Abstract
AIM: To study the therapeutic effects of transcatheter arterial three-segment chemoembolization for hepatocellular carcinoma (HCC).
METHODS: According to the anatomy of vessels, the tumor capillary networks, muscular arterioles and feeding arteries were successively occluded using lipiodol ultra-fluid (LUF), sinobufagin microsphere (SBMs) and particles of gelatin sponge (PGS). In this series of 80 cases, therapeutic effects were evaluated in 76 cases.
RESULTS: There were 22 cases (28.9%) with partial response and 41 (53.9%) with minor response in the 76 evaluated patients. The 6-month, 1-year, 2-year and 3-year survival rates were 97.4%, 86.8%, 46.1% and 27.6% respectively.
CONCLUSION: This regimen was a rational chemoembolization method for HCC patients.
Keywords: Liver neoplasm/therapy; Lipiodol; sinobufagin microsphere; gelatin sponge; chemoembolization, therapeutic
INTRODUCTION
Transcatheter arterial embolization was recommended for the treatment of unresectable HCC in the 70s. Recently, various embolic materials and anticancer agents have been developed. But these methods still have some unsolved problems such as the level of tumor vessels embolization, selection of embolic agents and anticancer drugs, etc. Therefore, we devised the three-segment chemoembolization of the tumor vassels ( TSCTV ) using lipiodol ultra-fluid, sinobufagin microsphere and particles of gelatin sponge for the treatment of HCC patients. The results of the clinical trials are reported below.
MATERIALS AND METHODS
Patients
Eighty consecutive patients with HCC were observed from March 1990 to March 1992. There were 72 males and 8 females, aged from 24 to 69 years (average 49 years). None of them underwent chemotherapy before. The diagnosis of HCC was established by various imaging techniques (including ultrasonography, US; computed tomography, CT; magnetic resonance imaging, MIR; and hepatic angiography, HAG;) and serum alpha-fetoprotein (AFP). The histologic diagnosis was made in 10 cases.
The cases were classified into four stages according to the Manual for Staging of Cancer[1], 2 cases as stage I (2.5%), 6 cases as Stage II (7.5%), 62 as Stage III (77.5%), and 10 as Stage IV (12.5%). The serum AFP levels were greater than 400 μg/L in 42 cases (52.5%). Hepatitis B surface antigen was positive in 62 cases (77.5%). The characteristics of these patients are shown in Table 1.
Table 1.
Clinical characteristcs of patients with hepatocellular carcinoma
| Average age in year (range) | 49 (24-69) |
| Sex (male: female) | 72:8 |
| Serum AFP level (μmol/L) | |
| < 50 | 18 (22.5%) |
| 50-399 | 20 (25%) |
| > 400 | 42 (52.5%) |
| HBsAg+ | 62 (77.5%) |
| TBILT(mg/L) | 16.9 ± 6.0 |
| ALT(u/L) | 68.9 ± 36.6 |
| ALB(g/L) | 38.4 ± 4.4 |
| Child’s grade (a:b:c) | 30:47:3 |
| Stage (I:II:III:IV) | 2:6:62:10 |
TBILT: bilirubin, ALT: glutamic-pyruvic transaminase, ALB: albumin.
Response criteria
The clinical response to the treatment was assessed objectively by the change in tumor size, which was estimated by US, CT and MRI before and after the therapy. The reduction in tumor size was measured at the same image level, presenting the maximum diameter. In cases of the multiple tumors, the largest mass was measured in the same way[2]. Response criteria were defined based on the reduction of the perpendicular diameter in the tumor as follows: (1) A complete response was total disappearance of tumor; (2) A partial response was a reduction in tumor size of more than 50%; (3) A minor response was a reduction of 25% to 50%; (4) No change was a change in tumor size less than ± 25%; (5) Progressive disease was an enlargement of more than 25%. Response criteria were determined once a month for 6 months after TSCTV. Response criteria had to be maintained for at least a month.
Preparation of chemoembolization agents
SBMs were prepared in the department of pharmacy of our college. The drug microsphere, with a mean diameter of 200 μm, was composed of approximately 6% (w/w) of sinobufagin in gelatin[3]. The doses of embolic and anticancer agents were determined by the tumor size and degree of liver dysfuction.
Usually, 10 mL-20 mL LUF and 50 mg-100 mg SBMs were used when the tumor diameter was less than 10 cm, and 20 mL-30 mL and 100 mg-200 mg respectively when the tumor size was more than 15 cm. The doses of the anticancer drugs were aclarubicin (ACR) 50 mg and cir-diaminedichloroplatin (CDDP) 80 mg. For patients with poor liver function anti-cancer drug doses should be reduced. The anticancer drugs were divided into two portions of same dose. A portion was suspended in LUF to make emulsion, the other portion was mixed in 10 mL of radiopaque contrast medium and SBMs to form mixture. The PGS, with a mean diameter of 1 mm, was prepared in our laboratory.
TSCTV
TSCTV was performed through the femoral artery using the technique of Seldinger. A 6.5-French (Cook Co., USA) angiographic catheter was inserted superselectively into the hepatic artery feeding the target tumor.
Initially, the emulsion of the LUF and anticancer drugs were slowly infused till the vessels within tumors were filled. Subsequently, the microsphere mixture was gently infused till the tumor arteries completely disappared. Finally, the PGS, approximatly 1 g-2 g, were infused until the feeding artery of the tumor was occluded. All of these procedures were performed under fluoroscopic guidance to avoid the refluence or spill-over of the embolic agents and anticancer drugs. The second therapy was performed about a month after the first TSCTV. TSCTV was repeated at 3-8 months depending on the patients’ condition. This therapy should be carried out immediately if any of the followings appeared: increase in tumor size, occurrence of new focus within the liver, inadequate accumulation of LUF in the tumor, reelevation of serum AFP ( > 200 μg/L) or GGT ( r-Glutamyltranspeptidase > 350 μg/L).
RESULTS
TSCTV courses and drugs doses
A total of 240 courses of TSCTV were performed, averaging 3 courses per patient, with a range of 1 to 6 courses. Fifty-six cases (70%) underwent more than 3 courses. The mean dose of embolic agents and anticancer drugs in TSCTV are shown in Table 2.
Table 2.
Mean doses of embolic agents and anticancer drugs
| TSCTV courses | No of patients |
Embolic agents |
Anticancer drugs |
|||
| SBMs (mg) | LUF (mL) | SB (mg) | ACR (mg) | CDDP (mg) | ||
| 1 | 80 | 145.0 | 22.3 | 8.7 | 41.8 | 80.0 |
| 2 | 72 | 114.3 | 13.7 | 6.9 | 37.9 | 75.6 |
| 3 | 56 | 107.8 | 15.4 | 6.5 | 40.8 | 76.4 |
| 4 | 24 | 91.7 | 11.7 | 5.5 | 40.0 | 80.0 |
| 5-6 | 6 | 100.0 | 11.2 | 6.0 | 40.0 | 80.0 |
| Mean | 240 | 111.8 | 14.9 | 6.7 | 40.1 | 78.4 |
SB: Sinobufagin
Response
Of 80 patients, 4 was excluded from this study because surgical operations were performed about a month after TSCTV. The remaining 76 patients (95%) were evaluated for responses. According to the response criteria, 22 (28.9%) had partial response, 41 (53.9%) minor responses, while no change in 10 cases (13.1%) and progressive diseases were observed in 3 (3.9%) cases (Table 3). The state of the tumor reduction maintained 4.5 to 49 months. The liquefaction and necrosis within tumors occurred in 60 (79%) cases after the first TSCTV. The necrosis area ranged from 2.5 cm to 6.5 cm (mean 3.8 cm × 3.2 cm). The second hepatectomy was performed in 6 cases within 3-6 months after 1-3 courses of TSCTV. The focuses were resected in 5 (6.6%) of 6 cases. The histological examination demonstrated that there were massive coagulation necrosis and fibrosis of tumor tissue in all cases. No living tumor cells could be seen in the specimens in 2 cases (Figure 1).
Table 3.
Response to TSCTV in evaluated patients
| Stage | CR | PR | MR | NC | PD | Total |
| I | 0 | 0 | 0 | 0 | 0 | 0 |
| II | 0 | 0 | 2 | 2 | 0 | 4 |
| III | 0 | 22 | 36 | 4 | 0 | 62 |
| IV | 0 | 0 | 3 | 4 | 3 | 19 |
| Response | 0 | 22 | 41 | 10 | 3 | 76 |
| Percentage | (0%) | (28.9%) | (53.9%) | (13.1%) | (3.9%) |
Figure 1.
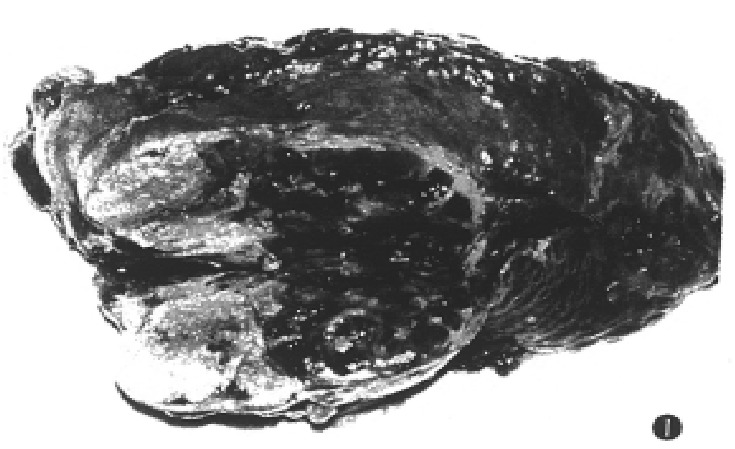
The resected specimen showing massive coagulation necrosis and fibrosis in tumor.
Survival rate
The starting point was defined as the day 0 initial TSCTV therapy. The 6-month, one-year, 2-year and 3-year survival rates were 97.4% , 86.8%, 46.1% and 27.6% (Table 4). The longest survival time was up to 49 months. During the disease palliation phase, the life quality of the patients with tumors was satisfactory.
Table 4.
Survival of 76 patients undergoing TSCTV
| Stage | No of patients |
No of survival (year) |
|||
| 0.5 | 1 | 2 | 3 | ||
| I | 0 | 0 | 0 | 0 | 0 |
| II | 4 | 4 | 4 | 3 | 2 |
| III | 62 | 62 | 60 | 32 | 19 |
| IV | 10 | 8 | 2 | 0 | 0 |
| Total | 76 | 74 | 66 | 35 | 21 |
| Percentage | (97.4%) | (86.8%) | (46.1%) | (27.6%) | |
Laboratory examinations
The liver function, peripheral white blood cell counts, platlet counts and AFP level were examined before and after TSCTV (1, 2 and 4 weeks). The serum AFP, which was more than 200 μg/L before treatment in 62 patients, apparently fell in 58 patients (94%) about 2 weeks after TSVCTV. Serum AFP was lowered to normal (AFP < 200 μg/L) after 2-4 courses of TSCTV in 28 (48.3%) cases. The changes of serum AFP level in 42 cases (AFP > 400 μg/L) are shown in Figure 2. The ALT level showed dropping tendency a week after TSCTV and then, elevated again in some patients after 2-3 weeks. The peripheral WBC of all patients increased after first TSCTV, < 3.5 × 109/L in 2 cases after the third TSCTV. The results of laboratory examinations are shown in Figure 3.
Figure 2.
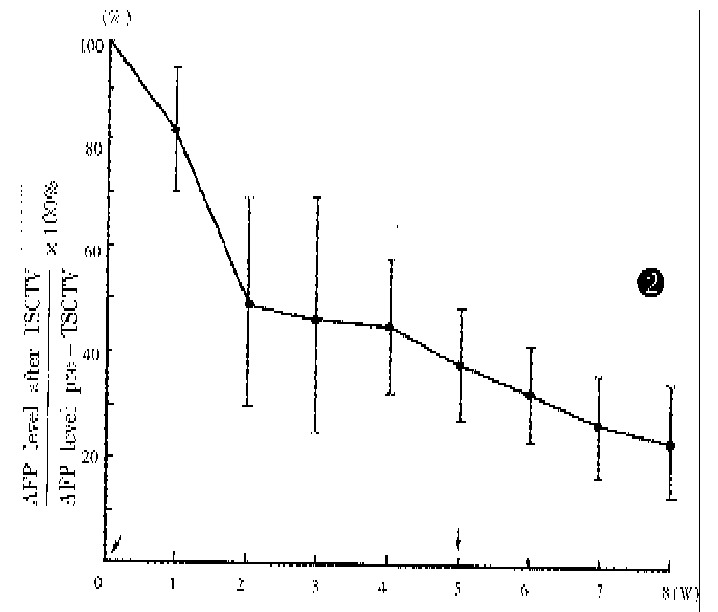
Serum AFP levels in 42 cases (AFP > 400 μmol/L) after TSCTV, assuming that pre-embolization value was 100% (↓TSCTV treatment).
Figure 3.
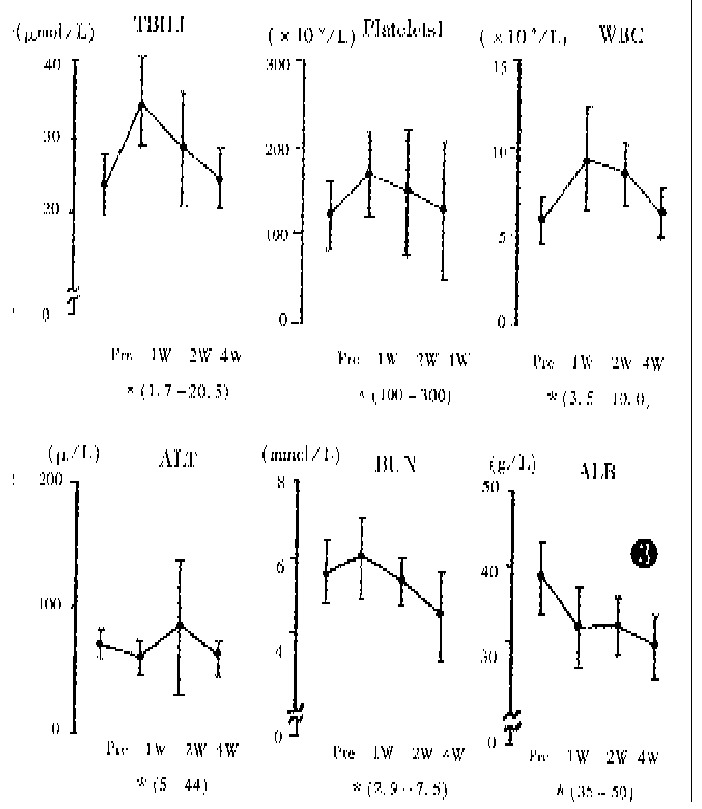
Changes in blood chemical data after first TSCTV (pre: level before TSCTV; W: week; *: normal range).
Side effects and complications
The postembolization syndrome (including fever, right hypochondrium pain, and nausea and vomiting) was seen in all patients after TSCTV. The serious degree of the syndrome was strongly correlated with the area of the liquefaction and necrosis within tumor and the doses of the embolic agents and anticancer drugs. After the first TSCTV, the syndrome was severe in some patients and generally maintained 1 to 3 weeks. The side effects and complications of TSCTV are shown in Table 5.
Table 5.
Side effects and complication
| Side effects and complications | Incidence No.(%) |
| Fever 38-39 °C | 71 (88.7) |
| > 39 °C | 18 (22.5) |
| Nausea and vomiting | 60 (75.0) |
| Right hypochondrium pain | 72 (90.0) |
| Cholecystitisa | 5 (6.2) |
| Liver abscess | 1 (1.2) |
| Hapatic failure | 4 (5.0) |
| Ascites | 6 (7.5) |
aThe floccule was found in gall bladder by US
DISCUSSION
According to the electronic microscopic observation, the arterial system of the HCC is composed of three kinds of successive vessels. There are massive neonate capillary networks within tumor consisting of single layer endothelium cells. Its angiographic imaging is revealed as vascular mass. The successive vessels are muscular arterioles with diameters of about 100 μm-250 μm. There are extensive arterio-portal vein shunts in these massive neonate vessels. The blood flow of the portal vein might reflux into the empty arterioles through these shunts as soon as the feeding arteries are occluded. The blood supply of the tumor renews in short time so that the inhibiting effect in tumors disappear soon. The successive feeding arteriols usually become wide and tortuous and are accompanied by “stealing blood” phenonenon[4,5]. In light of the vessels anatomy, we designed the TSCTV method. There are several possible mechanisms of TSCTV that can prolong the survival rates: (1) The capillary networks, muscular arterioles and feeding arteries of the tumor are successively occluded using three embolic agents of various diameters and properties so that direct ischemic changes within tumors are caused by liquid embolic agent, LUF, and solid embolic agent, SBMs; while proper occlusion of feeding arteries surrouding the tumor is also encountered by expansive embolic agent, PGS. Thus, the occlusion of tumor vessels is more rational and complete. (2) The less viscosity and better compatibility of LUF are favourable to occlusion of capillary networks in tumor and dispersion of anticancer drugs into oil phase[6]. (3) By complex chemical cross linking, the degradation of the gelatin microsphere is delayed in vivo. After infusion, SBMs can block blood flow, delay the “washout” of LUF (Figure 4), prevent reflux of the portal vein blood and, simultaneously, slowly releave the drug locally. (4) While occluding feeding and collateral arteries of the tumor, the PGS can avoid the refluxing of embolic agents and anticancer drugs into gallbladder and gastroduodenal arteries, resulting in severe side effects[7]. (5) Because anticancer drugs suspended in LUF and SBMs, forming the structure of “three-ply-board” after infusion introduced as regional chemotherapy, anticancer drugs seem to be delivered to and accumulated selectively within the tumor[8,9]. (6) Sinobufacin, as an anticancer drug of Chinese traditional materials, has its own efficiency on HCC treatment, which is superior to fluorouracil. The drug can cause vessels inflammation and the remarkable endovasculitis and leading secondary embolization in the embolized vassels, enhancing the effect of arterial occlusion. In addition, the drug is very effective for cardiotonic, diuresis, antisepsis, analgesia and increased peripheral white blood cells, enhancing body immunofunction, etc[3]. These complementary pharmocologic effects can augment the therapeutic effects and reduce systemic side effects. (7) ACR and CDDP are, at present, a better combination of chemotherapy, and have synergistic anticancer effects[7]. (8) This method caused relatively mild, damage to liver and renal functions so that it is acceptable to most patients.
Figure 4.
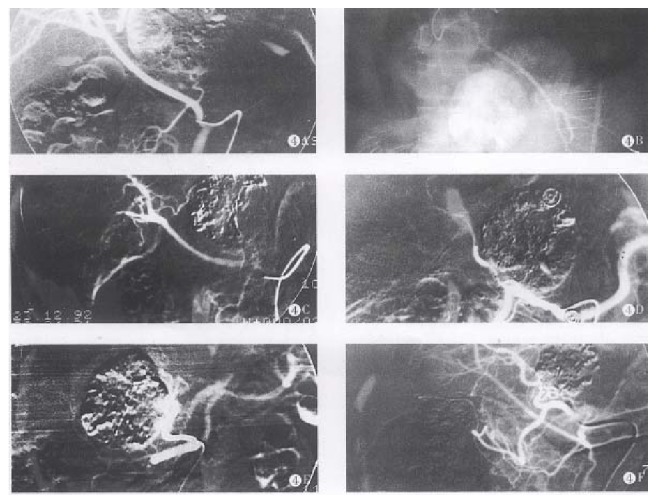
A. The hepatic right artery derived from superior mesenteric artery after TSCTV was performed. The left hepatic artery derived from gastroduodenal artery and supplying left and lower 1/3 of the tumor, to prevent embolic agents reflux, lipiodol chemoembolization (LCE) was performed. B. The tumor vessels disappeared completely and the lipiodol accumulated within tumor after embolization. C. The lipiodol was obviously washed out in the LCE area after 4 months, TSCTV and LCE were repeated. D. The lipiodol was washed out fourth in the LCE area after 6 months. E. LUF 5 mL and SBMs 50mg were infused through hepatic left artery, the tumor vessels completely disappeared, the LUF accumulated in the LCE area. F. The tumor obviously shrank (PR) and lipiodol accumulated within tumor after 19 months.
The outcomes of our clinical trial demonstrate that TSCTV can significantly improve the completeness of the tumor vassels occlusion, the area of li-quefaction and necrosis of the tumor, stay time of embolic agents within tumor vessels, partial response rate and survival rate, as compared with the treatment using microsphere or lipiodol alone.
It was found that after occlusion, the collateral circulation occurred sooner or later in most tumors. Where there was vessels occlusion, there was the formation of collateral circulation. Therefore, occlusion should be performed until the tumor vessel completely disappeared in each course of treatment so that the extensive and fast necrosis of the tumor cells can be induced. The necrosis of tumor cells might result in high response rates, survival rates and prevent metastasis within the liver[3,7]. The doses of embolic agents should be modest, as excessive embolic agents might cause reflux and side effects.
Tumor progression occurred in some patients with time. Timely re-TSCTV could usually inhibit or prevent the tumor progression. Therefore, repeated TSCTV was very important in ensuring the-rapeutic effects. We found that more than 3 couses of TSCTV was essential.
However, the effect of TSCTV was not so satisfactory in stage IV patients, especially in those with tumor thrombus of the major portal bronchus. In our study, there were 4 cases of hepatic failure. Therefore, this method should be carefully adopted in those patients.
The post-embolization syndrome was significant by this method. The causes might be as follows: (1) Complete occlusion of the tumor vessels resulted in remarkable necrosis of the tumor; (2) Sinobufagin caused the vasculitis of the embolized vessels; and (3)The embolic agents and anticancer drugs refluxed into gallbladder artery, leading to necrosis of the gallbladder mucosa (Figure 5). To prevent the occurrence of the second liver abscess and cholecystitis, it was essential to administer sufficient antibiotics after TSCTV.
Figure 5.
The necrosis of the gallbladded mucosa.
In conclusion, based on anatomy of the vessels, TSCTV combines with the advantages of the three embolic agents so that occlusion of the tumor vessels was more complete and rational. The embolization effect was strengthened by SBMs causing endovasculitis and second occlusion. The structure of the “three-ply-board” can form a high concentration region of anticancer drugs. Three anticancer drugs complement with each other in pharmocologic action, thus augmenting the anticancer effect and reducing systemic side effects. From these results, it is concluded that the TSCTV is a ideal therapeutic regimen for HCC patients.
Footnotes
Project supported by the Foundation of Jiangsu Province Public Health Bureau, No. H-93-24.
References
- 1.American Joint Committee on Cancer. Liver. In: Hermanek P, ed. manual for staging of cancer. Ed 3. Fhiladelphia: LB Lippincott. 1988:87–94. [Google Scholar]
- 2.Yamashita Y, Takahashi M, Koga Y, Saito R, Nanakawa S, Hatanaka Y, Sato N, Nakashima K, Urata J, Yoshizumi K. Prognostic factors in the treatment of hepatocellular carcinoma with transcatheter arterial embolization and arterial infusion. Cancer. 1991;67:385–391. doi: 10.1002/1097-0142(19910115)67:2<385::aid-cncr2820670212>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Fan J, Huang MK, Wu GY. An experimental study of regional chemotherapy with adriamycin and gelatin microsphere. Tiedao Yixue. 1995;14(6):434. [Google Scholar]
- 4.Akerman N. Experimental studies of the circutatory dynamics of intrahepatic tumor blood supply. Cancer. 1972;29(2):435–437. doi: 10.1002/1097-0142(197202)29:2<435::aid-cncr2820290227>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Li CL, Wang CE, Cao XH. [Vascular cast of primary liver cancer and scanning electron microscopic observations] Zhonghua Waike Zazhi. 1986;24:620–61, 640. [PubMed] [Google Scholar]
- 6.Shibata J, Fujiyama S, Sato T et al. Hepatic arterial injection chemotherapy with cisplatin suspended in an oily lymphographic agent for hepatocellular carcinoma. Cancer. 1989;64(12):1586–1589. doi: 10.1002/1097-0142(19891015)64:8<1586::aid-cncr2820640805>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Toru B, Chitoshi O, Yasuo Y et al. A new approach to chemoembolization for unresectable hepatocellular carcinoma using aclarubicin microspheres in combination with cisplatin suspended in iodized oil. Cancer. 1991;68(12):1555–1558. doi: 10.1002/1097-0142(19911215)68:12<2555::aid-cncr2820681204>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Fan J, Huang MK, Wu GY. An experimental study on hepatic arterial chemoem bolization with sinobufagin microspheres. Chin J Cancer. 1995;14(6):434–436. [Google Scholar]
- 9.Lin DY, Liaw YF, Lee TY, Lai CM. Hepatic arterial embolization in patients with unresectable hepatocellular carcinoma--a randomized controlled trial. Gastroenterology. 1988;94:453–456. doi: 10.1016/0016-5085(88)90436-2. [DOI] [PubMed] [Google Scholar]


