Abstract
Objective
To present our technique and experience of robot-assisted ureterocalycostomy (RAUC) in managing secondary pelvi-ureteric junction obstruction (PUJO) in adults.
Patients and methods
We retrospectively reviewed all patients from our centre who underwent RAUC, between 2011 and 2015, for secondary PUJO resulting from previous surgical intervention. Six procedures in five patients, including a bilateral RAUC were performed. The median (range) patient age was 33.7 (18–41) years. The outcome variables included operative time, duration of hospital stay, and objective evidence of unimpeded drainage on urography.
Results
The mean (range) operating time was 172 (144–260) min and estimated blood loss was 100 (50–250) mL. There were no conversions to open or laparoscopic surgery, and no intraoperative complications. Two patients had Clavien–Dindo Grade I complications that were managed conservatively and one patient had a Grade IIIb complication, which required balloon dilatation and re-stenting. After a median (range) follow-up of 11 (7–48) months, five of the six renal units had successful outcomes.
Conclusion
The robot-assisted approach appears to be ideally suited for redo cases demanding fine dissection with meticulous suturing. In our present series of adult patients, we could safely and successfully perform RAUC with minimal morbidity. However, a larger multi-institutional outcome analysis is required to substantiate the role of the robot-assisted approach in performing UC.
Abbreviations: PCNL, percutaneous nephrolithotomy; PUJO, pelvi-ureteric junction obstruction; RAUC, robot-assisted ureterocalycostomy; UC, ureterocalycostomy
Keywords: Robotics, Ureterocalycostomy, Pelvi-ureteric junction, Obstruction
Introduction
There are various management options for PUJ obstruction (PUJO), encompassing endoscopic to the definitive repairs, such as pyeloplasty and ureterocalycostomy (UC). The means of performing the latter repairs have transitioned from the era of open surgery to laparoscopy and recently to robot-assisted techniques. Although most PUJO can be specifically dealt with by Anderson–Hynes pyeloplasty [1], there are circumstances, such as failed prior pyeloplasty with minimal pelvis, PUJO with an intra-renal pelvis, obstructed horse-shoe kidney, and PUJO resulting from prior interventions, which may warrant UC [2]. Contemporary series have shown that endourological failures and complications thereof, have been an increasing indication for UC, consistent with the increased use of these minimally invasive procedures [3].
As far as the approach for performing UC is concerned, owing to the technical complexity, it has long been performed by open means. However, Gill et al. [4] published their first feasibility study of laparoscopic UC in a clinical context and brought UC into the realms of minimally invasive surgery. Similarly Korets et al. [5] reported the first robot-assisted procedure and their experience. The robot-assisted approach with its inherent unique attributes appears to be particularly appealing, owing to the technical complexity and meticulous suturing required for accomplishing the procedure. We herein present our technique and experience with robot-assisted UC (RAUC).
Patients and methods
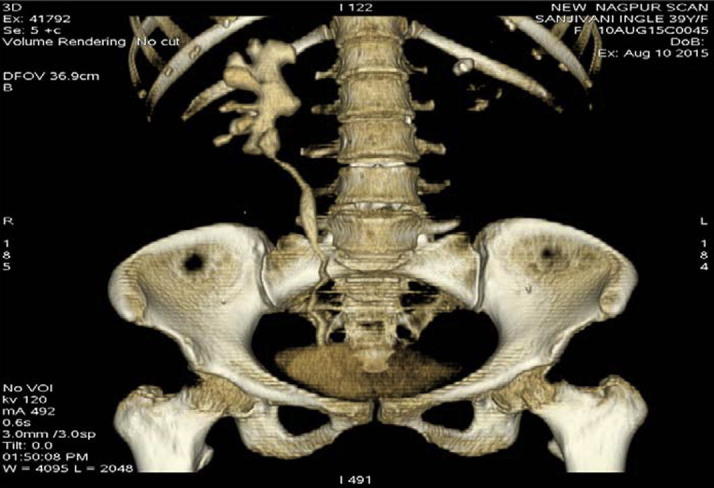
We retrospectively reviewed the records of all patients who underwent RAUC at our centre from March 2011 to February 2015. In all, six procedures on five patients, including a bilateral RAUC, were performed and followed. The patients’ presentations and demographics are shown in Table 1. The PUJO was evaluated and documented by CT-urography and/or antegrade dye study through the pre-placed nephrostomy tube (Fig. 1). Four renal units had undergone pyelolithotomy previously, one had a history of percutaneous nephrolithotomy (PCNL), and one had a previously failed pyeloplasty.
Table 1.
Patient presentation and demographics.
| Patient number | Age, years | Sex | Laterality | Cause of PUJO | Prior endourological attempt | Pre-placed nephrostomy |
|---|---|---|---|---|---|---|
| 1 | 41 | Male | Right | Secondary PUJO, post-pyelolithotomy | + | + |
| 2 | 28 | Female | Right | Secondary PUJO, post-PCNL | + | + |
| 3 | 36 | Female | Right | Secondary PUJO, post-pyelolithotomy | + | + |
| 4 | 40 | Male | Bilateral | Secondary PUJO, post-bilateral pyelolithotomy | + | + |
| 5 | 18 | Female | Right | Failed pyeloplasty | + | + |
Figure 1.
PUJO evaluated by CT-urography and/or antegrade dye study through the pre-placed nephrostomy tube.
The technique
After thorough discussion with the patient and a decision made to proceed with RAUC, an informed consent was obtained. Prophylactic antibiotic was administered 1 h before the induction of general anaesthesia. All the procedures were performed by the same surgeon.
After placement of 5-F open-ended ‘pigtail’ ureteric catheter over a 0.09-cm (0.035″) guidewire and 16-F Foley catheter, the patient was positioned for the RAUC. The patient was placed in a lateral flank position. Pneumoperitoneum was created using a Veress needle (closed technique). Port positioning comprised of two 12-mm (a camera and assistant port) and two 8-mm (robotic arms) ports. On the right side an additional 5-mm port was used for liver retraction. A 30° telescope was used in all cases.
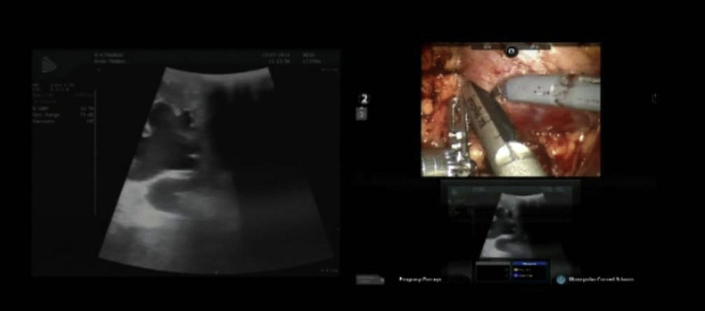
The bowel was mobilised along the white line of Toldt and the ureter was identified over the pre-placed ureteric catheter. The ureter was circumferentially dissected and vessel loops placed to provide traction avoiding devascularisation, while mobilisation continued up to the segment abutting the lower pole of the kidney. The latter was exposed by incising Gerota’s fascia and excising the perinephric fat, without resorting to hilar dissection. Intraoperative ultrasonography was used for delineation of the dilated lower pole calyceal system (Fig. 2). This was followed by performing a lower polar segmental nephrectomy and exposing the lower calyx (Fig. 3). Thereafter, the ureter abutting the lower pole calyx was divided. The pre-placed ureteric catheter with the guidewire in the ureter was then negotiated into the pelvicalyceal system through the exposed lower calyx. After lateral spatulation of the ureter, the anastomosis was made between the ureter and lower calyceal mucosa with 3–0 absorbable (copolymer of glycolic acid and trimethylene carbonate) barbed V-Loc™ suture in a continuous manner, finally achieving a dependent, tension-free anastomosis (Fig. 4). A drain was placed at the end of the procedure. After 48 h of surgery, the ureteric catheter was changed to a 6 F/26 cm JJ stent.
Figure 2.
Intraoperative ultrasonography and lower calyceal delineation.
Figure 3.
Lower polar nephrectomy and calyceal exposure.
Figure 4.
Completion of ureterocalyceal anastomosis.
Postoperative course
Patients were monitored for postoperative recovery and any complications. The pre-placed nephrostomy tube was removed on the third postoperative day and daily drain output was assessed. After drain removal patients were discharged on per urethral catheter, with the latter being removed on tenth postoperative day in the outpatient clinic. The JJ stent was removed after 1 month. Success was defined as patients being symptom free with documented unobstructed drainage on urography at 3- and 6-month intervals (Fig. 5).
Figure 5.
Follow-up urography image.
Results
The median (range) age of the five patients was 33.7 (18–41) years; two were men and three were women. Four patients had undergone right-sided RAUC, whereas one underwent a bilateral staged procedure with an intervening period of 2 months between the sides. All the patients presented with flank pain. Concomitantly, one patient had a UTI and another with a solitary functioning kidney had obstructive uropathy at presentation.
The mean (range) operating time was 172 (144–260) min and estimated blood loss was 100 (50–250) mL. There were no conversions to open or laparoscopic surgery, and no intraoperative complications. Two patients had Clavien–Dindo Grade I complications (fever), which were managed conservatively. One patient had a Grade IIIb complication (worsening renal function and recurrence of obstruction at the anastomotic site), which required balloon dilatation and re-stenting. The nephrostomy tube was removed on the third postoperative day and the abdominal drain removed on fourth day (3–6 days). The median (range) length of hospital stay was 6.5 (5–8) days. The mean (range) analgesic requirement was 325 (250–600) mg tramadol. The JJ stent was removed at 1 month after RAUC. After a median (range) follow-up of 11 (7–48) months, five of the six renal units were considered successes. At the latest follow-up, all but one patient had normal renal function and drainage. The latter is being managed presently with regular JJ stent change.
Discussion
Impairment of urinary drainage from the pelvis to the ureter ultimately culminates in deterioration of renal function, thus correction of the cause of obstruction, whether structural or functional, is of paramount importance. Cases of failed pyeloplasty, intra-renal pelvis with obstruction, and secondary PUJO from previous interventions, are some of the circumstances that warrant definitive correction in the form of UC. Although endoscopic intervention, such as endopyelotomy, may be tried, the outcomes are reported to be better with definitive techniques [6].
The technique of UC was first described by Neuwirt [7] in 1947; however, the surgical technique was later modified by Hawthorne et al. [8] in 1976, who advocated excision of the lower pole parenchyma and achieved good results using this modification. Subsequently, Mesrobian and Kelalis [9] emphasised and popularised the key technical facets of achieving a successful outcome: extensive excision of lower pole tissue to expose the calyceal lumen, performing a stented anastomosis, and ensuring mucosal continuity between the ureter and the exposed lower pole calyx.
The literature on UC comprises primarily of small case series and isolated case reports, with open surgery being the dominant treatment approach reported [10], [11], [12], [13]. Different authors report variable success rates [14] depending on the indication (better in the primary setting than in the setting of previous intervention), age group (better for paediatric ≈90% [9] versus adult series 60–75% [13]), and the endpoint chosen for follow-up (decrease with increase in the duration of follow-up).
Matlaga et al. [3] reviewed their experience in a series of 11 patients treated with open UC. The indications for the procedure were primary PUJO in patients with an intrarenal pelvis (four patients), failed cutting balloon incision of PUJO (three), proximal ureteric stricture after ureteroscopic stone manipulation (two), and obliterated PUJ after PCNL (one) and failed antegrade endopyelotomy (one). All 11 procedures were performed without complications with a mean operative time of 292 min, estimated blood loss of 373 mL, and an average hospital stay of 5.1 days. The investigators documented relief of obstruction in all patients by IVU or mercaptoacetyltriglycine renal scan. The perioperative variables in our present patients compare favourably with those in that open series, with a quicker convalescence but a comparable length of stay.
As the advantages of the minimally invasive nature of laparoscopy have been realised for other urological surgeries, the feasibility for performing UC by laparoscopic means was initially attempted in animal models [15]. Akin to other laparoscopic reconstructive procedures, it was realised that performance of meticulous suturing for ureterocalyceal anastomosis leading to a watertight tension-free repair, was the most demanding part of the surgery. This was soon followed by the first clinical case series of laparoscopic UC by Gill et al. [4], whereby the authors reported the performance of the procedure in two patients with PUJO, in whom previous surgical interventions had failed. The operative time for the first case was 5.2 h, which had decreased to 2.5 h for the second case. The estimated blood loss was 200 and 75 mL respectively, with both patients discharged 2 days postoperatively. The authors reported complete resolution of symptoms and radiographic improvement after 9 months of follow-up in the first case. In the second case, although there was improvement on imaging studies, the symptoms persisted and nephrectomy was performed 6 months later. In the largest laparoscopic series, reported by Arap et al. [16], the authors reported on six transperitoneal laparoscopic UCs performed for symptomatic complicated upper urinary obstruction. The causes being failed previous procedures (three patients), anatomical abnormalities (two), and a severe upper ureteric stenosis (one). The median (range) operative time was 215 (180–270) min. The authors reported no major complications and no conversions to open surgery, with clinical and radiographic improvement at a median follow-up of 30 months.
There has been a widespread increase in the use of the robotic surgical platform for almost every surgery that can been performed by open or laparoscopic means. The EndoWrist® technology, with its seven degrees of freedom, as well as the motion scaling feature, makes this an ideal platform for fine dissection and suturing, the very basis of reconstructive surgery. These features in particular, seem to render UC ideally suited for robot-assisted reconstruction.
The RAUC was first described in 2007 by Korets et al. [5], in a patient with refractory proximal ureteric stricture, secondary to multiple interventions for stones. The authors used laparoscopy for the initial dissection and exposure, and robotic techniques for lower pole amputation and the ureterocalyceal anastomosis. However, in our present series, we completed the entire procedure by robot-assisted means. Schimpf and Wagner [17] in 2009 described the case of a 32-year-old female with PUJO, with a history of open right nephrolithotomy, who was successfully treated with a RAUC.
In a large paediatric series of nine patients, Casale et al. [18] performed transperitoneal RAUC. Six of these patients underwent UC as a secondary procedure after failed pyeloplasty, two of which were found to have initially missed crossing vessels as a cause for obstruction. Primary UC was performed in three children who had an exaggerated intrarenal collecting system preventing conventional surgery. Ultrasonographic assessment performed 3 months after stent removal showed persistent severe dilatation. However, diuretic renography performed 12 months postoperatively showed no significant deterioration in function. The present series of RAUC comprised of adult patients, all of whom had a history of prior surgical intervention.
The present series demonstrates efficacy similar to other minimally invasive techniques. The use of robotic technology in the contemporary literature for UC is sparse and therefore additional experience from different authors is needed. However, the present series is limited by the few patients, single surgeon experience, short follow-up, and its retrospective nature. Further studies with longer follow-up involving more patients are required.
Conclusion
RAUC appears to be ideally suited for redo cases and permits fine dissection with meticulous suturing. In our present series of adult patients, we could safely and successfully perform RAUC. The outcomes appear to be encouraging with minimal morbidity. However, owing to the scarcity of cases warranting UC, a larger multi-institutional outcome analysis is required to substantiate the role of the robot-assisted approach in performing UC.
Conflicts of interest
None.
Source of Funding
None.
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Valla J.S., Breaud J., Griffin S.J., Sautot-Vial N., Beretta F., Guana R. Retroperitoneoscopic vs. open dismembered pyeloplasty for ureteropelvic junction obstruction in children. J Pediatr Urol. 2009;5:368–373. doi: 10.1016/j.jpurol.2009.02.202. [DOI] [PubMed] [Google Scholar]
- 2.Slaby D.J., Boeckman C., Nasrallah P. Ureterocalycostomy. Urology. 1982;20:377–381. doi: 10.1016/0090-4295(82)90459-9. [DOI] [PubMed] [Google Scholar]
- 3.Matlaga B.R., Shah O.D., Singh D., Streem S.B., Assimos D.G. Ureterocalicostomy: a contemporary experience. Urology. 2005;65:42–44. doi: 10.1016/j.urology.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Gill I.S., Cherullo E.E., Steinberg A.P., Desai M.M., Abreu S.C., Ng C. Laparoscopic ureterocalicostomy: initial experience. J Urol. 2004;171:1227–1230. doi: 10.1097/01.ju.0000114233.66534.b0. [DOI] [PubMed] [Google Scholar]
- 5.Korets R., Hyams E.S., Shah O.D., Stifelman M.D. Robotic-assisted laparoscopic ureterocalicostomy. Urology. 2007;70:366–369. doi: 10.1016/j.urology.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Rogers A., Hasan T. Management of secondary pelviureteric junction obstruction. Indian J Urol. 2013;29:294–302. doi: 10.4103/0970-1591.120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuwirt K. Implantation of the ureter into the lower calyx of the renal pelvis. In: VII Congress de la Societe Internationale d’Urologie, Part 2;1947:253–5.
- 8.Hawthorne N.J., Zincke H., Kelalis P.P. Ureterocalicostomy: an alternative to nephrectomy. J Urol. 1976;115:583–585. doi: 10.1016/s0022-5347(17)59290-3. [DOI] [PubMed] [Google Scholar]
- 9.Mesrobian H.G., Kelalis P.P. Ureterocalicostomy: indications and results in 21 patients. J Urol. 1989;142:1285–1287. doi: 10.1016/s0022-5347(17)39058-4. [DOI] [PubMed] [Google Scholar]
- 10.Selli C., Rizzo M., Moroni F., Dedola G., Amorosi A. Ureterocalicostomy in the treatment of pyeloplasty failures. Urol Int. 1992;48:274–277. doi: 10.1159/000282350. [DOI] [PubMed] [Google Scholar]
- 11.Ross J.H., Streem S.B., Novick A.C., Kay R., Montie J. Ureterocalicostomy for reconstruction of complicated pelviureteric junction obstruction. Br J Urol. 1990;65:322–325. doi: 10.1111/j.1464-410x.1990.tb14748.x. [DOI] [PubMed] [Google Scholar]
- 12.Ben Slama M.R., Zaafrani R., Ben Mouelli S., Derouich A., Chebil M., Ayed M. Ureterocalicostomy: last resort in the treatment of certain forms of ureteropelvic junction stenosis: report of 5 cases. Prog Urol. 2005;15:646–649. [PubMed] [Google Scholar]
- 13.Radford A., Thomas D., Subramaniam R. Role of ureterocalicostomy in children: a follow up study. J Pediatr Urol. 2009;5(Suppl. 1):S62. [Google Scholar]
- 14.Osman T., Eltahawy I., Fawaz K., Shoeib M., Elshawaf H., El Halaby R. Ureterocalicostomy for treatment of complex cases of ureteropelvic junction obstruction in adults. Urology. 2011;78:202–207. doi: 10.1016/j.urology.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 15.Cherullo E.E., Gill I.S., Ponsky L.E., Banks K.L., Desai M.M., Kaouk J.H. Laparoscopic ureterocalicostomy: a feasibility study. J Urol. 2003;169:2360–2364. doi: 10.1097/01.ju.0000058214.99086.37. [DOI] [PubMed] [Google Scholar]
- 16.Arap M.A., Andrade H., Torricelli F.C., Denes F.T., Mitre A.I., Duarte R.J. Laparoscopic ureterocalicostomy for complicated upper urinary tract obstruction: mid-term follow-up. Int Urol Nephrol. 2014;46:865–869. doi: 10.1007/s11255-013-0591-z. [DOI] [PubMed] [Google Scholar]
- 17.Schimpf M.O., Wagner J.R. Case report: robotic-assisted laparoscopic ureterocalicostomy with long-term follow-up. J Endourol. 2009;23:293–295. doi: 10.1089/end.2008.0165. [DOI] [PubMed] [Google Scholar]
- 18.Casale P., Mucksavage P., Resnick M., Kim S. Robotic ureterocalicostomy in the pediatric population. J Urol. 2008;180:2643–2648. doi: 10.1016/j.juro.2008.08.052. [DOI] [PubMed] [Google Scholar]