-cholangiocarcinoma. The patterns of intrahepatic cholangiocarcinoma manifested as tubulo-glandular structures, mostly the cuboidal cells arranging in small lumens and partly the high columnar cells taking the form of variously sized lumens with few mucus secretion. In some cases, the cells were in mass arrangement. The carcinoma cells were small in size with scanty cytoplasm. The fibrous interstitial tissues were more abundant. The above-mentioned tubulo-glandular structures and cellular masses showed irregular distribution within the fibrous interstitial tissue. Hepato-cholangiocarcinoma appeared as the tubulo-glandular structures mingling with hepatocellular carcinoma-cell cords or masses and the transition of each other was visible. In individual case, secretion of the bile was occasionally encountered. The carcinoma cells possessed a certain atypicality with relatively less mitotic figures. Of the 40 specimens of intrahepatic cholangiocarcinoma, the surrounding hepatic tissues were present in 33 cases, 22 of them were chronic active hepatitis (CAH), 7 chroni cpersistent hepatitis (CPH) and 4 liver cirrhosis (CIR).
Detection of HBV antigens
HBV antigens were detected in 20 cases of intrahepatic cholangiocarcinoma and 19 cases of the surrounding hepatic tissues. The HBV antigens included HBsAg, HBcAg, HBxAg, pre-S1 and pre-S2. Their positive results are shown in Table 1, Table 2. In addition, the positive coexistence of HBxAg, S1 and S2 antigens were present in 6 cases in the cancerous tissues, and positive HBxAg and HBsAg in 1 case. In the surrounding hepatic tissues, 7 cases showed the positive coexistence of HBxAg, S1 and S2 antigens, 4 cases with positive HBxAg and HBsAg, and 3 cases with positive HBxAg and HBcAg.
Table 1.
Positive results of 5 HBV antigens in cancerous tissues
| Pathological diagnosis | Cases | HBxAg | pre-S1 | pre-S2 | HBsAg | HBcAg |
| Chc | 12 | 8 | 5 | 5 | 0 | 0 |
| HChC | 8 | 7 | 3 | 3 | 2 | 0 |
| Total (%) | 20 | 15 (75) | 8 (40) | 8 (40) | 2 (10) | 0 |
Chc: intrahepatic cholangiocarcinoma. HChC: hepato-cholangiocarcinoma
Table 2.
Positive results of 5 HBV antigens in surrounding hepatic tissues
| Pathological diagnosis | Cases | HBxAg | pre-S1 | pre-S2 | HBsAg | HBcAg |
| CAH | 8 | 8 | 5 | 5 | 2 | 5 |
| CPH | 6 | 5 | 1 | 1 | 1 | 0 |
| CIR | 5 | 3 | 3 | 3 | 3 | 1 |
| Total (%) | 19 | 16 (84.2) | 9 (47.4) | 9 (47.4) | 6 (31.6) | 6 (31.6) |
CAH: Chronic active hepatis. CPH: Chronic persistent hepatitis. CIR: Liver cirrhosis
In the cancerous and surrounding hepatic tissues, positive HBxAg presented brown-yellow coloured evenly fine granules, mainly as in the cytoplasm (Figure 1). They gathered around the nuclei or on the cellular membranes, or in combined presence. The distribution and intensity of positive HBxAg varied in different cases, even in different places of the same case. In general, detection rate and intensity of the positive cells in the surrounding hepatic tissues were both higher than that in the cancerous tissues (Figure 2). In the above-mentioned cancerous and surrounding hepatic tissues, the positive HBxAg cells were scattered, local or diffuse in distribution. In this group, local distribution was most common and diffuse distribution was the next.
Figure 1.
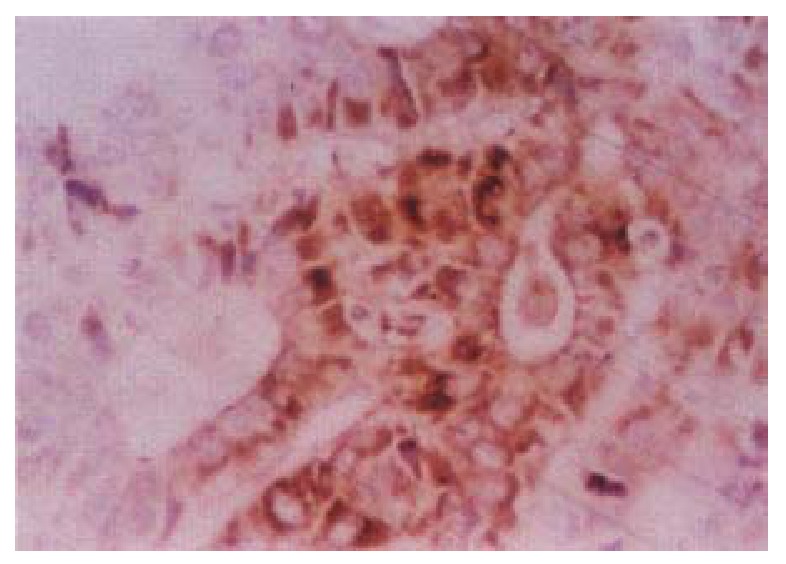
Cancerous surrounding hepatic tissue, HBxAg-positive substance located in the cytoplasms. ABC × 400
Figure 2.
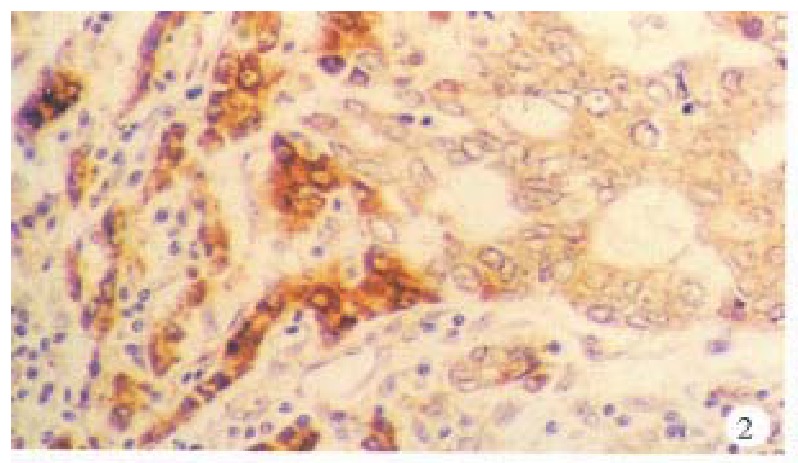
Cancerous and surrounding hepatic tissues, detection rate and intensity of the HBxAg-positive cells in the surrounding hepatic tissues were both higher than that in the cancerous tissues. ABC × 200
In the cancerous and surrounding hepatic tissues, HBsAg and the antigens of pre-S1 and pre-S2 appeared as brown-yellow coloured evenly fine granules as well, mainly intractytoplasmic, a few intranuclear or on the cellular membranes. The intracytoplasmic positive materials appeared frequently in shape of an inclusion body, located at one side of tie nucleus (Figure 3). In some cells nearly the whole cytoplasm was full of these materials with a few on the cellular membranes (Figure 4). These positive cells were mainly in local distribution.
Figure 3.
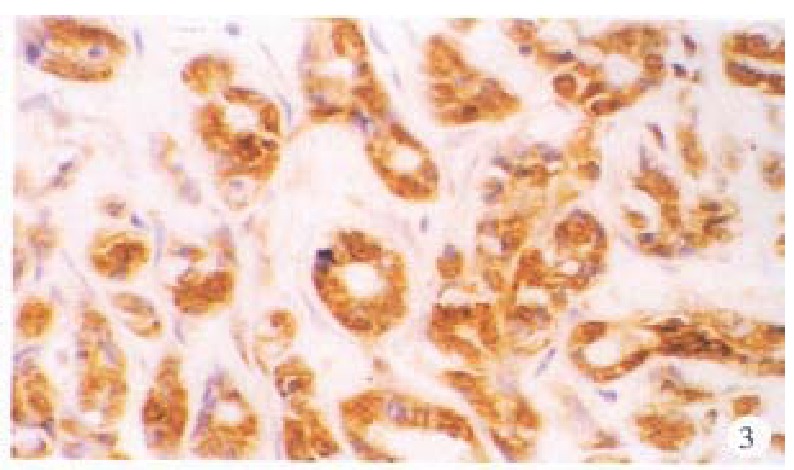
Cancerous tissue, HBsAg-positive substance located mainly in the cytoplasms. ABC × 200
Figure 4.
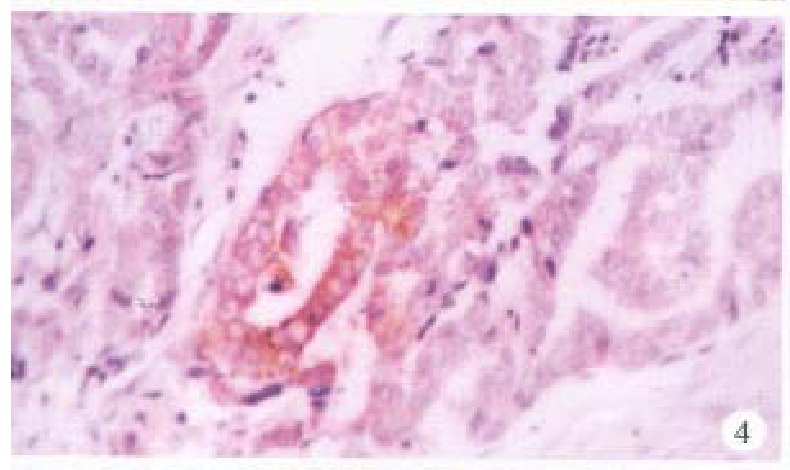
Cancerous tissus, pre-S1 antigens located mainly in the cytoplasms, some on cellular membranes. ABC × 400
HBcAg was chiefly present in the surrounding hepatic tissues, mostly seen in the nuclei, some in the cytoplasm or on the cellular membranes, even present in combination (Figure 5). The intranuclear HBcAg manifested as brown yellow coloured coarse granules, while fine granules were intracytoplasmic or on the cellular membranes. Positive HBcAg was frequently seen in chronic active hepatitis.
Figure 5.
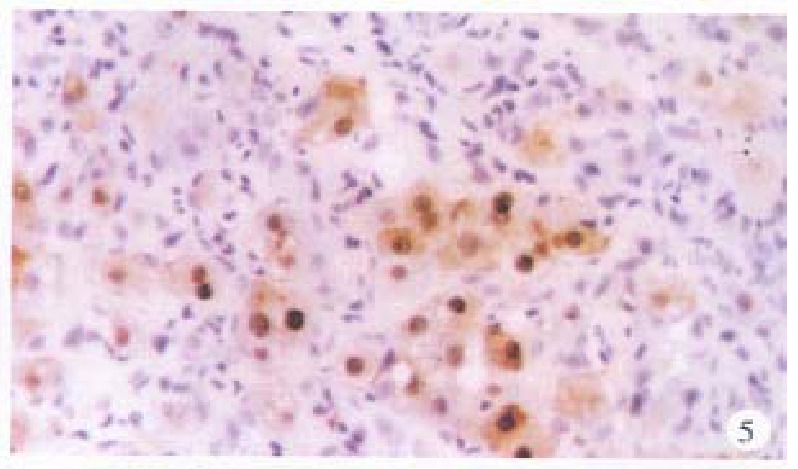
Surrounding hepatic tissue, HBc-Ag positive located mainly in the nuclei, some in the cytoplasms and on cellular membranes. ABC × 400
The detection of above-mentioned antigens was compared with several control experiments. The positive sections remained positive by repeated stainings, and the negative sections, the replacement and blank experiments were all negative.
Detection of HBV genes
The 5 kinds probes of were applied to detect HBV-DNA,X gene, pre S gene, S gene and C gene in the cancerous and surrounding hepatic tissues. The results are shown in Table 3, Table 4.
Table 3.
Positive results of hybridization by 5 kinds of probe for HBV genes in cancerous tissues
| Pathological diagnosis | Cases | HBV-DNA | X | pre-S | S | C |
| Chc | 30 | 23 | 20 | 13 | 12 | 9 |
| HChC | 10 | 8 | 8 | 6 | 4 | 8 |
| Total (%) | 40 | 31 (77.5) | 28 (70.0) | 19 (47.5) | 16 (40.0) | 17 (42.5) |
Table 4.
Positive results of hybridization by 5 kinds of probe for HBV genes in surrounding hepatic tissues
| Pathological diagnosis | Cases | HBV-DNA | X | pre-S | S | C |
| CAH | 22 | 18 | 18 | 12 | 14 | 13 |
| CPH | 7 | 7 | 6 | 5 | 5 | 5 |
| CIR | 4 | 4 | 4 | 4 | 4 | 4 |
| Total (%) | 33 | 29 (87.9) | 28 (84.8) | 21 (63.6) | 23 (69.7) | 22 (66.7) |
On the basis of statistics of positive cases, among 40 cases of intrahepatic cholangiocarcinoma, HBV-DNA was positive in 33 cases (82.5%), X gene, 31 (77.5%), pre S gene 26 (65.0%), S gene 24 (60.0%) and C gene 27 (67.5%). In cancerous tissues, 9 cases had coexistent positive expression of X gene, pre S gene, S gene and C gene, 6 had positive X and S genes, and 4 had only positive X gene. In the surrounding hepatic tissues, 16 cases showed the coexistent expression of X gene, S gene, pre-S gene and C gene, 5 cases postive X and S genes, and 2 cases only positive X gene.
In the cancerous and surrounding hepatic tissues, the positive hybridized signal of HBV-DNA and X gene appeared as blue-purplish coloured fine granules in the cytoplasm (Figure 6) and the nuclei, or on the cellular membranes, or present in combination (Figure 7). The main manifestation was the nuclear type or nucleocytoplasmic type, while the purely cytoplasmic type was less encountered. The distribution and reaction intensity of positive hybridized signal varied in different cases, results were even different in different places of the same case. In general, the detection rate of the positive cells and intensity of positive reaction in the surrounding hepatic tissues were higher than that in cancerous tissues. The distribution of the positive cells could generally be classified into 3 types: scattered type, only individual positive cells were visible in section; local type, a small number of positive cells gathered in clusters located at certain regions in section; and diffuse type, numerous positive cells distributed diffusely in section. In this study, the local type was chiefly observed, the diffuse type was the next. In the cancerous and surrounding hepatic tissues, the positive signal of pre s gene, s gene and c gene also appeared as blue-purplish coloured fine granules, mainly located in the cytoplasm or nucleocytoplasm (Figure 8 and Figure 9), a few on the cellular membranes. Mostly, nearly the whole cytoplasm was permeated with the positive materials. Most positive cells were local in distribution, and the remainings in diffuse distribution, with scattered distribution least encountered.
Figure 6.
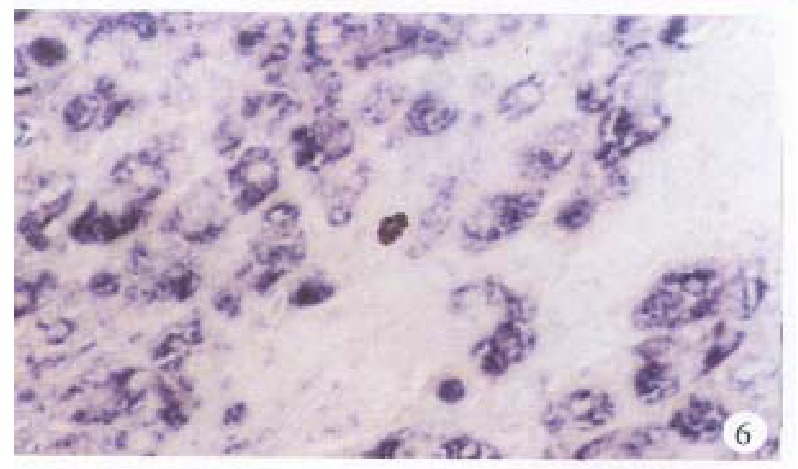
Surrounding hepatic tissue, HBV-DNA gene located in the cytoplasms, some in the nuclei. ISH × 400
Figure 7.
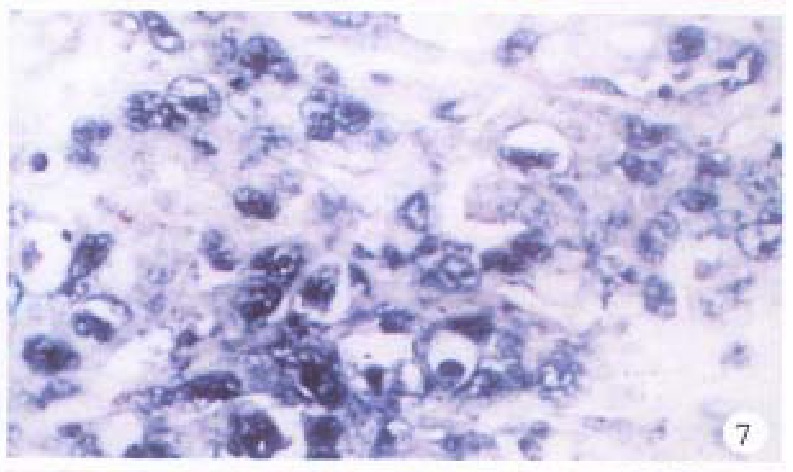
Surrounding hepatic tissue, pre-S gene located in the cytoplasms, some in the nuclei. ISH × 400
Figure 8.
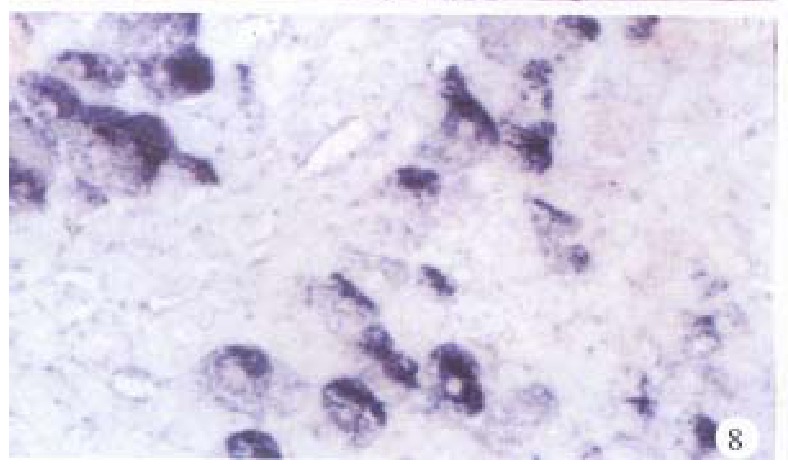
Surrounding hepatic tissue, S gene located mainly in the cytoplasms. ISH × 400
Figure 9.
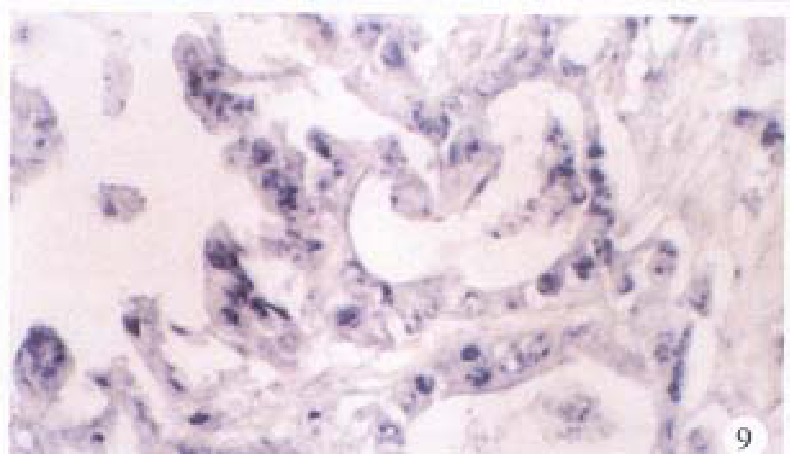
Cancerous tissue, C gene located mainly in the nuclei, some in the cytoplasms. ISH × 400
The abovementioned detection of genes was compared with several control experiments. The positive sections still presented positive by repeated hybridizations. The negative sections and the blank and replacement controls were all negative in results.
DISCUSSION
In human primary hepatic carcinoma, intrahepatic cholangiocarcinoma is relatively less encountered, and few reports were involved in the study of etiology and pathogenesis. This disease was considered to be related to colloidal thoriun dioxide (ChO2)[6]. Hou reported that the development of intrahepatic cholangiocarcinoma bore relationship with Clonorchis sinensis infection by mechanic stimulation of worm-moving and chemical irritation of the bile and tyrosinase from the worm body[6]. In our study, these patients had[5] neither taken colloidal thorium dioxide, nor infected with Clonorchis sinensis. Moreover, the cancerous and surrounding hepatic tissues showed no pathological pattern of parasitic infection. In the cancerous and surrounding hepatic tissues in this series, the detection rate of HBV antigens was obviously high, being 85%, especially the HBxAg, being above 80%. The detection rate of HBV-DNA was 82.5%. Among the genes of X, pre S, C and S, X gene had the highest detection rate of 77.5%. By pathological examination of the surrounding hepatic tissues, CAH was 66.7%, CPH 21.2% and CIR 12.1%. 75.0% of the patients with hepatic carcinoma had a history of chronic hepatitis. This result indicates that the development of intrahepatic cholangiocarcinoma is closely related to HBV infection. The persistent HBV infection plays an important role not only in hepatocellular carcinoma, but also in intrahepatic cholangiocarcinoma.
Up to now, the mechanism of development of intrahepatic cholangiocarcinoma by HBV is still unclear. The HBV and reverse transvirus are homologous in the origin of evolution[7], and it is generally considered that the X gene of HBV and its protein product may play an important role in malignant transformation. It has been discovered that the sera of patients with hepatic carcinoma contained higher anti-HBx with a detection rate of 85.7%[8]. The X gene region might be the important region or HBV integrated into the chromosomes of primary hepatic carcinoma, and the integration of DNA of the host cells with HBV-DNA was completed through the peculiar recombination in the X gene region[9]. The translation product of X gene has a transactive expression of genes[10]. The experiment of transgenic mice manifested that the HBx might directly induce primary hepatic carcinoma[11]. These facts state clearly that HBV may possess probably the direct carcinogenic effect, and HBx gene is the importain factor. Thus, intrahepatic cholangiocarcinoma may be closely related to HBV infection.
About 80% hepatocellular carcinoma cases are accompanied by liver cirrhosis. It can be assumed that HBV infection may induce chronic active hepatitis, further progress to live cirrhosis and finally result in malignant transformation. Fewer intrahepatic cholangio-carcinoma cases are complicated with liver cirrhosis[12]. In our study, only 12% of intrahepatic cholangiocarcinoma were associated with liver cirrhosis, while 87.8% with chronic active hepatitis and chronic persistent hepatitis. This indicates that intrahepatic cholangiocarcinoma is not chiefly developed through liver cirrhosis but mainly through persistent hepatitis. The precise relationship of HBV and development of intrahepatic cholangiocarcinoma remains to be further studied.
Footnotes
Project supported by the National Natural Science Foundation of China, No.39270744.
References
- 1.Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942–1956. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.London WT, Buetow K. Hepatitis B virus and primary hepatocellular carcinoma. Cancer Invest. 1988;6:317–326. doi: 10.3109/07357908809080654. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima T, Kondo Y, Miyazaki M, Okui K. A histopathologic study of 102 cases of intrahepatic cholangiocarcinoma: histologic classification and modes of spreading. Hum Pathol. 1988;19:1228–1234. doi: 10.1016/s0046-8177(88)80156-4. [DOI] [PubMed] [Google Scholar]
- 4.CHANG HP. HEPATIC CLONORCHIASIS AND CARCINOMA OF THE BILE DUCT IN A DOG. J Pathol Bacteriol. 1965;89:365–367. [PubMed] [Google Scholar]
- 5.HOU PC. The relationship between primary carcinoma of the liver and infestation with Clonorchis sinensis. J Pathol Bacteriol. 1956;72:239–246. doi: 10.1002/path.1700720130. [DOI] [PubMed] [Google Scholar]
- 6.Kew MC. Tumors of the liver. In: Zakim D and Boyer TD. Hepatology: a text-book of liver disease. Philadelphia WB Saunders Company. 1982:1065. [Google Scholar]
- 7.Miller RH, Robinson WS. Common evolutionary origin of hepatitis B virus and retroviruses. Proc Natl Acad Sci USA. 1986;83:2531–2535. doi: 10.1073/pnas.83.8.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suqihara S, Kojiro M. Pathology of Cholangiocarcinoma. In: Okudak , Ishak KG, eds , editors. Neoplasma of liver. Japan: Springer Verlag; 1987. p. 143. [Google Scholar]
- 9.KLATSKIN G. ADENOCARCINOMA OF THE HEPATIC DUCT AT ITS BIFURCATION WITHIN THE PORTA HEPATIS. AN UNUSUAL TUMOR WITH DISTINCTIVE CLINICAL AND PATHOLOGICAL FEATURES. Am J Med. 1965;38:241–256. doi: 10.1016/0002-9343(65)90178-6. [DOI] [PubMed] [Google Scholar]
- 10.Zahm P, Hofschneider PH, Koshy R. The HBV X-ORF encodes a transactivator: a potential factor in viral hepatocarcinogenesis. Oncogene. 1988;3:169–177. [PubMed] [Google Scholar]
- 11.Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 12.Ellis EF, Gordon PR, Gottlieb LS. Oral contraceptives and cholangiocarcinoma. Lancet. 1978;1:207. doi: 10.1016/s0140-6736(78)90640-2. [DOI] [PubMed] [Google Scholar]


