Abstract
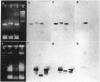
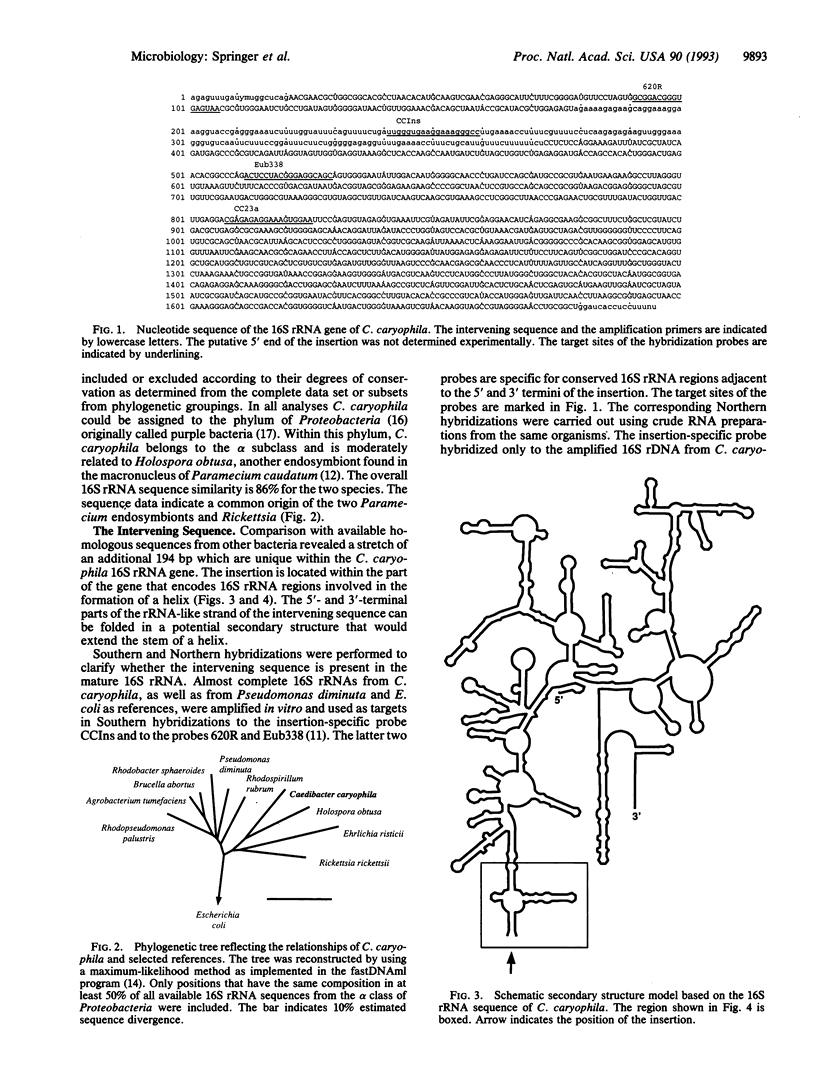
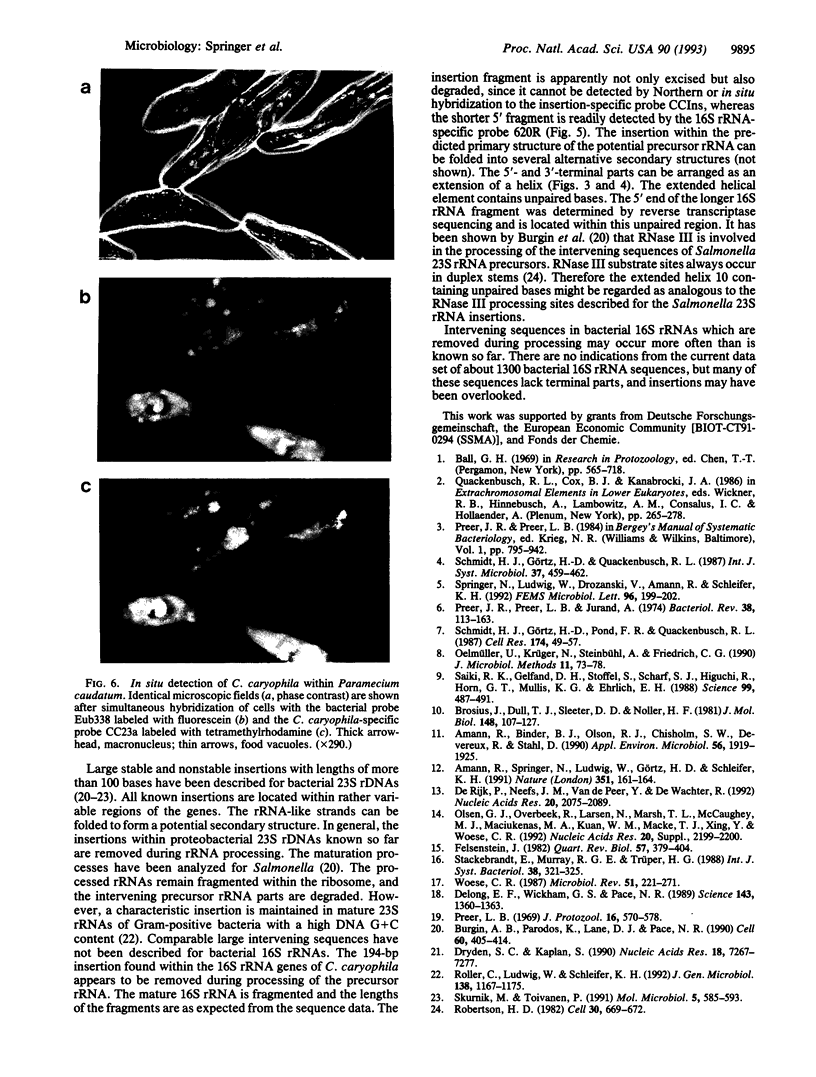
The phylogenetic position of Caedibacter caryophila, a so far noncultured killer symbiont of Paramecium caudatum, was elucidated by comparative sequence analysis of in vitro amplified 16S rRNA genes (rDNA). C. caryophila is a member of the alpha subclass of the Proteobacteria phylum. Within this subclass C. caryophila is moderately related to Holospora obtusa, which is another obligate endosymbiont of Paramecium caudatum, and to Rickettsia. A 16S rRNA targeted specific hybridization probe was designed and used for in situ detection of C. caryophila within its host cell. Comparison of the 16S rDNA primary structure of C. caryophila with homologous sequences from other bacteria revealed an unusual insertion of 194 base pairs within the 5'-terminal part of the corresponding gene. The intervening sequence is not present in mature 16S rRNA of C. caryophila. It was demonstrated that C. caryophila contained fragmented 16S rRNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann R. I., Binder B. J., Olson R. J., Chisholm S. W., Devereux R., Stahl D. A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990 Jun;56(6):1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann R., Springer N., Ludwig W., Görtz H. D., Schleifer K. H. Identification in situ and phylogeny of uncultured bacterial endosymbionts. Nature. 1991 May 9;351(6322):161–164. doi: 10.1038/351161a0. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Burgin A. B., Parodos K., Lane D. J., Pace N. R. The excision of intervening sequences from Salmonella 23S ribosomal RNA. Cell. 1990 Feb 9;60(3):405–414. doi: 10.1016/0092-8674(90)90592-3. [DOI] [PubMed] [Google Scholar]
- De Rijk P., Neefs J. M., Van de Peer Y., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1992 May 11;20 (Suppl):2075–2089. doi: 10.1093/nar/20.suppl.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong E. F., Wickham G. S., Pace N. R. Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science. 1989 Mar 10;243(4896):1360–1363. doi: 10.1126/science.2466341. [DOI] [PubMed] [Google Scholar]
- Dryden S. C., Kaplan S. Localization and structural analysis of the ribosomal RNA operons of Rhodobacter sphaeroides. Nucleic Acids Res. 1990 Dec 25;18(24):7267–7277. doi: 10.1093/nar/18.24.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen G. J., Overbeek R., Larsen N., Marsh T. L., McCaughey M. J., Maciukenas M. A., Kuan W. M., Macke T. J., Xing Y., Woese C. R. The Ribosomal Database Project. Nucleic Acids Res. 1992 May 11;20 (Suppl):2199–2200. doi: 10.1093/nar/20.suppl.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preer J. R., Jr, Preer L. B., Jurand A. Kappa and other endosymbionts in Paramecium aurelia. Bacteriol Rev. 1974 Jun;38(2):113–163. doi: 10.1128/br.38.2.113-163.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preer L. B. Alpha, an infectious macronuclear symbiont of Paramecium aurelia. J Protozool. 1969 Aug;16(3):570–578. doi: 10.1111/j.1550-7408.1969.tb02315.x. [DOI] [PubMed] [Google Scholar]
- Quackenbush R. L., Cox B. J., Kanabrocki J. A. Extrachromosomal elements of extrachromosomal elements of Paramecium and their extrachromosomal elements. Basic Life Sci. 1986;40:265–278. doi: 10.1007/978-1-4684-5251-8_21. [DOI] [PubMed] [Google Scholar]
- Robertson H. D. Escherichia coli ribonuclease III cleavage sites. Cell. 1982 Oct;30(3):669–672. doi: 10.1016/0092-8674(82)90270-7. [DOI] [PubMed] [Google Scholar]
- Roller C., Ludwig W., Schleifer K. H. Gram-positive bacteria with a high DNA G+C content are characterized by a common insertion within their 23S rRNA genes. J Gen Microbiol. 1992 Jun;138(6):1167–1175. doi: 10.1099/00221287-138-6-1167. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schmidt D. A. Jet injection: high speed infiltration anesthesia. ASDC J Dent Child. 1970 Nov-Dec;37(6):459–462. [PubMed] [Google Scholar]
- Schmidt H. J., Görtz H. D., Pond F. R., Quackenbush R. L. Characterization of Caedibacter endonucleobionts from the macronucleus of Paramecium caudatum and the identification of a mutant with blocked R-body synthesis. Exp Cell Res. 1988 Jan;174(1):49–57. doi: 10.1016/0014-4827(88)90141-3. [DOI] [PubMed] [Google Scholar]
- Skurnik M., Toivanen P. Intervening sequences (IVSs) in the 23S ribosomal RNA genes of pathogenic Yersinia enterocolitica strains. The IVSs in Y. enterocolitica and Salmonella typhimurium have a common origin. Mol Microbiol. 1991 Mar;5(3):585–593. doi: 10.1111/j.1365-2958.1991.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Springer N., Ludwig W., Drozański W., Amann R., Schleifer K. H. The phylogenetic status of Sarcobium lyticum, an obligate intracellular bacterial parasite of small amoebae. FEMS Microbiol Lett. 1992 Sep 15;75(2-3):199–202. doi: 10.1016/0378-1097(92)90403-b. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]