Abstract
Objectives
To test the hypothesis that testosterone replacement therapy (TRT) improves the long-term health-related quality of life (HRQoL) of men with late-onset hypogonadism (LOH), as studies have shown that sub-physiological testosterone levels have a negative impact on psychological (e.g. mood, vitality, libido and sexual interest) and physical features (e.g. erectile function and physical strength), all of which contribute to a sense of well-being.
Patients and methods
In all, 261 patients (mean age 58 years) diagnosed with LOH were treated with long-acting intramuscular testosterone undecanoate (TU) for up to 5 years. Health quality indicators including the International Prostate Symptom Score (IPSS), the five-item version of the International Index of Erectile Function (IIEF-5), the Aging Males’ Symptoms (AMS) scale, and the percentage of patients reporting joint and muscle pain were measured at baseline and at each visit. The means were then plotted over time in parallel with mean total testosterone (TT) levels.
Results
Both the mean IPSS and AMS scores fell significantly within the first 3 months and the mean IIEF-5 score and TT levels increased within the first 3 months. All four parameters continued to improve over the course of the trial. The percentage of patients reporting both joint and muscle pain decreased during TRT.
Conclusions
This prospective, observational and longitudinal analysis shows a clear improvement in both psychological and physical characteristics as physiological testosterone levels are reached and maintained contributing to an improvement in the HRQoL in men with diagnosed LOH.
Abbreviations: AMS, Aging Males’ Symptoms (scale); ED, erectile dysfunction; HRQoL, health-related quality of life; IIEF-5, five-item version of the International Index of Erectile Function; LOH, late-onset hypogonadism; TRT, testosterone replacement therapy; TT, total testosterone; TU, testosterone undecanoate
Keywords: Testosterone, Late-onset hypogonadism, Erectile dysfunction, Depression, Obesity
Introduction
Late-onset hypogonadism (LOH), as defined by a serum total testosterone (TT) level of ⩽12 nmol/L, is diagnosed when declining testosterone concentrations in the ageing male cause unwanted symptoms such as erectile dysfunction (ED), obesity, lack of physical strength, and depressed mood [1], [2], [3], [4]. It is thought that almost 40% of men aged >45 years are hypogonadal to some degree [5].
Numerous studies have shown that testosterone levels are closely associated with both erectile function and obesity [6], [7], [8]. In fact, the presence of visceral obesity can predict ED [9] and weight loss improves erectile function and testosterone levels [10], [11]. Increasing evidence shows that testosterone can reduce both total and visceral body fat [12], [13], [14], [15], [16], and is an effective treatment for ED, as measured by the International Index of Erectile Function (IIEF) questionnaire [17], [18], [19].
Testosterone also maintains psychological features such as mood, vitality, libido and sexual interest, which contribute greatly to an overall sense of well-being and health-related quality of life (HRQoL). Consequently, in men with LOH there is an increase in symptoms such as dysphoria, low vigour and vitality, diminished libido and orgasm, irritability, poor cognitive function, and increased risk of depression [20], [21], [22], [23]. Associations have also been reported between both ED and depression [24], and obesity and depression where obese patients at baseline were at an increased risk of depression at follow-up [25]. Evidence that testosterone can effectively treat symptoms of depression comes from a randomised, placebo-controlled phase III trial in which 184 men with hypogonadism and metabolic syndrome were treated with testosterone undecanoate (TU) for 30 weeks. At the end of the trial period TU was found to have significantly improved depressive symptoms as measured by the Beck Depression Inventory (BDI-IA) [26].
In men undergoing androgen-deprivation therapy for prostate cancer, mood disturbances, anxiety, fatigue, and lack of drive are observed [27]. In a cohort of elderly hypogonadal men in the European Ageing Male Study, low testosterone levels were found to be associated with ED, low sexual desire, poor morning erections, fatigue, and depression [28]. Thus, low testosterone-associated sexual dysfunction has a major impact on HRQoL and emotional well-being [29], [30], [31].
Hypogonadal men undergoing testosterone replacement therapy (TRT) show improved parameters of well-being, bone density, muscle mass, physical strength, sexual function, and libido [32]. In a study of men undergoing treatment with TU, there was an increase in libido, vigour and vitality, sleep quality, a reduction in waist circumference, and a decrease in severity of ED [33]. Therefore, TRT in hypogonadal men may be a valuable tool to restore a physiological balance and achieve sexual pleasure as a component of well-being.
We hypothesised that TRT would improve both psychological and physical features that contribute to the long-term HRQoL in men with LOH.
Patients and methods
From November 2004, 261 patients (mean age 58 years) diagnosed with LOH were treated with long-acting TU (Nebido®, Bayer Pharma, Berlin, Germany) in a prospective observational and longitudinal registry study. All patients gave their written informed consent to be included in the study, which was conducted according to ethical guidelines as formulated by the German ‘Ärztekammer’ (the German Medical Association) for observational studies and followed the principles outlined in the Helsinki Declaration of 1975, as revised in 1983. Men with a TT concentration of ⩽3.5 ng/mL (12 nmol/L) and documented symptoms of ED (Sexual Health Inventory for Men (SHIM) score of ⩽21) met the inclusion criteria. Men received i.m. injections of 1000 mg TU at day 1 (≈3 weeks after diagnosis), week 6 and every 3 months thereafter. Patients were entered into a cumulative registry database once they had received treatment for at least 1 year and followed for up to 5 years. The average treatment period was 4.25 years.
Assessment of outcome
To assess the effect of long-term TU treatment, health quality indicators including the IPSS, the five-item version of the IIEF (IIEF-5), the Aging Males’ Symptoms (AMS) scale, and the percentage of patients reporting joint and muscle pain were measured among the study population at baseline and at every subsequent visit. The means were then calculated and plotted over time in parallel with mean TT levels. A P < 0.05 was considered to indicate statistical significance.
Results
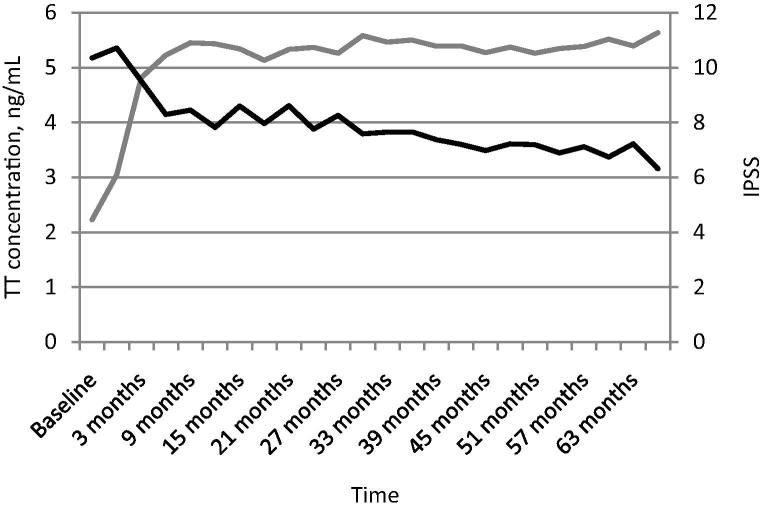
The mean IPSS measured at baseline was 10.35, which fell significantly within the first 3 months to 9.5 (P < 0.05). This then decreased steadily over the remainder of the trial period and measured 7.22 after 63 months of TRT (Fig. 1).
Figure 1.
Long-term TU treatment reduces the mean IPSS over time. The mean IPSS is shown in black; TT level (ng/mL) is shown in grey. Time is shown along the x-axis.
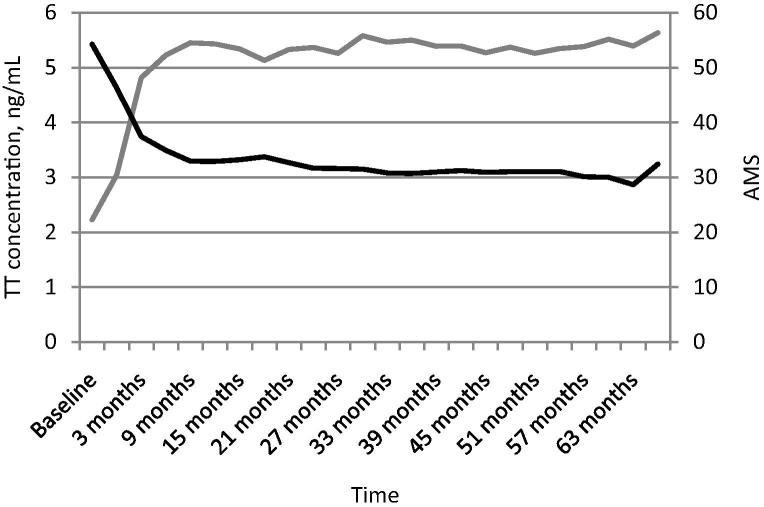
The mean AMS score measured 54.27 at baseline and dropped dramatically to 37.42 within the first 3 months of the trial (P < 0.05). The mean AMS score subsequently fell gradually to 28.66 by the end of the trial (Fig. 2).
Figure 2.
Long-term TU treatment reduces the mean AMS. The mean AMS score is shown in black; TT level (ng/mL) is shown in grey. Time is shown along the x-axis.
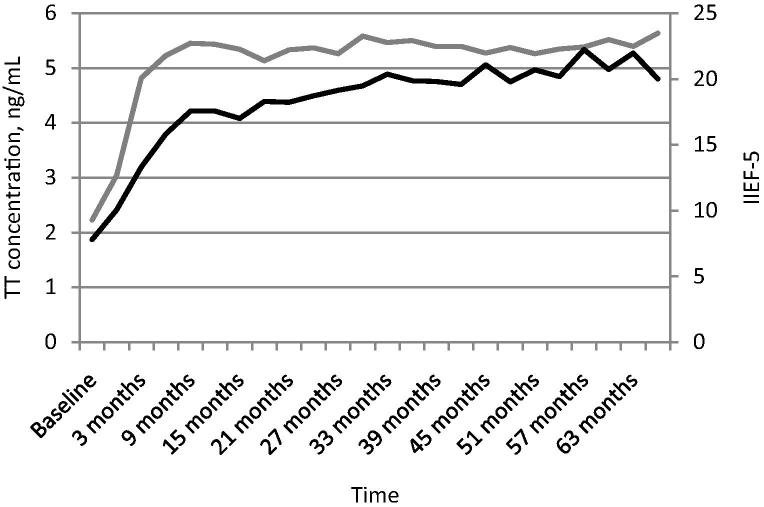
At baseline the mean IIEF-5 score was 7.8 and increased to 13.31 over the first 3 months of the trial. By 9 months the mean IIEF-5 score was 17.55 and by the end of the trial was 21.96 (Fig. 3).
Figure 3.
Long-term TU treatment improves the mean IIEF-5 score over time. The mean IIEF-5 score is shown in black; TT level (ng/mL) is shown in grey. Time (years) is shown along the x-axis.
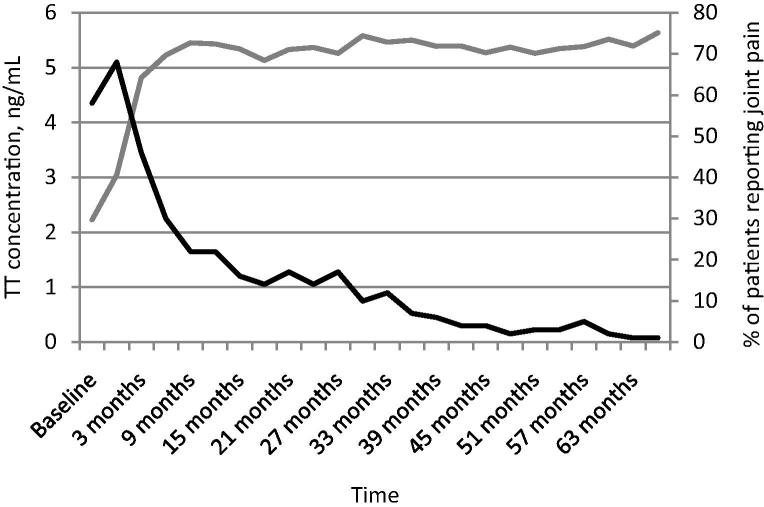
In all, 58% of patients reported joint pain at the beginning of the study, which dropped to 46% after 3 months of TRT, and dropped again to 22% after 9 months. By the end of the trial period at 63 months the percentage of patients reporting joint pain was just 1% (Fig. 4). Additionally, ≈60% of patients reported muscle pain at the start of the study, which dropped to ≈50% after 3 months and fell to a remarkably low level by the end of the study period.
Figure 4.
Long-term TU treatment reduces the percentage of patients reporting joint pain. The percentage of patients reporting joint pain is shown in black; TT level (ng/mL) is shown in grey. Time (years) is shown along the x-axis.
TT levels increased over the first 3 months of the trial from 2.23 ng/mL (7.73 nmol/L) at baseline to 4.82 ng/mL (16.71 nmol/L) after 3 months, reflecting the changes seen in the health quality indicator scores. After 63 months of treatment, the mean TT levels were 5.39 ng/mL (18.69 nmol/L; Figure 1, Figure 2, Figure 3, Figure 4).
Discussion
In the present prospective, observational and longitudinal study, we have shown that long-term treatment with TU in men with LOH results in a sustained improvement in health quality indicators including the IPSS, AMS, IIEF-5 scale, and reported joint and muscle pain, alongside increased TT levels, contributing to an improved, long-term HRQoL.
Our present findings confirm previous studies in which testosterone is shown to significantly improve sexual function and vitality, physical pain, general health, and the overall HRQoL in men with testosterone deficiency [33], [34], [35], [36], [37], [26], [38]. Such studies provide evidence that testosterone improves the HRQoL in hypogonadal men [39], [40], [41] and may explain the increase in physical activity that is often associated with TRT [42].
In the present study, both the AMS score and IPSS fell significantly within the first 3 months of the trial. In a previous study, erectile function was found to improve as early as 3 weeks and the AMS score as early as 6 weeks after the initiation of TU treatment [43]. However, it can take up to 12 months for maximum results [43], showing clearly that long-term treatment with TU is required to achieve optimal effects. Thus, the duration of the present study, which allows time for the optimal effects of TU to be observed, provides a useful timescale to gain a thorough understanding of the time course of testosterone effects from which hypogonadal patients will benefit. The time course of IPSS improvement has been less well studied than other parameters of HRQoL. One study found a significant reduction in the IPSS at 3 months after commencing TU treatment [44].
The results of several randomised, placebo-controlled trials observing the effect of TRT on symptoms of depression and HRQoL, as assessed by various subjective questionnaires, have produced mixed results; some found no effect of testosterone [45], [46], whereas other studies did find beneficial effects of testosterone on depression in men [26], [47]. However, these trials were short-term and therefore any indirect actions of testosterone to improve HRQoL, e.g. by improving the symptoms of metabolic syndrome and reducing obesity [39], may not be apparent. As such, longer trials may be required for the positive effects of testosterone to materialise. Moreover, several studies included healthy men with TT levels of >12.5 nmol/L [45], [48]. In the present study, the greatest improvements were seen within the first 3 months, an effect that was sustained throughout the remainder of the trial period, which lasted on average 4.25 years.
There are some potential limitations of the present study. The TT levels measured were trough levels, as blood samples were taken before the next TU injection, i.e. 3 months after the previous TU injection. Evidence from pharmacokinetic studies shows that TT levels between injections peak in the second week after injection [49], [50] and thus any dose-dependent effects seen may reflect this. A recent study demonstrated that in addition to testosterone, oestradiol plays an important role in the regulation of body fat and sexual function, thus a deficiency in this hormone may underlie a number of sequelae of male hypogonadism [51]. Oestradiol levels were not measured in the present study and so this may be considered a further limitation of the study. One final limitation of the present study is the nature of the registry design. This single-centre, open-label study is not a randomised controlled study and therefore limits the scope of interpretation of the presented findings. However, our present findings were recently confirmed in a small placebo-controlled study in which 20 obese hypogonadal men with LUTS were treated for 5 years with TU [52]. Although no changes in IPSS were seen, TT levels increased significantly with TRT [52].
In conclusion, this prospective, observational and longitudinal analysis shows a clear improvement in the long-term HRQoL, both physically and psychologically, in men with LOH treated with TU.
Conflicts of interest
Farid Saad, beside his position as University Professor, is an employee at Global Medical Affairs Men’s Healthcare, Bayer Pharma AG, Berlin, Germany.
All other authors have no conflicts of interest to declare.
Source of funding
None.
Acknowledgements
Editorial support for the manuscript was provided by Astra-Health, www.astra-health.co.uk.
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Dohle GR, Arver S, Bettocchi C, Jones TH, Kliesch S, Punab M. EAU Guidelines on male hypogonadism. Available at: <http://uroweb.org/guideline/male-hypogonadism/>; [accessed 27.7.2015].
- 2.Lunenfeld B., Mskhalaya G., Zitzmann M., Arver S., Kalinchenko S., Tishova Y. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18:5–15. doi: 10.3109/13685538.2015.1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harman S.M., Metter E.J., Tobin J.D., Pearson J. Longitudinal study of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 4.Purifoy F.E., Koopmans L.H., Mayers D.M. Age differences in serum androgen levels in normal adult males. Hum Biol. 1981;53:499–511. [PubMed] [Google Scholar]
- 5.Mulligan T., Frick M.F., Zuraw Q.C., Stemhagen A., McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762–769. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corona G., Rastrelli G., Monami M., Melani C., Balzi D., Sforza A. Body mass index regulates hypogonadism-associated CV risk: results from a cohort of subjects with erectile dysfunction. J Sex Med. 2011;8:2098–2105. doi: 10.1111/j.1743-6109.2011.02292.x. [DOI] [PubMed] [Google Scholar]
- 7.Corona G., Mannucci E., Fisher A.D., Lotti F., Petrone L., Balercia G. Low levels of androgens in men with erectile dysfunction. J Sex Med. 2008;5:2454–2463. doi: 10.1111/j.1743-6109.2008.00856.x. [DOI] [PubMed] [Google Scholar]
- 8.Esposito K., Giugliano F., Di Palo C., Giugliano G., Marfella R., D’Andrea F. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. J Am Med Assoc. 2004;291:2978–2984. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 9.Riedner C.E., Rhoden E.L., Ribeiro E.P., Fuchs S.C. Central obesity is an independent predictor of erectile dysfunction in older men. J Urol. 2006;176:1519–1523. doi: 10.1016/j.juro.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 10.Wing R.R., Rosen R.C., Fava J.L., Bahnson J., Brancati F., Gendrano I.N., III Effects of weight loss intervention on erectile dysfunction in older men with type 2 diabetes in the Look AHEAD trial. J Sex Med. 2010;7:156–165. doi: 10.1111/j.1743-6109.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96:2341–2353. doi: 10.1210/jc.2011-0118. [DOI] [PubMed] [Google Scholar]
- 12.Lyon C.J., Law R.E., Hsueh W.A. Mini-review: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144:2195–2200. doi: 10.1210/en.2003-0285. [DOI] [PubMed] [Google Scholar]
- 13.Trayhurn P., Wood I.S. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 14.Eckel R.H., Grundy S.M., Zimmet P.Z. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 15.Corona G., Monami M., Rastrelli G., Aversa A., Tishova Y., Saad F. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2005;8:272–283. doi: 10.1111/j.1743-6109.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- 16.Aversa A., Bruzziches R., Francomano D., Spera G., Lenzi A. Efficacy and safety of two different testosterone undecanoate formulations in hypogonadal men with metabolic syndrome. J Endocrinol Invest. 2010;33:776–783. doi: 10.1007/BF03350341. [DOI] [PubMed] [Google Scholar]
- 17.Yassin A.A., Saad F. Improvement of sexual function in men with late-onset hypogonadism treated with testosterone only. J Sex Med. 2007;4:497–501. doi: 10.1111/j.1743-6109.2007.00442.x. [DOI] [PubMed] [Google Scholar]
- 18.Yassin A.A., Saad F. Treatment of sexual dysfunction of hypogonadal patients with long-acting testosterone undecanoate (Nebido) World J Urol. 2006;24:639–644. doi: 10.1007/s00345-006-0120-0. [DOI] [PubMed] [Google Scholar]
- 19.Jannini E.A., Screponi E., Carosa E., Pepe M., Lo Giudice F., Trimarchi F. Lack of sexual activity from erectile dysfunction is associated with a reversible reduction in serum testosterone. Int J Androl. 1999;22:385–392. doi: 10.1046/j.1365-2605.1999.00196.x. [DOI] [PubMed] [Google Scholar]
- 20.Barrett-Connor E., Von Mühlen D.G., Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1999;84:573–577. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- 21.Rizvi S.J., Kennedy S.H., Ravindran L.N., Giacobbe P., Eisfeld B.S., Mancini D. The relationship between testosterone and sexual function in depressed and healthy men. J Sex Med. 2010;7:816–825. doi: 10.1111/j.1743-6109.2009.01504.x. [DOI] [PubMed] [Google Scholar]
- 22.Shores M.M., Moceri V.M., Sloan K.L., Matsumoto A.M., Kivlahan D.R. Low testosterone levels predict incident depressive illness in older men: effects of age and medical morbidity. J Clin Psychiatry. 2005;66:7–14. doi: 10.4088/jcp.v66n0102. [DOI] [PubMed] [Google Scholar]
- 23.Almeida O.P., Yeap B.B., Hankey G.J., Jamrozik K., Flicker L. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch Gen Psychiatry. 2008;65:283–289. doi: 10.1001/archgenpsychiatry.2007.33. [DOI] [PubMed] [Google Scholar]
- 24.Atlantis E., Sullivan T. Bidirectional association between depression and sexual dysfunction: a systematic review and meta-analysis. J Sex Med. 2012;9:1497–1507. doi: 10.1111/j.1743-6109.2012.02709.x. [DOI] [PubMed] [Google Scholar]
- 25.Luppino F.S., de Wit L.M., Bouvy P.F., Stijnen T., Cuijpers P., Penninx B.W. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 26.Giltay E.J., Tishova Y.A., Mskhalaya G.J., Gooren L.J., Saad F., Kalinchenko S.Y. Effects of testosterone supplementation on depressive symptoms and sexual dysfunction in hypogonadal men with the metabolic syndrome. J Sex Med. 2010;7:2572–2582. doi: 10.1111/j.1743-6109.2010.01859.x. [DOI] [PubMed] [Google Scholar]
- 27.Shahinian V.B., Kuo Y.F., Freeman J.L., Goodwin J.S. Risk of the ‘androgen deprivation syndrome’ in men receiving androgen deprivation for prostate cancer. Arch Intern Med. 2006;166:465–471. doi: 10.1001/.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu F.C., Tajar A., Beynon J.M., Pye S.R., Silman A.J., Finn J.D. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 29.Feldman H.A., Longcope C., Derby C.A., Johannes C.B., Arujo A.B., Coviello A.D. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 30.Bacon C.G., Giovannucci E., Testa M., Glass T.A., Kawachi I. The association of treatment-related symptoms with quality-of-life outcomes for localized prostate carcinoma patients. Cancer. 2002;94:862–871. doi: 10.1002/cncr.10248. [DOI] [PubMed] [Google Scholar]
- 31.Brooke J.C., Walter D.J., Kapoor D., Marsh H., Muraleedharan V., Jones T.H. Testosterone deficiency and severity of erectile dysfunction are independently associated with reduced quality of life in men with type 2 diabetes. Andrology. 2014;2:205–211. doi: 10.1111/j.2047-2927.2013.00177.x. [DOI] [PubMed] [Google Scholar]
- 32.Snyder P.J., Peachey H., Hannoush P., Berlin J.A., Loh L., Holmes J.H. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966–1972. doi: 10.1210/jcem.84.6.5741. [DOI] [PubMed] [Google Scholar]
- 33.Yassin D.J., Doros G., Hammerer P.G., Yassin A.A. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med. 2014;11:1567–1576. doi: 10.1111/jsm.12523. [DOI] [PubMed] [Google Scholar]
- 34.Hackett G., Cole N., Bhartia M., Kennedy D., Raju J., Wilkinson P. Testosterone replacement therapy with long-acting testosterone undecanoate improves sexual function and quality-of-life parameters vs. placebo in a population of men with type 2 diabetes. J Sex Med. 2013;10:1612–1627. doi: 10.1111/jsm.12146. [DOI] [PubMed] [Google Scholar]
- 35.Yassin D.J., El Douaihy Y., Yassin A.A., Kashanian J., Shabsigh R., Hammerer P.G. Lower urinary tract symptoms improve with testosterone replacement therapy in men with late-onset hypogonadism: 5-year prospective, observational and longitudinal registry study. World J Urol. 2014;32:1049–1054. doi: 10.1007/s00345-013-1187-z. [DOI] [PubMed] [Google Scholar]
- 36.Zitzmann M., Mattern A., Hanisch J., Gooren L., Jones H., Maggi M. IPASS: a study on the tolerability and effectiveness of injectable testosterone undecanoate for the treatment of male hypogonadism in a worldwide sample of 1438 men. J Sex Med. 2013;10:579–588. doi: 10.1111/j.1743-6109.2012.02853.x. [DOI] [PubMed] [Google Scholar]
- 37.Tong S.F., Ng C.J., Lee B.C., Lee V.K., Khoo E.M., Lee E.G. Effect of long-lasting testosterone undecanoate treatment on quality of life in men with testosterone deficiency syndrome: a double blind randomized controlled trial. Asian J Androl. 2012;14:604–611. doi: 10.1038/aja.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yassin A., Saad F., Traish A. Testosterone undecanoate restores erectile function in a subset of patients with venous leakage: a series of case reports. J Sex Med. 2006;3:727–735. doi: 10.1111/j.1743-6109.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 39.Traish A.M., Haider A., Doros G., Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2013;68:314–329. doi: 10.1111/ijcp.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corona G., Rastrelli G., Maggi M. Diagnosis and treatment of late-onset hypogonadism: systematic review and meta-analysis of TRT outcomes. Best Pract Res Clin Endocrinol Metab. 2013;27:557–579. doi: 10.1016/j.beem.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Corona G., Rastrelli G., Morelli A., Vignozzi L., Mannucci E., Maggi M. Hypogonadism and metabolic syndrome. J Endocrinol Invest. 2011;34:557–567. doi: 10.3275/7806. [DOI] [PubMed] [Google Scholar]
- 42.Sattler F., Bhasin S., He J., Chou C.P., Castaneda-Sceppa C., Yarasheski K. Testosterone threshold levels and lean tissue mass targets needed to enhance skeletal muscle strength and function: the HORMA trial. J Gerontol A Biol Sci Med Sci. 2011;66:122–129. doi: 10.1093/gerona/glq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saad F., Aversa A., Isidori A.M., Zafalon L., Zitzmann M., Gooren L. Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur J Endocrinol. 2011;165:675–685. doi: 10.1530/EJE-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haider A., Gooren L.J., Padungtod P., Saad F. A safety study of administration of parenteral testosterone undecanoate to elderly men over minimally 24 months. Andrologia. 2010;42:349–355. doi: 10.1111/j.1439-0272.2009.01016.x. [DOI] [PubMed] [Google Scholar]
- 45.Emmelot-Vonk M.H., Verhaar H.J., Nakhai Pour H.R., Aleman A., Lock T.M., Bosch J.L. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. J Am Med Assoc. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 46.Seidman S.N., Spatz E., Rizzo C., Roose S.P. Testosterone replacement therapy for hypogonadal men with major depressive disorder: a randomized, placebo-controlled clinical trial. J Clin Psychiatry. 2001;62:406–412. doi: 10.4088/jcp.v62n0602. [DOI] [PubMed] [Google Scholar]
- 47.Shores M.M., Kivlahan D.R., Sadak T.I., Li E.J., Matsumoto A.M. A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression) J Clin Psychiatry. 2009;70:1009–1016. doi: 10.4088/jcp.08m04478. [DOI] [PubMed] [Google Scholar]
- 48.Steidle C., Schwartz S., Jacoby K., Sebree T., Smith T., Bachand R. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–2681. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- 49.Schubert M., Minnemann T., Hübler D., Rouskova D., Christoph A., Oettel M. Intramuscular testosterone undecanoate: pharmacokinetic aspects of a novel testosterone formulation during long-term treatment of men with hypogonadism. J Clin Endocrinol Metab. 2004;89:5429–5434. doi: 10.1210/jc.2004-0897. [DOI] [PubMed] [Google Scholar]
- 50.Von Eckardstein S., Nieschlag E. Treatment of male hypogonadism with testosterone undecanoate injected at extended intervals of 12 weeks: a phase II study. J Androl. 2002;23:419–425. [PubMed] [Google Scholar]
- 51.Finkelstein J.S., Lee H., Burnett-Bowie S.A., Pallais J.C., Yu E.W., Borges L.F. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Francomano D., Ilacqua A., Bruzziches R., Lenzi A., Aversa A. Effects of 5-year treatment with testosterone undecanoate on lower urinary tract symptoms in obese men with hypogonadism and metabolic syndrome. Urology. 2013;83:167–173. doi: 10.1016/j.urology.2013.08.019. [DOI] [PubMed] [Google Scholar]