Abstract
AIM: To study the radioimmunoimaging (RAII) using the human/mouse chimeric Ab to evaluate its targeting activity in animal models.
METHODS: To chimeric Ab was labeled with 131I. RAII was performed at different intervals after injection of radio-labeled Abs in nude mice with human hepatoma xenograft, and tissue distribution of radioactivity was measured. Comparison was made in the chimeric Ab between the single segment Ab and previous murine mAb against HBxAg.
RESULTS: The experimental objects developed tumor-positive image after 2 days of radio-labeled Abs injection, and the peak accumulation of radioactivity fell on the 7th day. The tumor/liver ratioactivity of the chimeric Ab, single segment Ab, anti-HBx mAb, and the control group was 281 ± 0.21, 2.44 ± 0.16, 4.60 ± 0.19, and 0.96 ± 0.14, respectively.
CONCLUSION: The genetic engineering Abs have a considerable targeting activity which can be used as a novel humanized vector in the targeting treatment of liver cancer.
Keywords: liver neoplasms, experimental; carcinoma, hepatocellular; chimeric antibody; mice, nude; hepatitis B virus; disease models, animal; radioimmunodetection; radioimmunotherapy
INTRODUCTION
Human hepatocellular carcinoma (HCC) is one of the most common malignant tumors in China. Patients with such a tumor can not, by and large, be diagnosed in its early stage and, therefore, lose the chance for operation. The purpose of this study was to explore the potential value of chimeric human/ mouse Ab applied to the treatment of HCC in targeting therapy. Clinical evidence showed that the anti-HBx mAb prepared by HCC-related hepatitis virus X protein was effective vectors[1-3]. However, because of its murine-derived nature, the protein had certain immunogenecity, thus hindering its cli-nical applications in targeting therapy considerably. In order to circumvent the obstacle, modifications have been made to humanize the Abs using genetic engineering techniques. The light- and heavy-chain variable region (VH, VL) gene of the cloned anti-HBx mAb were ligated with the constant region ( CH ) gene of human IgG, resulting in the construction of a human/mouse chimeric Ab gene and itsexpression in the prokaryotic vector[4]. In this study, we carried out the radioimmunoimaging (RAII) on nude mice models bearing human HCC using 131I-labeled chimeric Ab, aiming at characterizing the localization of the chimeric Ab in the HCC, and eva-luating its potential use as a vector in targeting therapy.
MATERIAL AND METHODS
Preparation of the chimeric human/mouse Ab and the single segment Ab
The chimeric Ab gene was cloned into pUC19, and subcloned into expressing plasmid PBLMVL2, which was a gift from Professor Wu (Shanghai Institute of Biochemistry, Chinese Academy of Sciences, China). A single colony from a fresh plate was incubated in a 3 mL culture and grown overnight at 30 °C; then transferred into fresh medium, incubated at 30 °C for 2 h-3 h until A600 = 0.5, and then induced in a medium containing 15 mM MgSO4 at 42 °C for 3 h. The bacteria were collected, cytolysed by ultrasonication, and aspirated. The supernatant was dried by vaccum and stored at - 20 °C.
Labeling of the Abs
The chimeric Ab, single segment Ab, anti-HBx mAb and expression protein of E. coli (TG1) were labeled with 131I (provided by the Chinese Atomic Energy Institute) by the Iodogen method. Unincorporated 131I was separated from the bound iodine by gel filtration on a Sephadex G-50 column. The whole process of labeling was a bacteria free and pyrogen free event[5].
Animal model
22 LTNM4 nude mice models bearing human HCC by subcutaneous implantation were established in the authors’ institute. Of these models, 5 mice were injected with chimeric Ab, 5 with single segment Ab, 5 with anti-HBx mAb as positive control, 5 with 131I as negative control, and 2 with TG1. A small amount tumor sample (2 mm3-3 mm3) of HCC tissue originated from a nude mouse was transplanted subcutaneously into the 22 nude mice, and tumors measuring 1 cm in diameter were used for the immunoimaging study[6].
Radioimmunoimaging and biodistribution analysis
The experimental and control groups were injected intraperitoneally with 131I-labeled human/mouse chimeric Ab, single segment Ab, anti-HBx mAbs, TG1 and 131I respectively at a dose of 250 μCi per mouse. And γ imaging was operated on the 1st, 5th, and 7th day after injection. All animals were killed on the 7th day following the injection in order to determine the biodistribution of their radioactivity.
RESULTS
Immunoactivity assay of the radio-labeled Abs
The human/mouse chimeric Ab, single segment Ab, anti-HBx mAb, and TG1 were labeled by the Iodogen method. After separation of unincorporated 131I from bound iodine, the binding activity of the chimeric Ab, single segment Ab, and anti-HBx mAb remained unchanged by ELISA (Figure 1).
Figure 1.
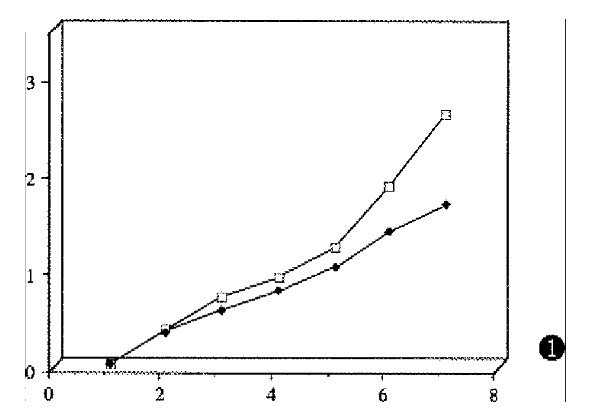
The binding activity curve of chimeric Ab by ELISA. MoAbs: anti-HBx mAb; sfv: anti-HBx human/mouse chimeric Ab
Radioimmunoimaging of nude mice models bearing HCC
One day after injection of the 131I-labeled human/ mouse chimeric Ab and single segment Ab, the experimental animals showed distinct radio-accumulation in their abdominal cavities, whereas a faint -image was developed in tumor region. From the 5th day following injection, the distinct background image gradually disappeared, and an obvious radio-accumulation developed in tumor region. This phenomenon lasted until the 7th day. The positive control displayed tumor-positive image on the 5th day following injection of anti-HBx mAb, and even more clear tumor image was obtained on the 7th day which was in conformity with the previous reports. No accumulation was found in the negative control with injection of TG1 and 131I. Since the thyroid glands of the nude mice were not blocked, they showed obvious radio-accumulation in the experiment (Figure 2).
Figure 2.
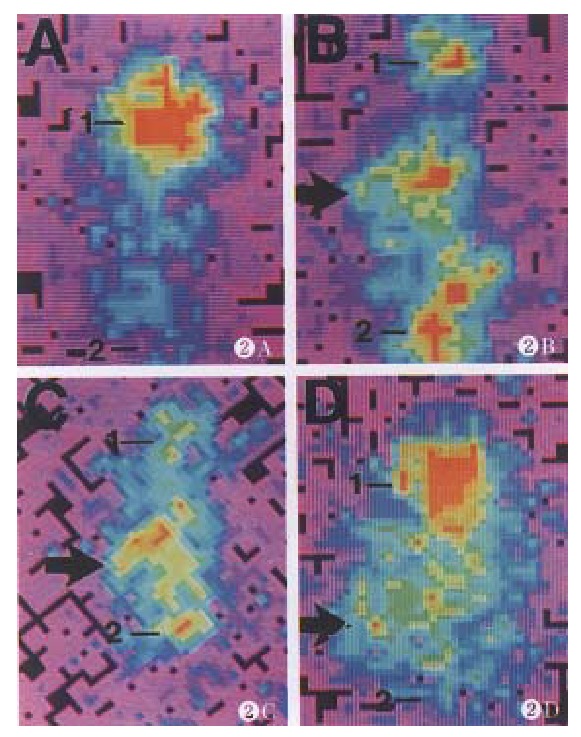
The localization of 131I-labeled chimeric Ab in nude mice bearing human HCC. A: 131I-TG1; B: 131I-chimeric Ab; C: 131I-single segment Ab; D: 131I-anti-HBx mAb. Arrow: the tumor image. 1. The thyroid gland area; 2. The bladder area
Biodistribution of the radio-labeled Abs
Table 1 shows the radioactivity of tumor versus other tissues expressed as T/NT ratio following the injection of radio-labeled Abs after 7 days.
Table 1.
T/NT ratio of 131I in nude mice models bearing human HCC (-x ± s )
| 131I-labeled chimeric Ab | 131I-labeled segment Ab | 131I-labeled anti-HBx mAb | 131I | 131I-TG1 | |
| Blood | 1.81 ± 0.35 | 3.26 ± 0.09 | 1.70 ± 0.05 | 0.76 ± 0.14 | 1.18 |
| Liver | 2.81 ± 0.21 | 2.44 ± 0.12 | 4.60 ± 0.20 | 0.96 ± 0.14 | 0.41 |
| Kidney | 0.48 ± 0.09 | 2.22 ± 0.07 | 2.66 ± 0.15 | 0.42 ± 0.05 | 0.69 |
| Spleen | 0.64 ± 0.09 | 1.59 ± 0.13 | 1.74 ± 0.01 | 0.97 ± 0.09 | 0.37 |
| GI | 1.06 ± 0.03 | 0.40 ± 0.08 | 2.67 ± 0.33 | 0.90 ± 0.01 | 0.07 |
| Heart | 3.84 ± 0.37 | 3.23 ± 0.23 | 6.10 ± 0.51 | 1.86 ± 0.15 | 2.39 |
| Bone | 4.25 ± 0.20 | 5.71 ± 0.11 | 6.70 ± 0.24 | 1.84 ± 0.13 | 1.97 |
| Muscle | 10.86 ± 0.76 | 11.39 ± 0.26 | 10.38 ± 0.16 | 3.62 ± 0.22 | 4.07 |
The tumor/liver ratio in human/mouse chimeric Ab and single segment Ab was 2.81 and 2.44, respectively in the experimental group, which was significantly higher than that of 131I and TG1 in the negative control group (0.96 and 0.41). But they are lower than the tumor/liver ratio of murine derived mAb (4.6) in the positive control group. In addition, there has been a high accumulation of radioactivity in blood, gastrointestinal tract and kidney, especially predominant in the human/mouse chimeric Ab.
DISCUSSION
Targeting therapy has been applied to the clinical treatment of liver cancer as a powerful means to kill tumor cells for more than a decade. In this context, mAbs were usually the first choice. However, because of the murine origin of these mAbs, their repeated use often led to generation of anti-antibodies which would markedly reduce their bio-efficiency. Although several methods were adopted such as changing species and varieties of antibodies’ origin, adding immunosuppressors, etc., the incidence of human anti-mouse Abs (HAMA) was 63%-95% after the treatment with murine derived antibodies. The incidence of HAMA was 34.4% in our report[7]. The ultimate approach to solving this problem is, perhaps to reduce the immnogenicity of antibodies, that is to humanize the antibodies.
Based on our previous results, the expression level of X gene of hepatitis B virus was much higher than that of s gene and c gene, the T/NT ratio of anti-HBx- mAb approached 4.5 in the nude mice models, clinical targeting therapy trial confirmed that anti-HBx mAb was an exciting vector, and a humanized chimeric Ab gene had been established[4,5,8], we are trying to evaluate its potential value in the targeting therapy as a novel vector.
The results indicate that a distinct accumulation of tumor radioactivity developed on day 5 after injection of radio-labeled chimeric Ab, and an even more obvious accumulation was obtained on day 7. In bio-distribution analysis, the T/NT ratio on day 7 of the chimeric Ab, single segment Ab, positive control anti-HBx mAb, negative control TG1 and 131I was 2.81, 2.44, 4.60, 0.41 and 0.96, respectively. This promising result suggests that recombinant Abs do have affinity to liver cancer tissues. Therefore, they can specifically direct against liver cancer tissues.
Although the chimeric Ab displayed certain high T/NT ratio, it did not surpass that of murine derived mAb. The reason behind this may be as follows: (1) The Ab used in this experiment was the expression product of E. coli. When it was extracted from the bacteria by sonic cytosis, the extracts were none other than primary yields of E. coli. They contained not only the heat-induced Ab protein, but also other impure proteins derived from the expression of E. coli itself. For fear of possible inactivity of the Ab by gel filtration method, the expression product was not further separated and purified. This may account for the relatively low T/ NT ratio in E. coli; (2) The chimeric Ab was the product of prokaryotic expression vector. It is short of post-translational processing toward foreign protein by a eukaryotic system, such as glucolation, methylation, carboxylation, etc,. This may result in the production of an imperfect protein from which the experimental results may suffer.
In regard to the relatively high accumulation of radioactivity in blood, kidney, spleen, etc., the possible answer may be due to the impure Ab protein containing foreign substances which were apt to be uptaken by mononuclear cells, leading to the increase in the nonspecific binding.
The labeling ability was quite low for the chimeric Ab and single segment Ab in this experiment. Initially, we attributed this fact to fewer lysine groups of the recombinant Ab than those of murine derived mAb, for the principle of Iodogen method is to incorporate iodine with lysine groups of protein. Later, we found the amount of lysine groups of the genetic engineering Ab were no less than those of murine derived mAb. Therefore, our conclusion in terms of the low labeling ability of the recombinant Ab was the impurity of the protein with excess of foreign substances, and the lack of modification during post-translational processing. In a word, recombinant chimeric Ab has the advantages of low immunogenicity and high bio-efficiency of antibody-mediated ADCC and CDC, which certainly approve its qualification in clinical use. There is, however, much to be desired in terms of the practical use of chimeric Ab in clinical targeting therapy. Currently, work is underway in our institute to further separate and purify these chimeric Ab, make it expressed in eukaryotic systems in order to obtain intact secretory Ab.
In line with the advance of novel technologies in biomedical sciences, the increasing use of phage display antibody library technique, the antibodies, derived from the total antibody library, can be constructed in vitro without immunization in vivo . It can be well predicted that in the near future, certain active proteins with high affinity will be produced to benefit the mankind.
Footnotes
Project supported by the National Natural Science Foundation of China, No.39370681.
References
- 1.Li KD. [Evaluation of radioimmunotherapy in the multimodality treatment of hepatocellular carcinoma (HCC)] Zhonghua Zhongliu Zazhi. 1992;14:430–432. [PubMed] [Google Scholar]
- 2.Liu KD, Tang ZY, Fan Z, Lu JZ, Yu YQ, Zhou XD. Radioimmunotherapy in treatment of unresectable hepatoma: a report of 43 cases. Chin J Cancer Res. 1994;6(1):74–78. [Google Scholar]
- 3.Sands H. Experimental studies of radioimmunodetection of cancer: an overview. Cancer Res. 1990;50:809s–813s. [PubMed] [Google Scholar]
- 4.Zhou G, Liu KD, Tang ZY, Chen YH, Wu XF, Schroeder CH. Reconstruction and expression of chimeric anti-HBx antibody in Escherichia coli. J Cancer Res Clin Oncol. 1997;123:325–330. doi: 10.1007/BF01438308. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Tang Z, Liu K. [Application of radiolabeled anti-HBx monoclonal antibody for HCC targeting therapy] Zhonghua Yixue Zazhi. 1996;76:271–274. [PubMed] [Google Scholar]
- 6.Liu KD. [Targeting study of human hepatocellular carcinoma (HCC) using human HCC model in nude mice] Zhonghua Zhongliu Zazhi. 1988;10:414–416. [PubMed] [Google Scholar]
- 7.Liu KD, Tang ZY, Lu JZ, Fan Z, Zeng SC, Xie H et al. Long-term results of targeting therapy using radiolabeled antibodies in multimodality treatment of hepatocellular carcinoma (HCC)-analysis of 75 cases. Acta Acad Med Shanghai. 1995;22(suppl):14–18. [Google Scholar]
- 8.Li J, Tang ZY, Liu KD, Schroder CH. [Preparation of monoclonal antibody directed against hepatitis B virus X protein and detection of reactive antigen in hepatocellular carcinoma] Zhonghua Yixue Zazhi. 1994;74:533–55, 582. [PubMed] [Google Scholar]


