Graphical abstract
This review furnishes an overview of all naturally isolated compounds, especially diterpenoids as well as biological activities of these species such as anticancer, immunomodulatory, antiviral, antimicrobial, and anti-inflammatory activities.
Keywords: Cespitularia, Terpenoids, Steroids, Anticancer, Anti-inflammatory
Abstract
Soft corals belonging to the genus Cespitularia have been well recognized as a rich source of bioactive secondary metabolites especially diterpenoids. This review furnishes an overview of all naturally isolated compounds from Cespitularia genus as, diterpenoids, nitrogen-containing diterpenes, sesquiterpenoids and steroids as well as biological activities of these species. Cespitularia species have been studied for their anticancer, immunomodulatory, antiviral, antimicrobial, and anti-inflammatory activities. This work is the first review published on this topic.
Introduction
Marine organisms have developed a variety of bioactive secondary metabolites [1]. Chemically, the bioactive metabolites isolated from marine animals could be divided into steroids, terpenoids, isoprenoids, nonisoprenoids, quinones, halogenated compounds, nitrogen heterocyclics, and nitrogen sulfur heterocyclics [2], [3], [4], [5]. The bioactive metabolites that are adjectives of that kind of interest have been mainly isolated from corals, marine sponges, jellyfish, sea anemones, bryozoans, molluscs, echinoderms, tunicates and crustaceans [3].
Octocorals (phylum Cnidaria) have been widely studied, as they are responsible for the production of a huge array of skeletal different classes of secondary metabolites. Family Xeniidae (order Alcyonacea) which involves 17 genera of soft corals such as Heteroxenia, Cespitularia, Xenia, Anthelia, Asterospicularia, Bayerxenia, Sympodium, is a very large family distributed in all over the marine environments [6], [7].
Cespitularia genus involves almost 18 species such as C. erecta, C. hypotentaculata, C subviridus, C. taeniata, C. infirmata [8] (Fig. 1). They live in tropical reefs, in areas with strong currents and with good light intensity like in the Indo-Pacific Ocean from the East African coast to Australia, New Guinea and southern Japan [8].
Fig. 1.
Photographs of some Cespitularia species.
Several biological studies on different extracts and isolated secondary metabolites from Cespitularia species have reported activities such as anticancer, immunomodulatory, antiviral, antimicrobial, and anti-inflammatory [9], [10], [11]. Soft corals of the genus Cespitularia are rich in novel and diverse chemical structures with interesting biological activities [12]. Reports related metabolites chemistry of the genus Cespitularia is scarce. Earlier studies of the genus Cespitularia led to the isolation of a diverse array of diterpenoids including alcyonolides, caryophyllanoids, cembranolides, cespitularanoids, dolabellanoids, norverticillanoids, verticillanoids, and xenicanoids [3], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30].
Biological activities of Cespitularia species
Anticancer activity
It was reported that some isolated compounds from C. taeniata have significant cytotoxic activity. Cespitulactone A (60) exhibited significant cytotoxicity against human cervical epithelioid carcinoma (HeLa) and colon adenocarcinoma (DLD-1) cancer cells with IC50 of 3.69 and 9.98 μg/ml, respectively. Flaccidoxide-13-acetate (62) showed mild activity against human medulloblastoma (Daoy) and colon (WiDr) cancer cells 16.9 and 13.8 μg/ml, respectively [10], [16].
Cheng et al. [14] have reported that some isolated sesquiterpene lactams from EtOH extract of the soft coral C taeniata exhibited cytotoxic activity. 8β-methoxyatractylenolide (83) was also reported to exhibit cytotoxicity against KB and Daoy cancer cell lines with ED50 values of 10.71 and 7.93 μg/ml [14], [17].
Some isolated cespitulactams from C. taeniata have been reported to exhibit significant cytotoxicity against some human cancer cells. Cespitulactam A (19) was reported to exhibit significant cytotoxicity against human Widr and Daoy cancer cells with the IC50 values of 2.72 and 6.34 μg/ml, respectively [18]. Duh et al. [9] have reported that some of isolated cespitularin derivatives showed cytotoxic activity against A-549; P-388 and HT-29. Cespitularin B (33) and D (35) showed moderate cytotoxicity against P-388 cells with ED50 values of 3.23 and 3.86 μg/ml respectively. Cespitularin C (34) was stated to exhibit potent cytotoxicity against P-388 and A-549 cells at ED50 values of 0.12 and 0.01 μg/ml respectively while cespitularin E (36) exhibits potent cytotoxicity against A-549 cells at ED50 value of 0.034 μg/ml [9].
Shen et al. [19], stated that some isolated cespihypotin diterpene derivatives showed significant cytotoxic activity against human Daoy and WiDr tumor cell lines. Cespihypotin T (18), a Cespitularia norditerpene with a keto and two adjacent hydroxy groups, showed significant cytotoxic activity against human tumor cells exhibited significant cytotoxicity against Daoy and WiDr cell lines with ED50 values of 9.3 and 7.5 μg/ml, respectively [19].
Some of nitrogen-containing verticillene diterpenoids from the soft coral C. taeniata were reported to exhibit in vitro antitumor activity against human oral epidermoid carcinoma (KB) and murine L1210 leukemia tumor cell lines. Cespitulactam K (31) was stated to have a significant in vitro cytotoxic activity against both human cancer cell lines at 3.7 and 5.1 μg/ml respectively [12].
Duh et al. [11] reported that some isolated cespitularin diterpenoids and secosteroids exhibited cytotoxic activity. It was stated that cespitularin O (47) showed cytotoxicity against P-388 cells with ED50 value of 3.4 μg/ml. While 3β,11-dihydroxy-5β,6β-epoxy-9,11-secocholestan-9-one (83) exhibited cytotoxicity against HT-29 cells with an ED50 of 1.0 μg/ml [11]. Some of reported cespitularines and cespihypotins from C. hypotentaculata have exhibited cytotoxicity against leukemia (P-388 and A-549) cells [9], [11], [18], [20]. Recently, Roy et al. [29] stated that the two alcyonolide derivatives, trisnorditerpenoid 1 (72) and 2 (73), showed cytotoxicity against HCT116 cancer cells with the IC50 values of 6.04 and 47.0 μM, respectively, and a dose dependent [21].
Recently, Lin et al. [15] stated that the isolated diterpenoid from CH2Cl2/EtOH extract of C. taeniata, cespitulon A (74), exhibited significant cytotoxicity against human medulloblastoma and colon adenocarcinoma cancer cells with IC50 values of 8.7 and 6.7 μM, respectively by a comparison with a positive control with IC50 at 0.3 μM [15].
Immunomodulatory and antiviral activities
Some isolated cespihypotin diterpenes from C. hypotentaculata have exhibited weak antiviral activity. Cespihypotin K (11) showed significant enhancement of cell proliferation, while cespihypotin L (12) exhibited inhibition on peripheral blood mononuclear cells (PBMC) proliferation induced by phytohemagglutinin (PHA). The antiviral activities of these compounds were achieved by a comparison with the positive control, cyclosporine A [20].
Anti-inflammatory activity
Some isolated compounds from C. hypotentaculata were reported to have a significant anti-inflammatory activity in vitro. Cespitularin F (37), cespitularines I (41) and cespitularin S (51) showed significant inhibition of iNOS protein expression [21]. Roy et al. [29] stated that the two alcyonolide derivatives, trisnorditerpenoid 1 (72) and 2 (73), showed anti-inflammatory effect in LPS/IFN-c-stimulated inflammatory RAW 264.7 macrophage cells and showed anti-inflammatory activity in low concentrations and a dose dependent of 2–8 μM. The lack of cytotoxicity against RAW 264.7 macrophage cells in the test concentration range indicated that inhibition of nitric oxide production was due to the effects of these compounds [21].
The isolated compounds from C. taeniata, cespitulins E–G (56–58) were reported to have inhibitory effects of superoxide anion generation and elastase release by human neutrophils in response to FMLP/CB. Cespitulin G (56) exhibited significant inhibitory activity against elastase release with an IC50 value of 2.7 μg/ml and inhibition of superoxide anion with an IC50 value of 6.2 μg/ml. Cespitulin E (58) exhibited moderate activities at the concentration of 10 μg/ml (30.6 ± 6.0 and 33.8 ± 4.1% inhibition, respectively) with the use of genistein as a positive control [26].
Antimicrobial activity
Some of the isolated nitrogen-containing verticillene diterpenoids isolated from the Taiwanese soft coral C. taeniata were reported to exhibit a antimicrobial activity [12]. Cespitulactam G (27) was stated to exhibit potent antimicrobial activity against Trichophyton mentagrophytes (IFM45110) with an MIC value of 2.08 μg/ml. Cespitulactam D (24), cespitulactam J (30), and cespitulactam K (31) were reported to have significant antimicrobial activity against M. luteus (IFM2066) and C. neoformans (IFM46914) (6–8) and T. mentagrophytes (2 and 7) with MIC value of 4.16 μg/ml [12], [20]
Chemical constituents of Cespitularia species
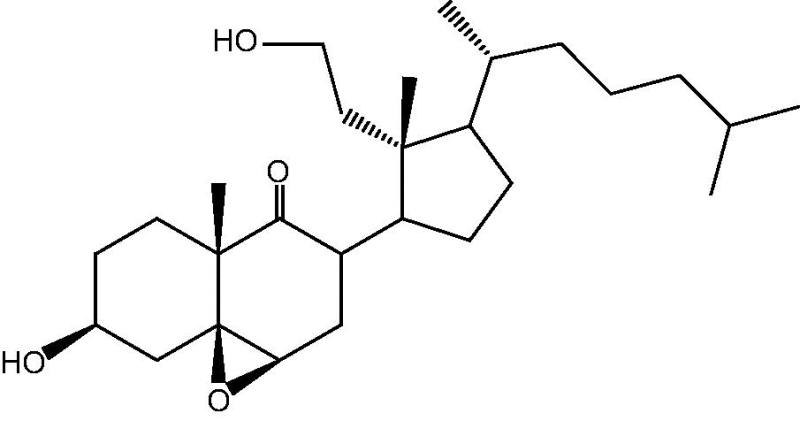
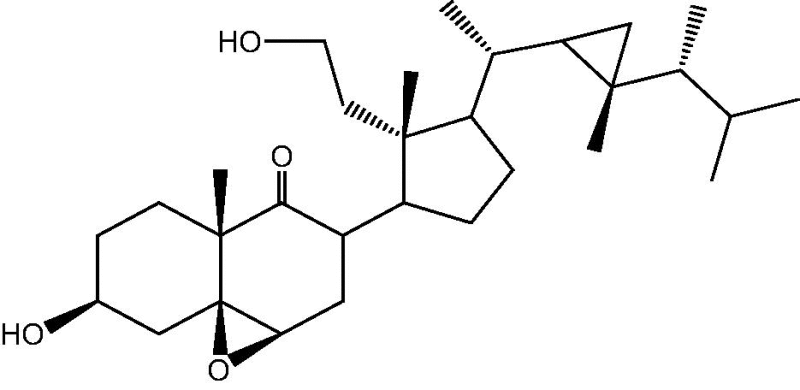
Soft corals of the genus Cespitularia are rich in novel and diverse chemical structures with interesting biological activities. This genus elaborates varied diterpenoids of cembrane, neodolabellane, cespitularane, and verticillane skeleton [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. Few numbers of sesquiterpenes were also reported [23]. The previously isolated diterpenoids, sesquiterpenoids and steroids from Cespitularia species are summarized in Table 1, Table 2, Table 3.
Table 1.
Diterpenoids of Cespitularia species.
| Source | Structure | Compd. name |
|---|---|---|
| C. hypotentaculata[11], [24] | 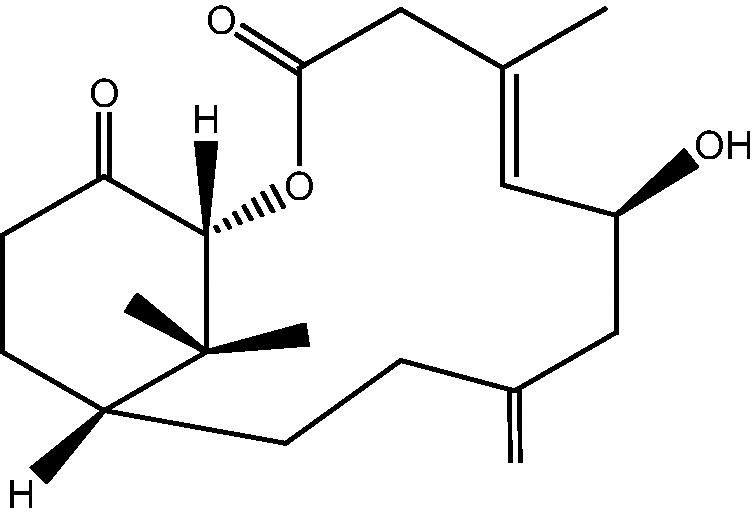 |
1: Cespihypotin A |
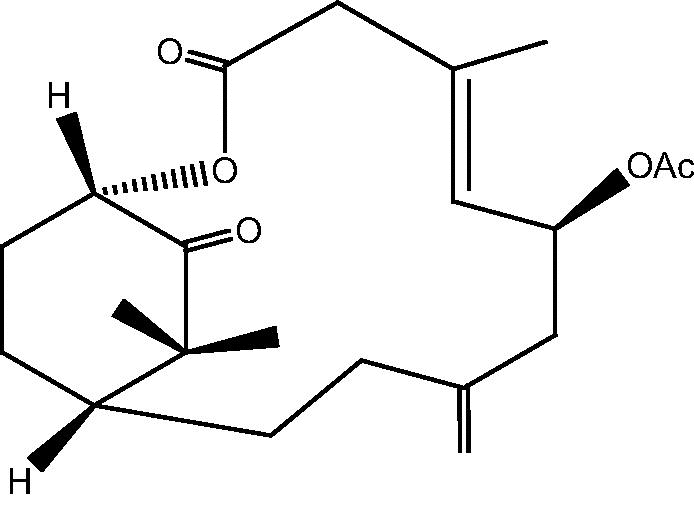 |
2: Cespihypotin B | |
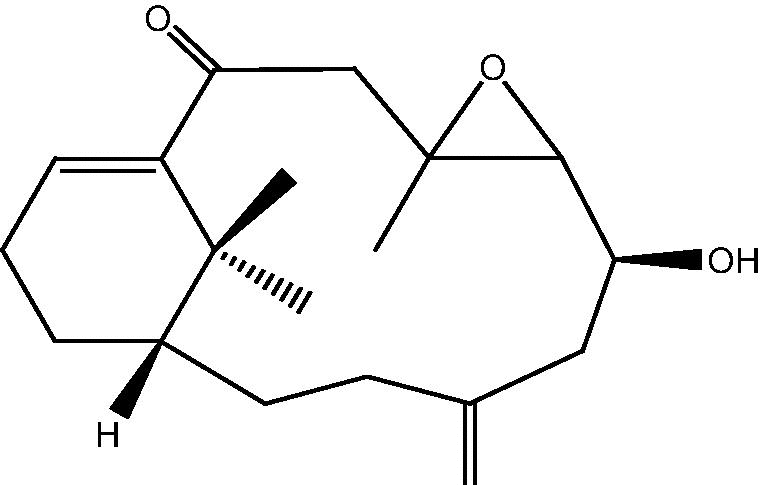 |
3: Cespihypotin C | |
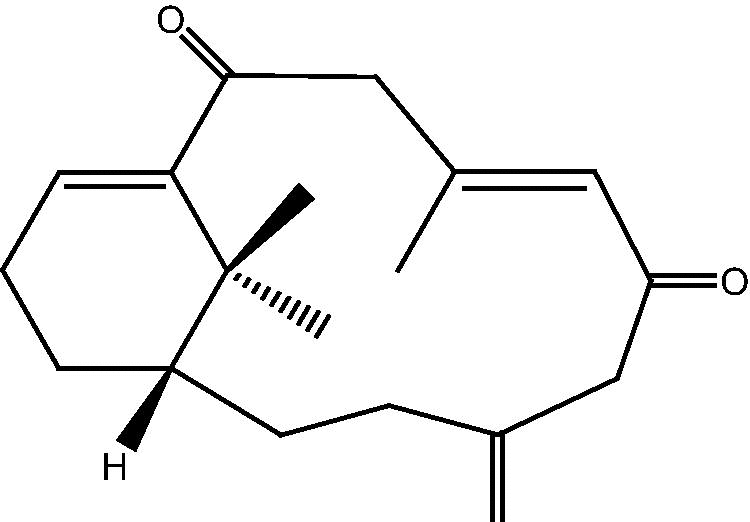 |
4: Cespihypotin D | |
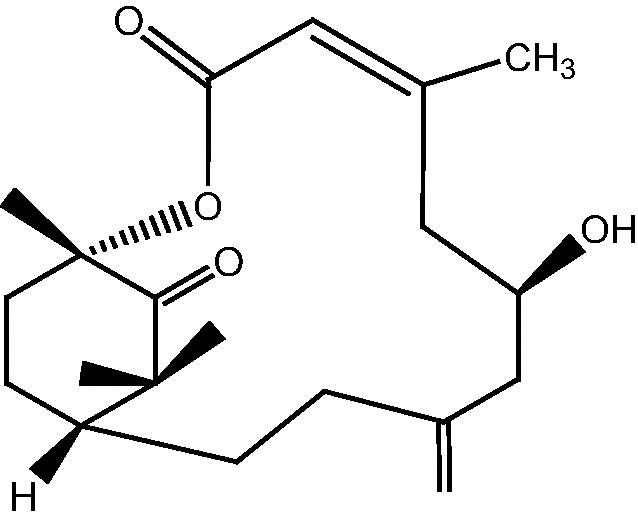 |
5: Cespihypotin E | |
| C. hypotentaculata[20] | 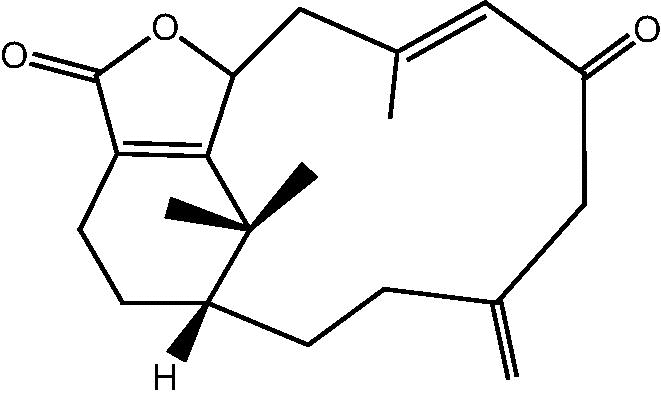 |
6: Cespihypotin F |
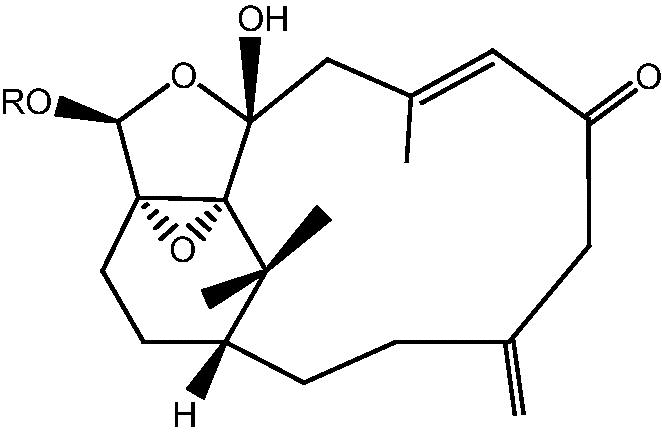 |
7: R H – Cespihypotin G | |
| 8: R COCH CH2 – Cespihypotin H | ||
| 9: R Ac – Cespihypotin I | ||
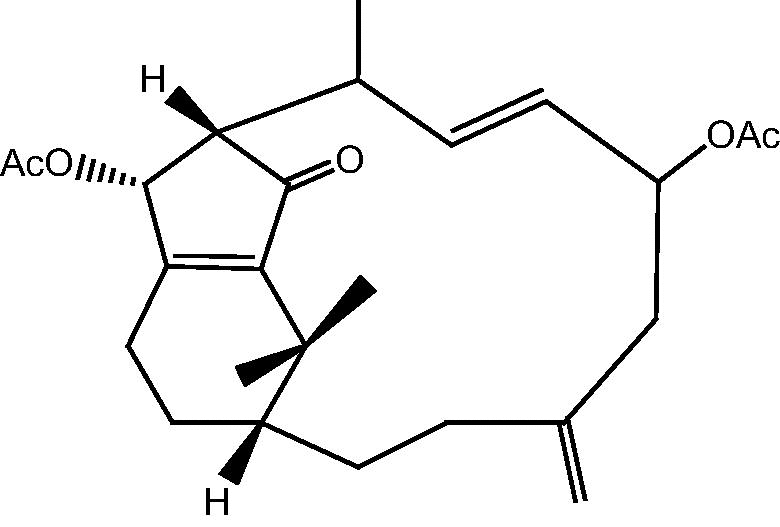 |
10: Cespihypotin J | |
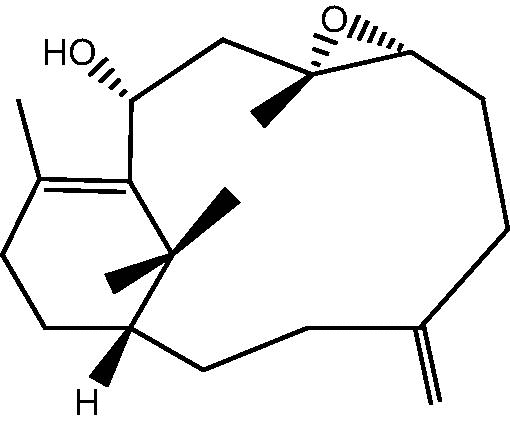 |
11: Cespihypotin K | |
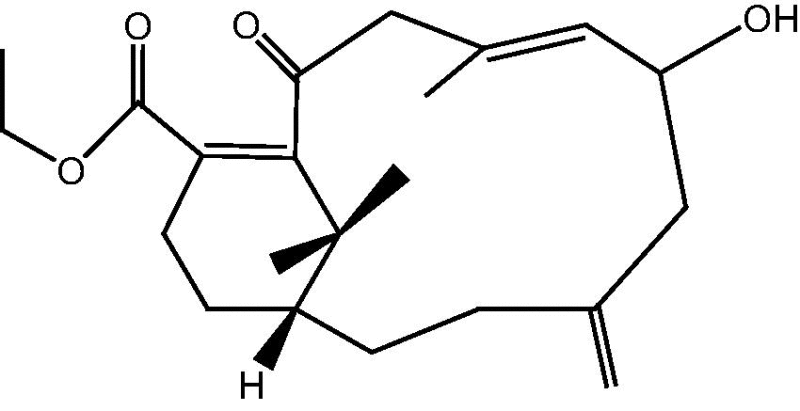 |
12: Cespihypotin L | |
| C. hypotentaculata[19] | 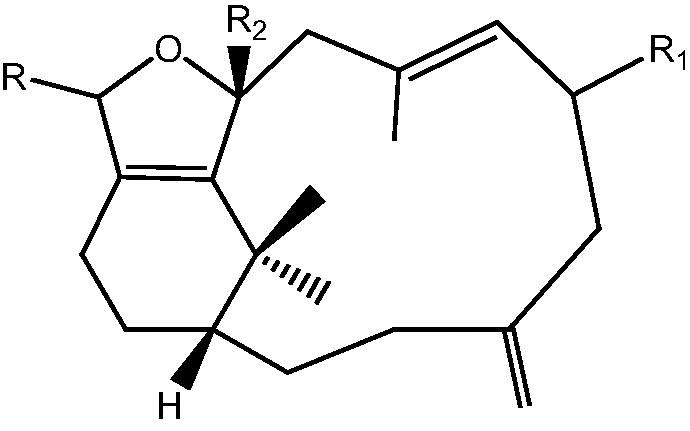 |
13: R R1 OMe, R2 OH – Cespihypotin Q |
| 14: R R2 OMe, R1 H – Cespihypotin R | ||
| 15: R O, R1 R2 OH – Cespihypotin V | ||
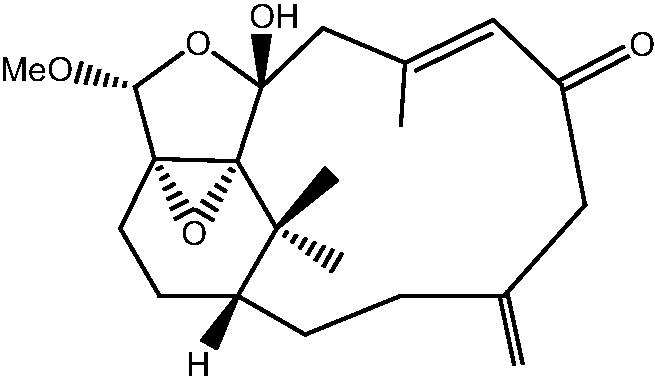 |
16: Cespihypotin S | |
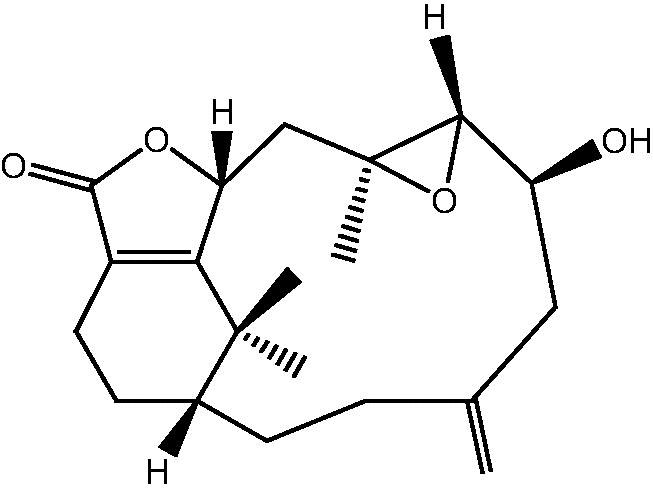 |
17: Cespihypotin U | |
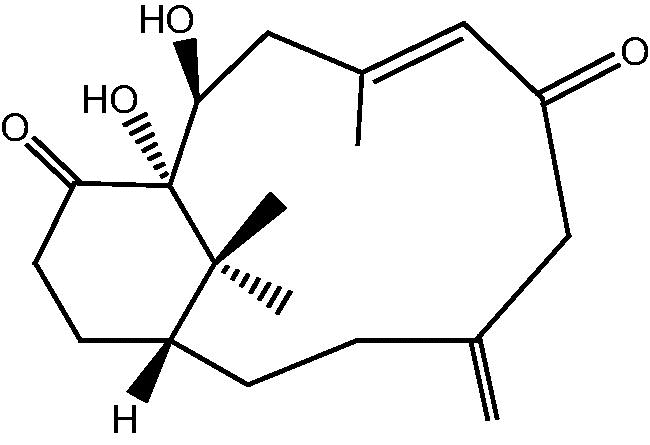 |
18: Cespihypotin T | |
| C. taeniata[20], [16], [25] | 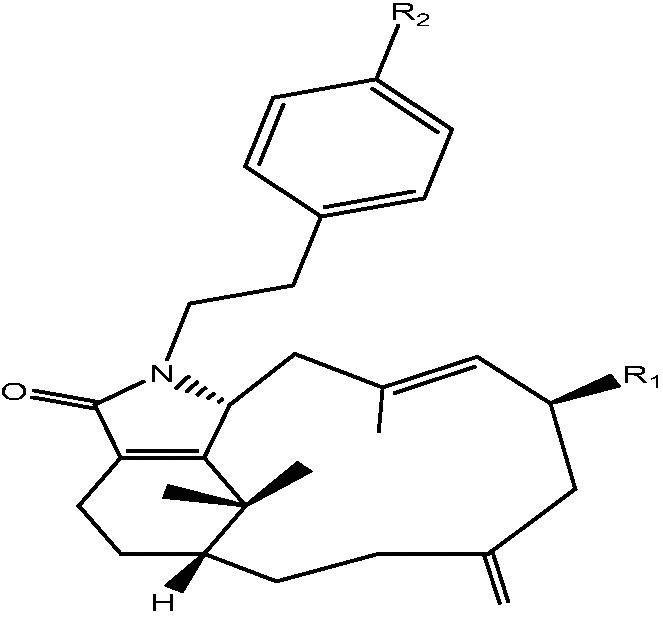 |
19: R1 OH, R2 H – Cespitulactam A |
| 20: R1 R2 OH – Cespitulactam C | ||
| 21: R1 OAc, R2 H – Cespitulactam A-monoacetate | ||
| 22: R1 R2 OAc – Cespitulactam A-diacetate | ||
| 23: R1 H, R2 H – Cespitulactam B | ||
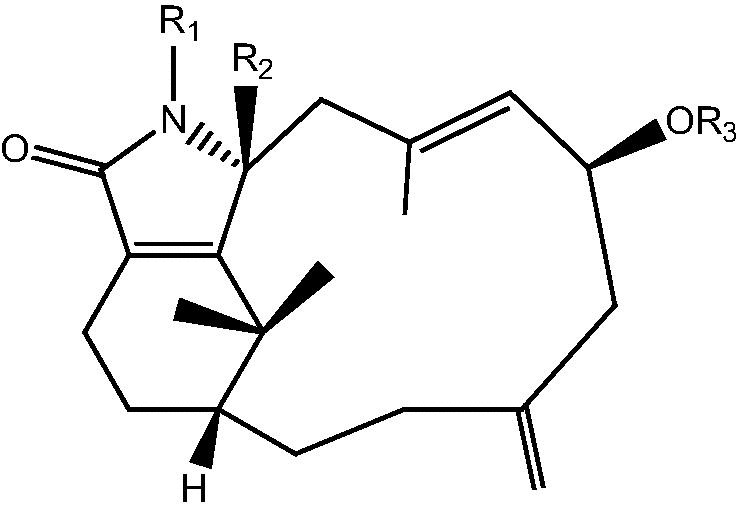 |
24: R1 R2 R3 H – Cespitulactam D | |
| 25: R1 R2 H, R3 Ac – Cespitulactam E | ||
| 26: R1 R3 H, R2 OH – Cespitulactam F | ||
| 27: R1 CH2CH3, R2 OH, R3 H –Cespitulactam G | ||
| 28: R1 CH3, R2 R3 H – Cespitulactam H | ||
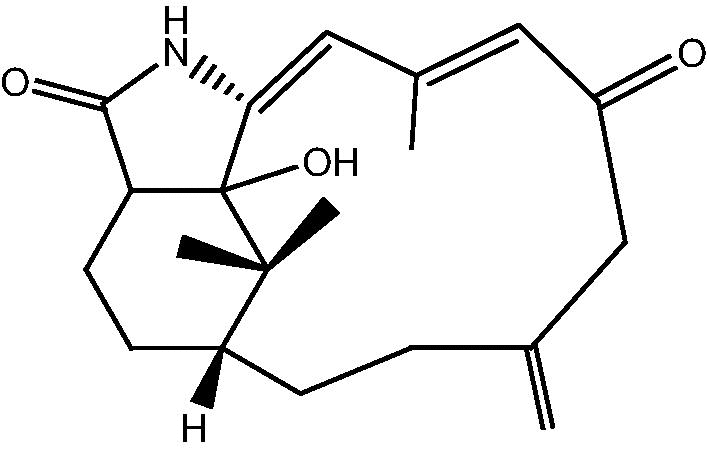 |
29: Cespitulactam I | |
| C. taeniata[12] | 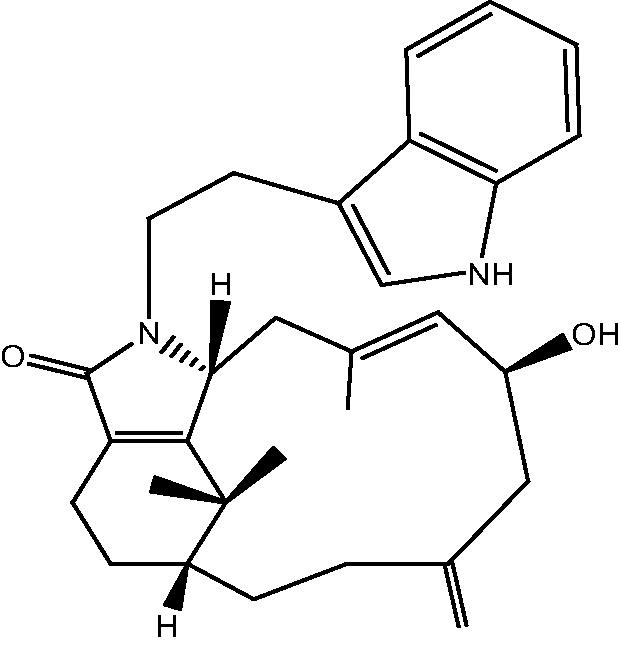 |
30: Cespitulactam J |
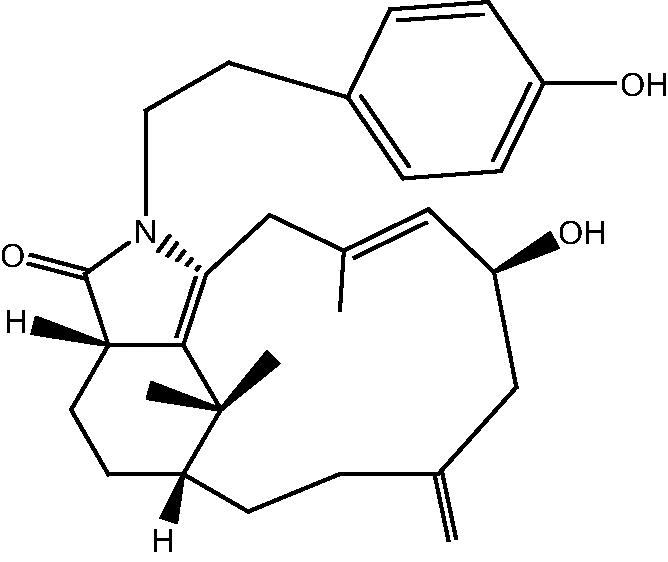 |
31: Cespitulactam K | |
| C. hypotentaculata and C. taeniata[9], [12] | 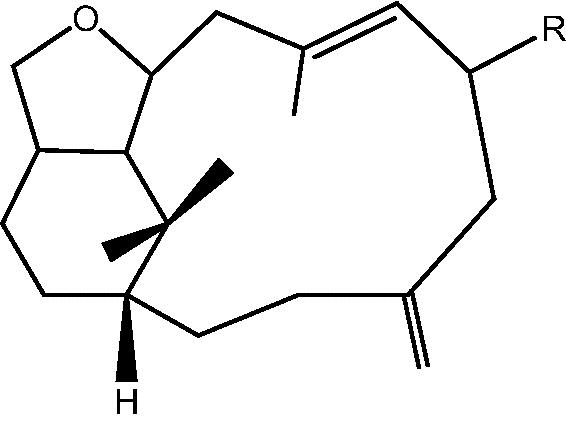 |
32: R OH – Cespitularin A |
| 33: R H – Cespitularin B | ||
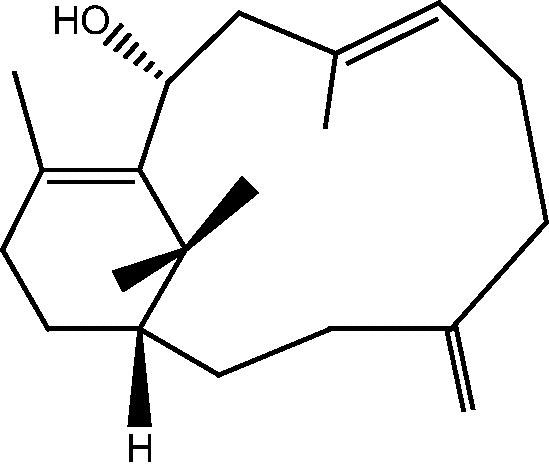 |
34: Cespitularin C | |
| C. hypotentaculata and C. taeniata[9], [12], [20], [24] | 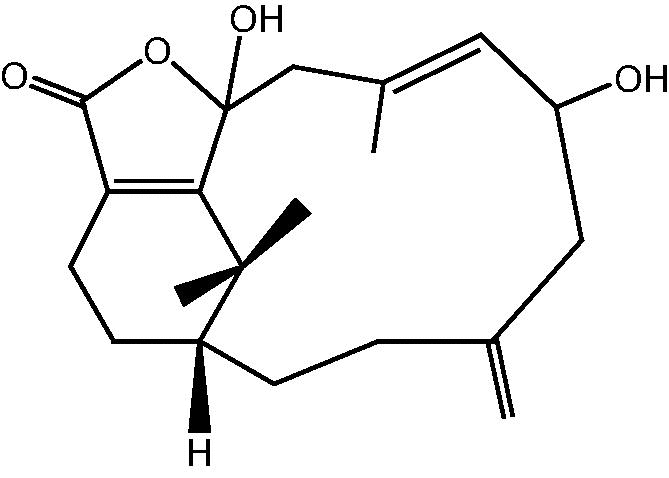 |
35: Cespitularin D |
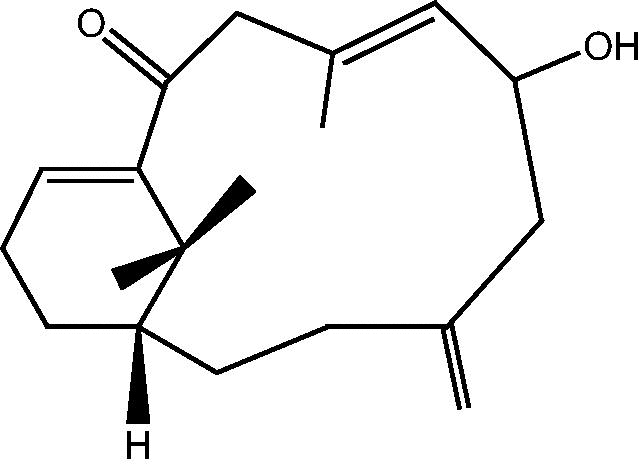 |
36: Cespitularin E | |
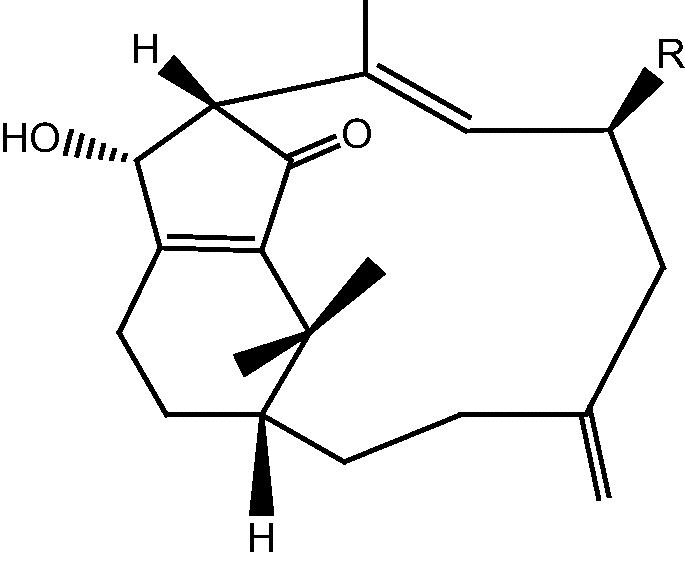 |
37: R OH – Cespitularin F | |
| 38: R OAc – 6-O-acetylcespitularin F | ||
| 39: R H – Cespitularin G | ||
| 40: R O – Cespitularin H | ||
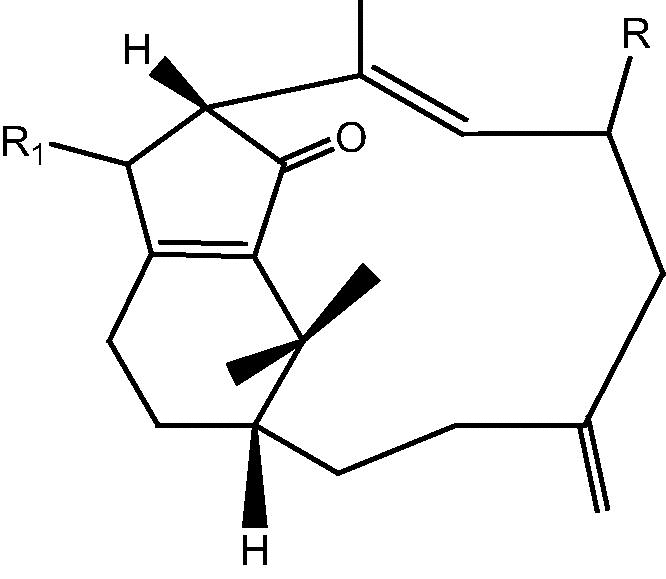 |
41: R OH, R1 O – Cespitularin I | |
| 42: R OAc, R1 α-OH – Cespitularin J | ||
| 43: R OAc, R1 O –Cespitularin K | ||
| C. hypotentaculata[11], [22] | 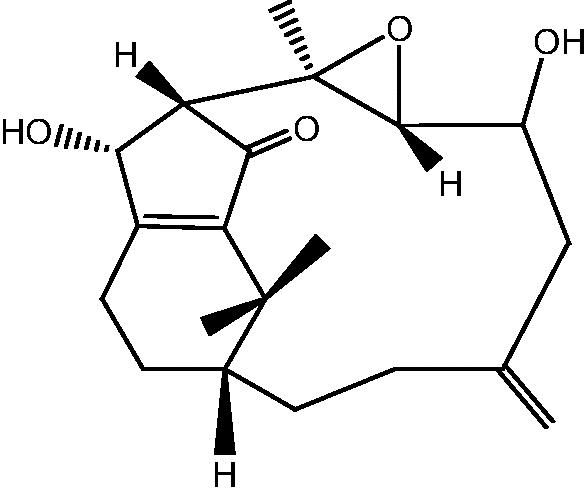 |
44: Cespitularin L |
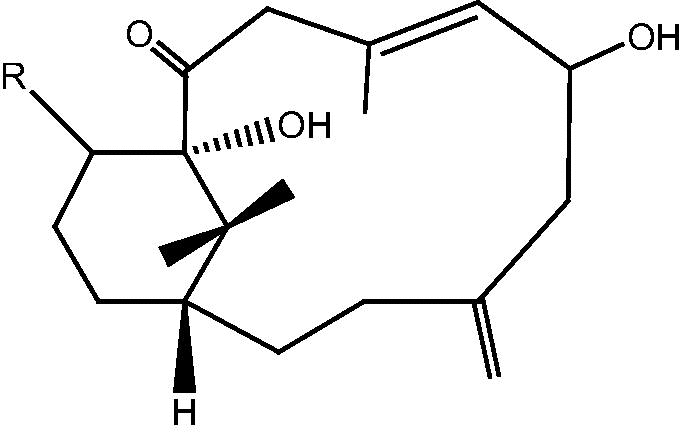 |
45: R α-OH – Cespitularin M | |
| 46: R β-OH – Cespitularin N | ||
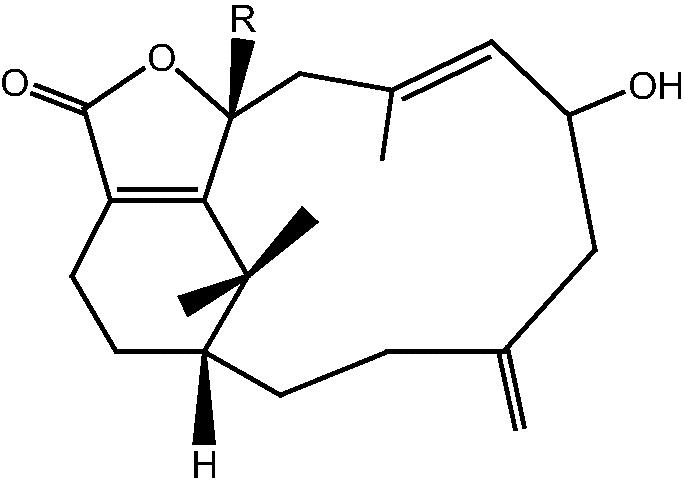 |
47: R H – Cespitularin O | |
| 48: R OMe – Cespitularin P | ||
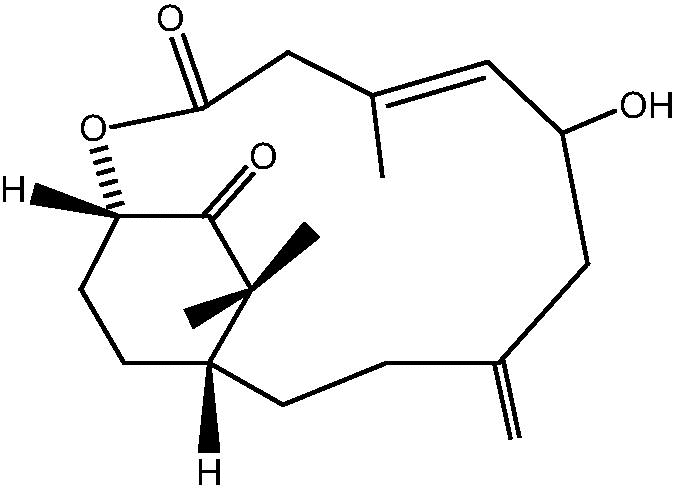 |
49: Cespitularin Q | |
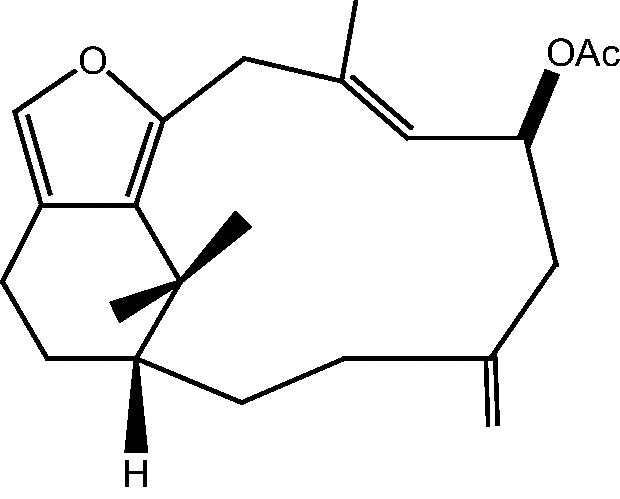 |
50: Cespitularin R | |
| C. hypotentaculata[22] | 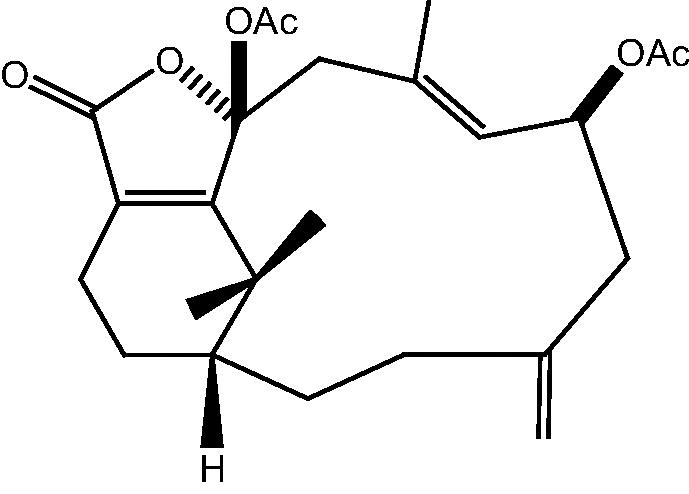 |
51: Cespitularin S |
| C. taeniata[25] | 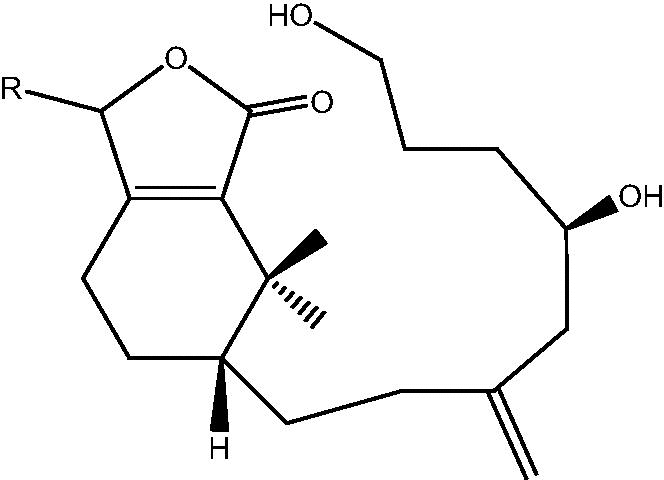 |
52: R α-OH – Cespitulin A |
| 53: R β-OH – Cespitulin B | ||
| 54: R α-OEt – Cespitulin C | ||
| 55: R β-OEt – Cespitulin D | ||
| C. taeniata[26] | 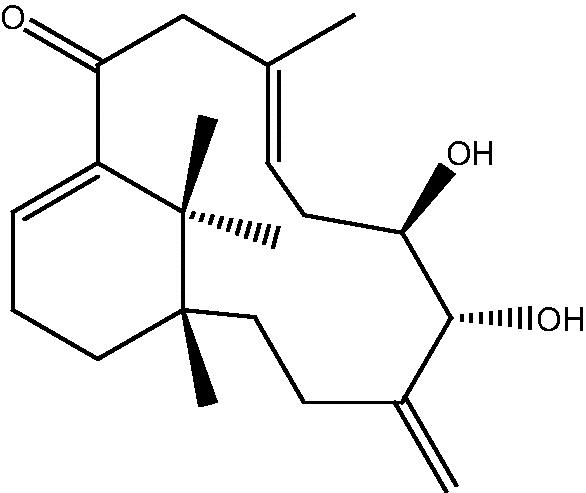 |
56: Cespitulin E |
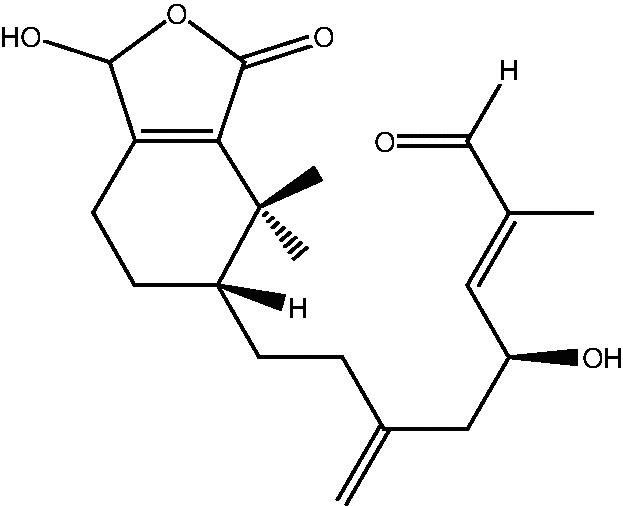 |
57: Cespitulin F | |
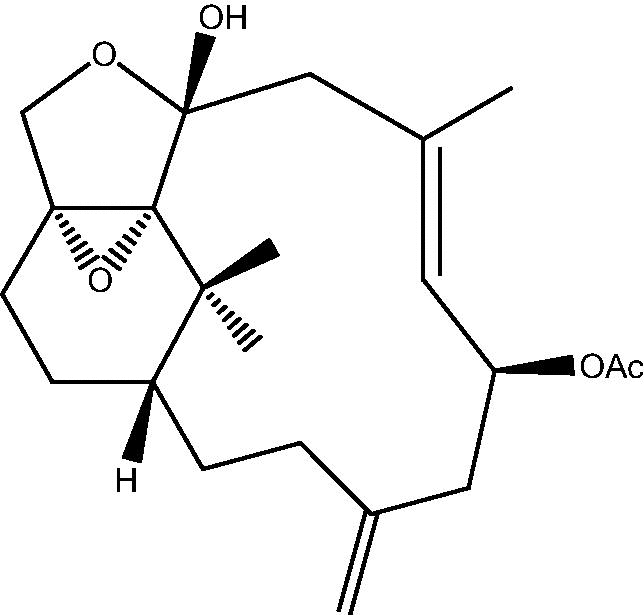 |
58: Cespitulin G | |
| C. hypotentaculata[22], [24] | 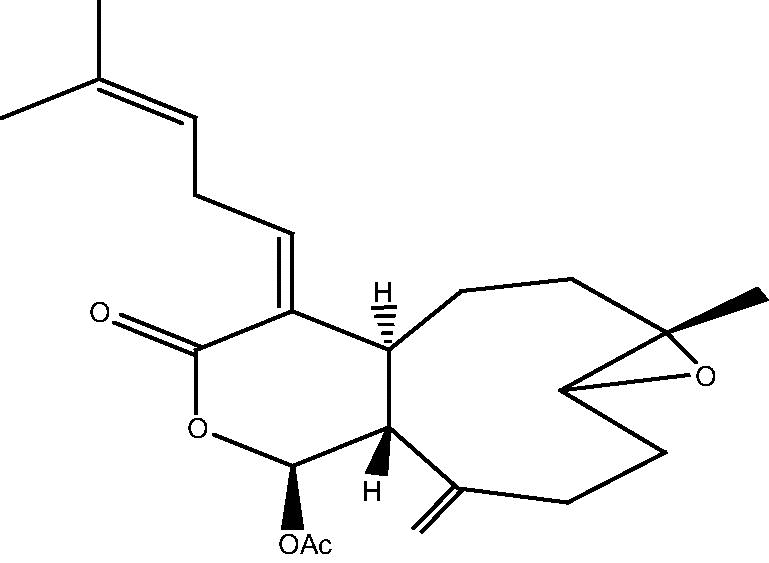 |
59: Cespitolide |
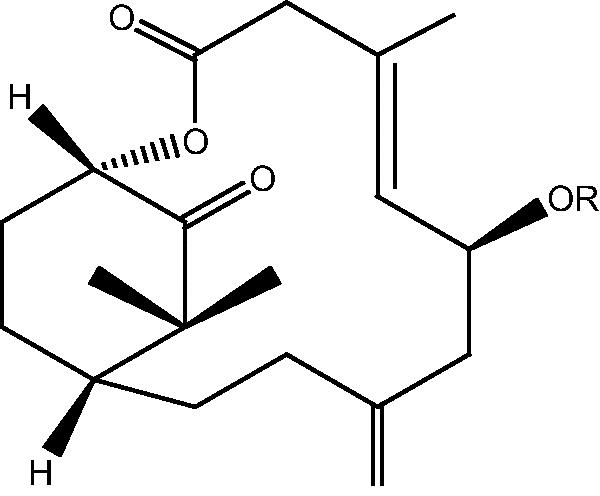 |
60: R H – Cespitulactone A | |
| 61: R Bz – Cespitulactone B | ||
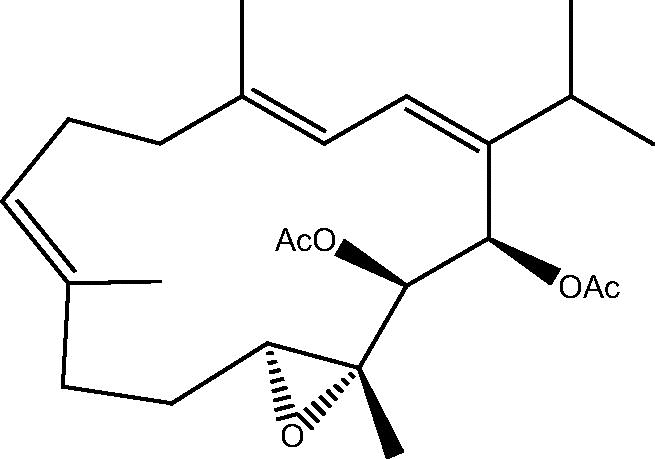 |
62: Flaccidoxide-13-acetate | |
| C. sp [28] | 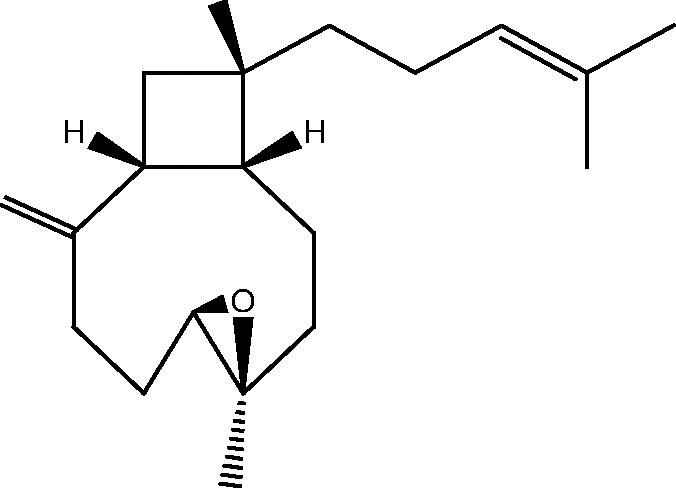 |
63: 4β,5β-epoxyxeniaphylla-8(19),14-diene |
| C. erecta[29] | 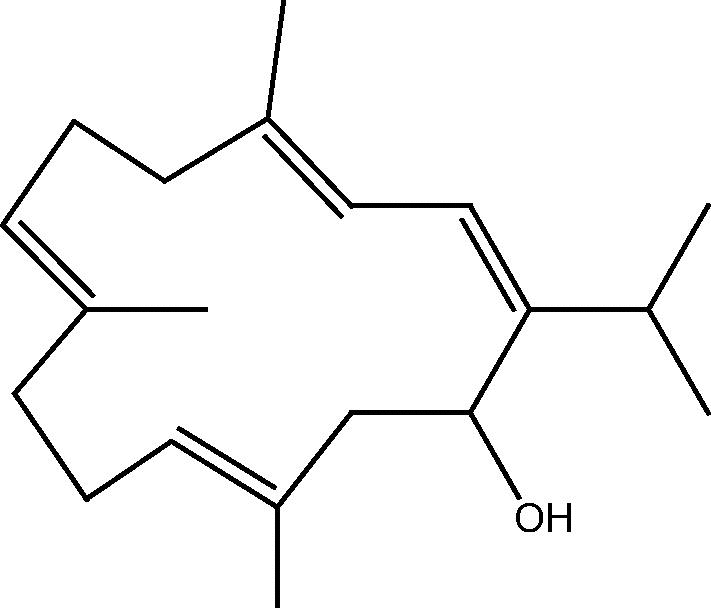 |
64: Sarcophytol A |
| C. erecta[29] | 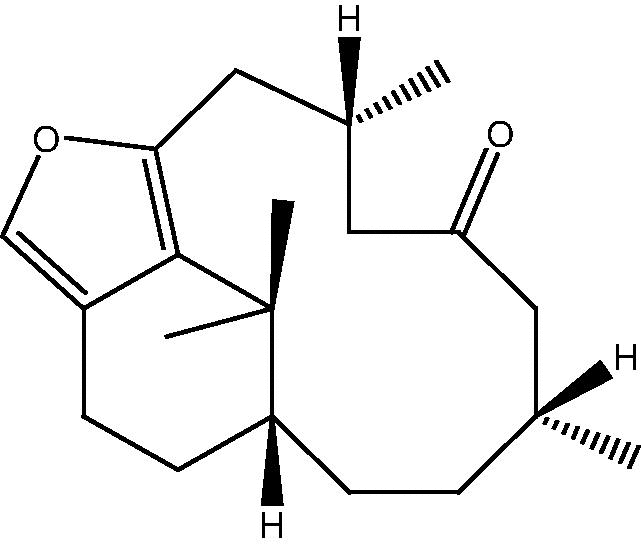 |
65 |
| C. sp [21] | 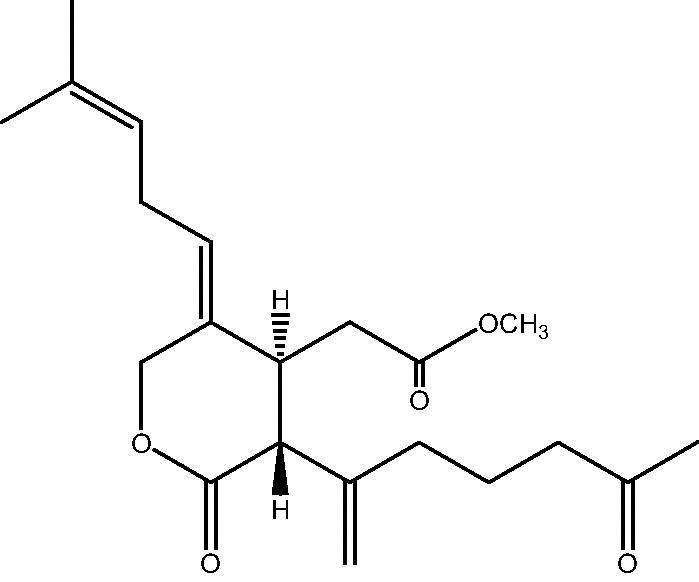 |
66 |
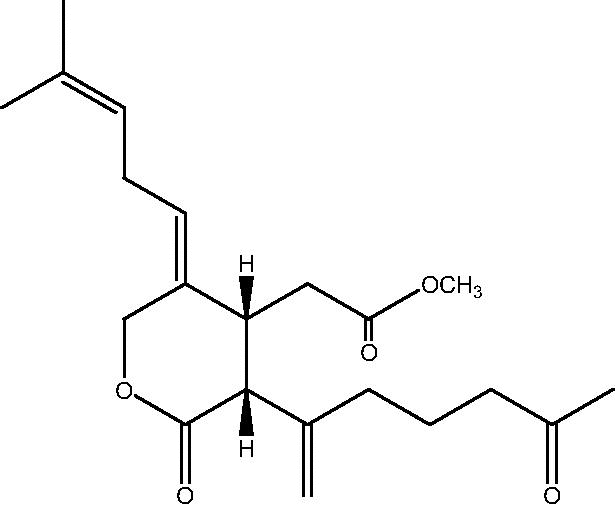 |
67 | |
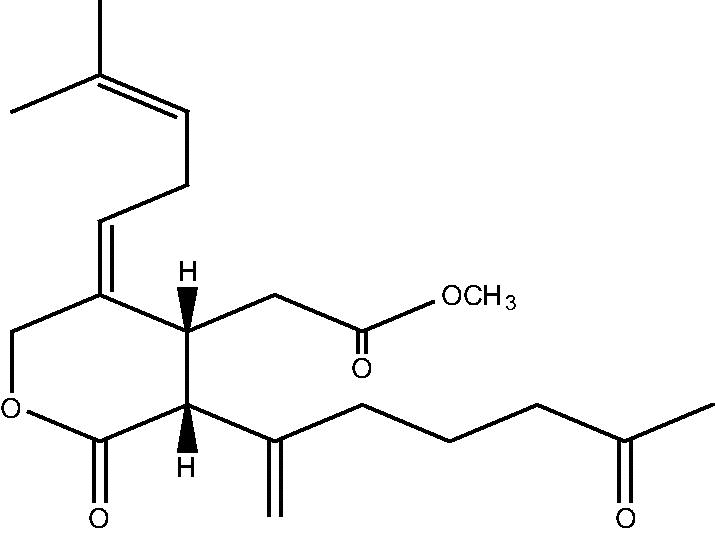 |
68 | |
| C. sp [21] | 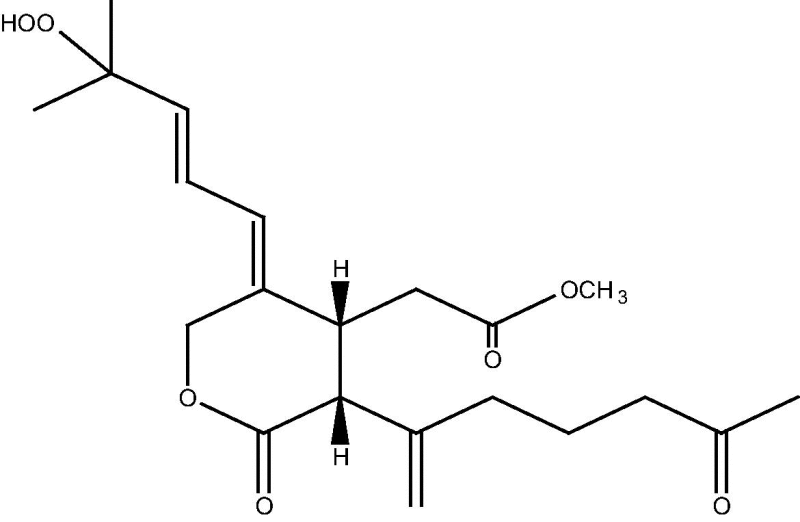 |
69 |
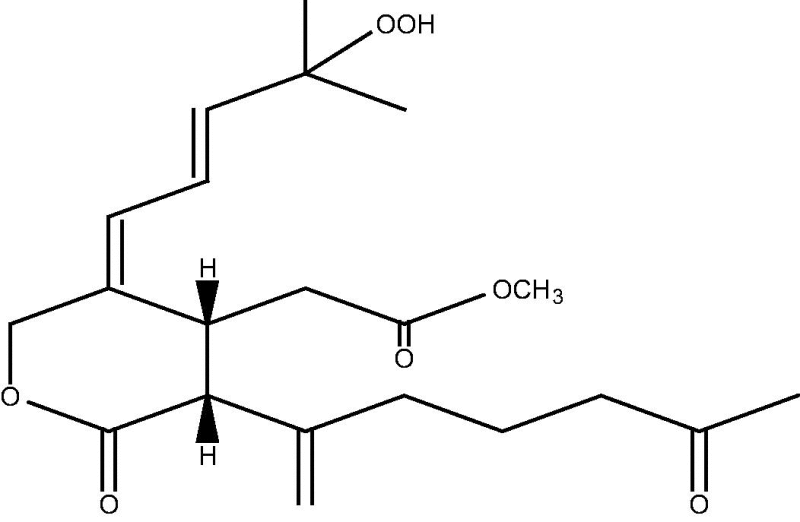 |
70 | |
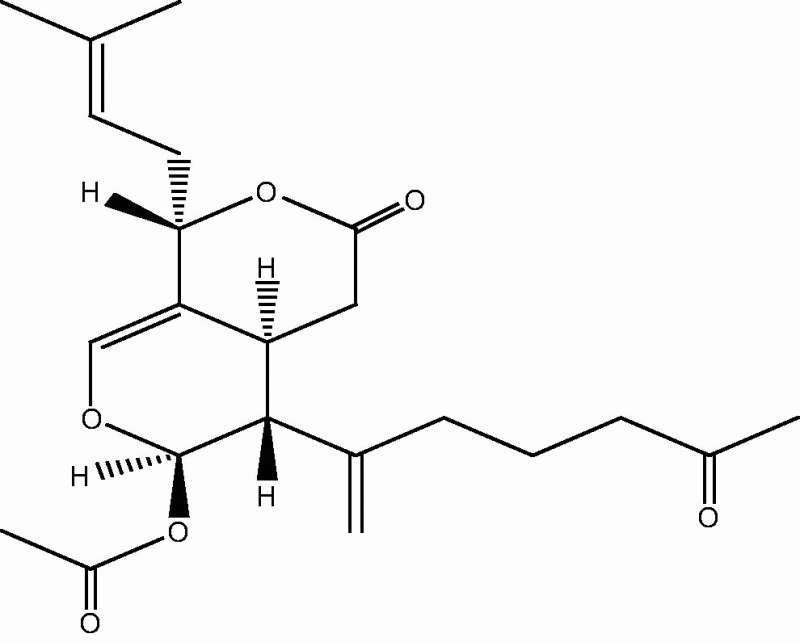 |
71: Alcyonolide | |
| C. sp [21], [29] | 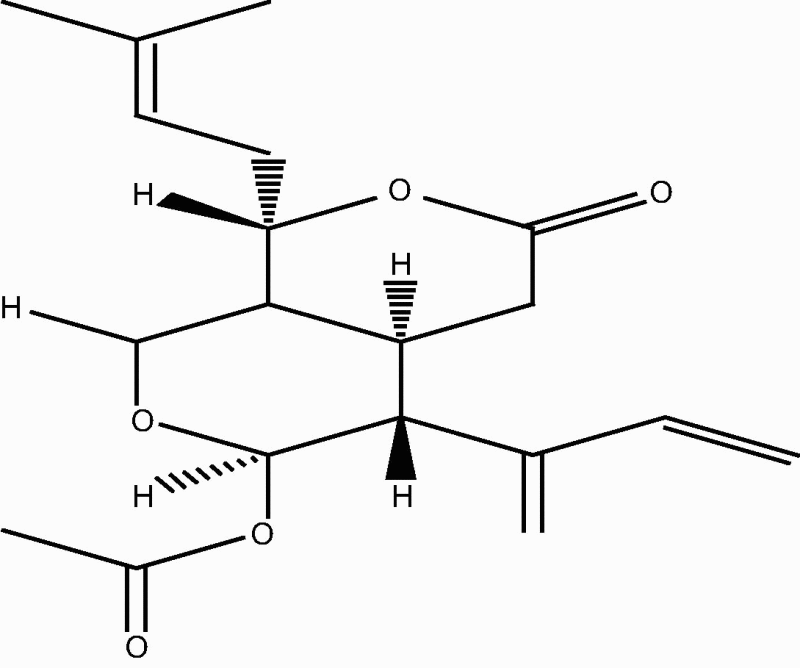 |
72: Trisnorditerpenoid 1 |
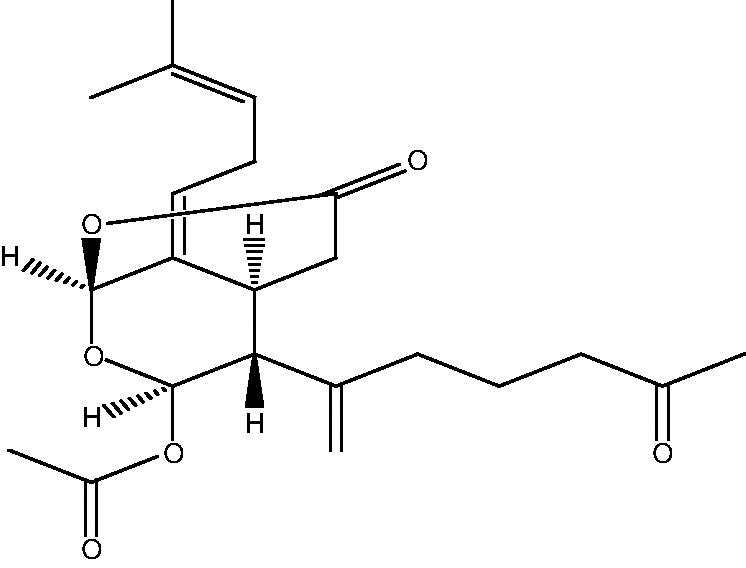 |
73: Trisnorditerpenoid 2 | |
| C. taeniata (15) | 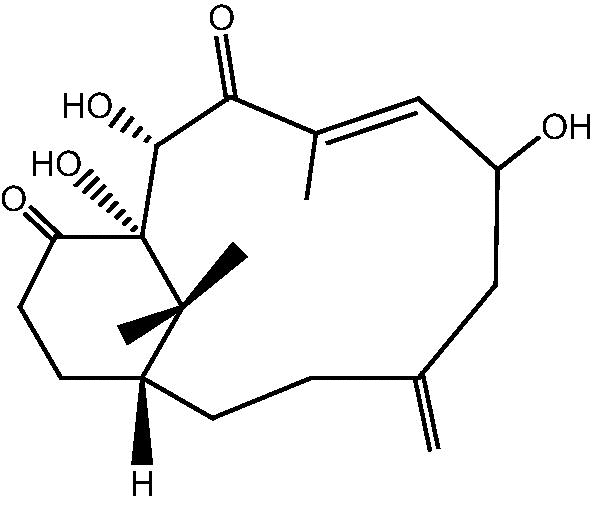 |
74: Cespitulone A |
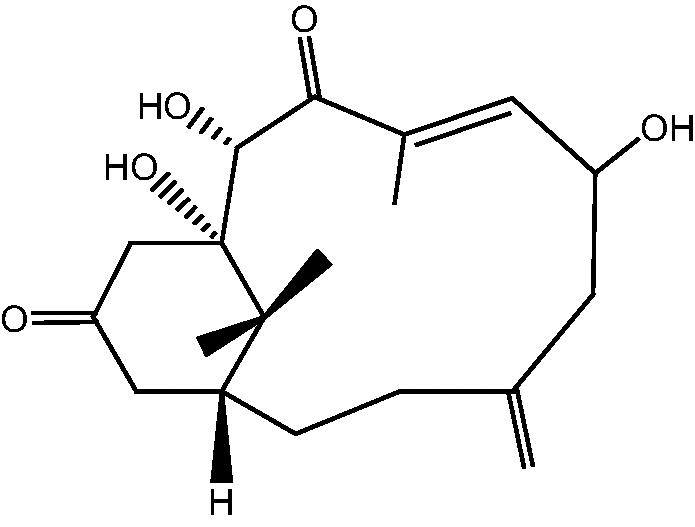 |
75: Cespitulone B | |
Table 2.
Sesquiterpenoids of Cespitularia species.
| Source | Structure | Compd. name |
|---|---|---|
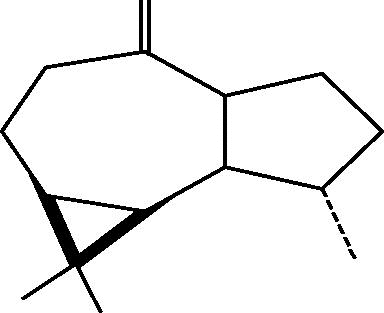
| C. aff. subviridis[23], [27] | 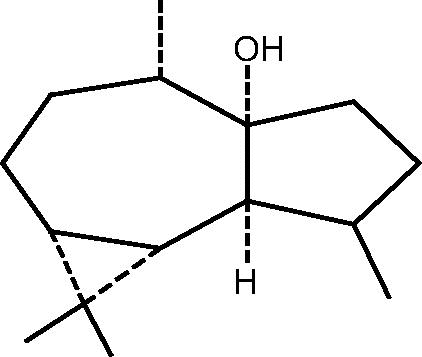 |
76: (+)Palustrol |
 |
77: (−)Alloaromadendrene | |
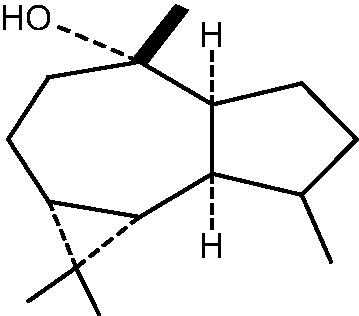 |
78: (−)Viridiflorol | |
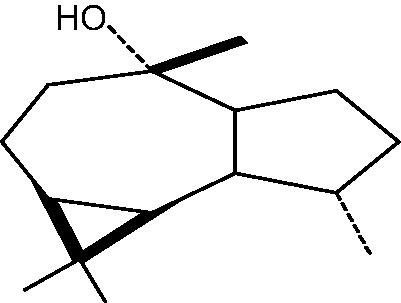 |
79: (+)Ledol | |
| C. taeniata[14] | 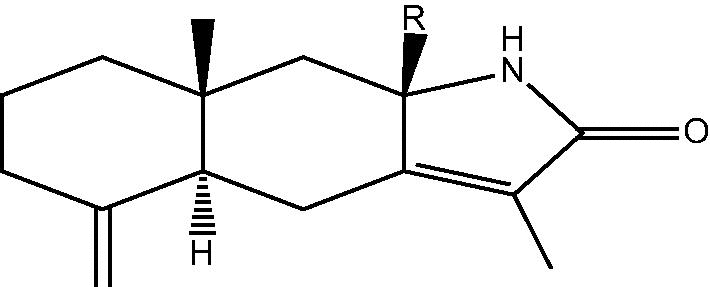 |
80: R H – Taenialactam A |
| 81: R OH – Taenialactam B | ||
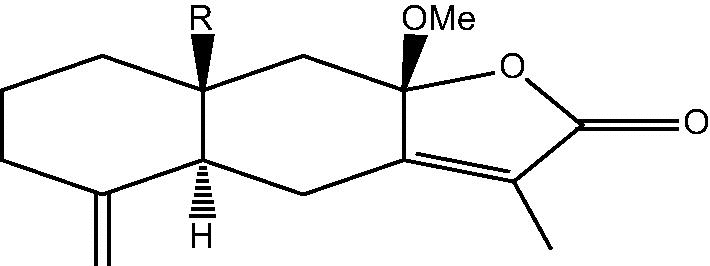 |
82: R α-Me – Taenialactone A | |
| 83: R β-Me – 8β-methoxyatractylenolide | ||
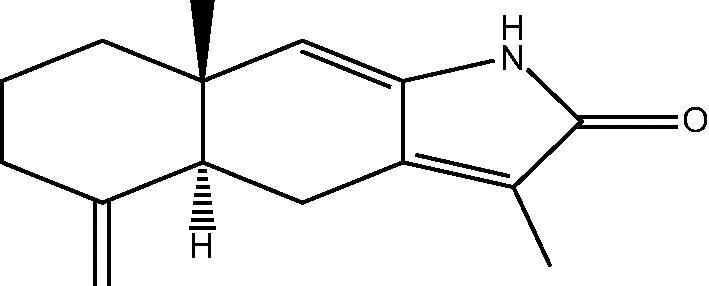 |
84: Atractylenolactam | |
| C. sp [26], [30] | 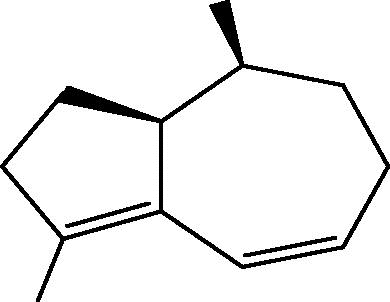 |
85: Trinorsesquiterpene |
Table 3.
Steroids of Cespitularia species.
Diterpenoids
Marine invertebrates are a rich source of structurally unique terpenoids with interesting biological activities. The biological activity of some isolated Cespitularia diterpenoids has demonstrated remarkable cytotoxicity against various cancer cell lines. Several chemical studies on the Cespitularia species led to the isolation of a diverse array of diterpenoids as shown in Table 1, including alcyonolides, caryophyllanoids, cembranolides, cespitularanoids, dolabellanoids, norverticillanoids, verticillanoids, and xenicanoids.
Biogenetic pathways of Cespitularia diterpenoids
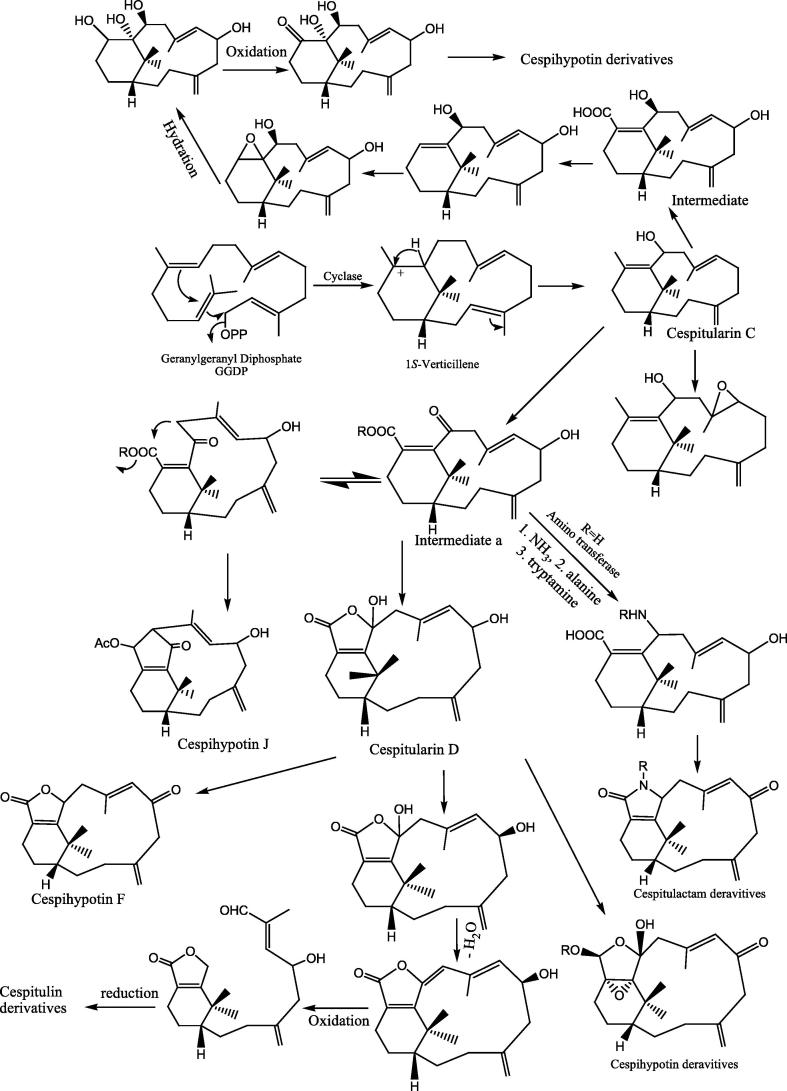
The Cespitularia species are characterized with a special type of diterpenoids such as cespitularia, cespihypotin, cespitulactam, cespitularin, and cespitulons. The biogenetic pathways of these diterpenoids were described in few reports [15], [16]. The biogenetic pathways of Cespitularia diterpenoids, as shown in Fig. 2, were derived from the starting amino acid geranylgeranyl diphosphate (GGDP). The amino acid (GGDP) was enzymatically converted by cyclization to 1S-verticillene that might be the main precursor of all Cespitularia diterpenoids. Firstly, cespitularin C, a basic diterpenoid for biogenesis of Cespitularia diterpenoids, was synthesized from 1S-verticillene via biogenetically rearrangement. Then cespitularin C was biogenetically rearranged to give the intermediate a that could be converted to different Cespitularia diterpenes such as cespitularin, cespihypotin and cespitulactams [16], [19], [20], [26].
Fig. 2.
Biogenetic pathways of Cespitularia diterpenoids.
Sesquiterpenoids
A few reported sesquiterpenoids were identified from Cespitularia species that include sesquiterpenoids, N-containing sesquiterpenes (sesquiterpene lactams), and sesquiterpene lactones (Table 2). Cheng et al. [10] stated the biogenesis of the Cespitularia sesquiterpenoids starting by (E,E)-Farnesyl cation [10].
Steroids
Soft corals belonging to family Xeniidae have been shown to be an extraordinarily rich source of sterols displaying unconventional nuclear structures and side chains, as well as unusual oxygenation patterns of the A-D rings such as petrosterols [31], gorgosterols [32], cholesterols, ergosterols [33], [34] and secosteroids [35], [36], [37]. The first marine secosteroid to be described was encountered in the gorgonian Pseudopterogorgia americana in 1972 [36]. Cespitularia species are not rich soft corals with steroids. Only two cytotoxic 9,11-secosteroids were isolated from C. hypotentaculata [11].
Conclusions
Cespitularia species (family Xeniidae) are interesting marine organisms as rich sources in novel and diverse chemical structures such as terpenoids and steroids. These species are characterized by special types of diterpenoids that may be named Cespitularia diterpenoids such as cespitulins, cespitularines, cespitulactams, cespitulactones and cespihypotins. As well as Cespitularia species are characterized by a very rare type of sesquiterpene lactams. Biologically, Cespitularia species produce novel secondary metabolites with very interesting biological activities especially anticancer activity.
Conflict of Interest
The authors have declare no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Abdelsamed I. Elshamy, Researcher in National Research Centre, Egypt. The research experiences focused on isolation and identification of phenanthrenes, flavonoids, sterols, terpenes, coumarines, volatile oils, ceramides from medicinal plants and marines by different isolation and identification methods such as structural elucidation by modern techniques of spectroscopic analysis, MS, HRMS, 1D and 2D NMR and X-ray. Synthesis of derivatives of natural products. Bioactive assay in vivo and in vitro of natural products against different diseases.

Mahmoud I. Nassar, Professor of Natural Products Chemistry, National Research Centre, Egypt. His research experiences focused on isolation, identification of phenanthrenes, flavonoids, sterols, coumarines, volatile oils, and ceramides from medicinal plants and marines. Bioactive evaluation of natural products such as plant extracts and compounds.

Tarik A. Mohamed, Researcher in National Research Centre, Egypt. His research interest focused on Chemical Constituents of Medicinal Plants and Marine Organisms, Extraction, Isolation and Purification of Natural Bioactive Compounds, Structural Elucidation of Natural Products by Modern Techniques of Spectroscopic Analysis, MS, HRMS, 1D and 2D NMR and X-ray analysis, Biological Activities of Natural Products against different common diseases and Biotransformation for Natural Compounds.

Mohamed-Elamir F. Hegazy, Associate Professor in Chemistry of Medicinal plant Department, National Research Center, who has two Ph.D. degrees: A Ph.D. degree from Hiroshima University, Japan, and a Ph.D. degree from Minia University, Egypt. Dr. Hegazy is working in the field of natural products chemistry and biotransformation of natural compounds with cultured plant cells ten years ago and he had a strong experience in the isolation, purification and identification of natural compounds from medicinal plants and marine organisms using high technique for identification (1D and 2D NMR analysis).
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Faulkner D. Marine natural products. Nat Prod Rep. 2001;18:1. doi: 10.1039/b006897g. [DOI] [PubMed] [Google Scholar]
- 2.Faulkner D. Marine natural products. Nat Prod Rep. 1990;7:613. doi: 10.1039/np9900700269. [DOI] [PubMed] [Google Scholar]
- 3.Bhakuni D.S., Rawat D.S. Anamaya Publishers; New Delhi, India: 2005. Bioactive Marine Natural Products. [Google Scholar]
- 4.Scheuer P.J. vol. 4. Academic Press; New York: 1980. (Marine Natural Products, Chemical and Biological Perspectives). [Google Scholar]
- 5.Scheuer P.J. vol. 5. Academic Press; New York: 1983. (Marine Natural Products, Chemical and Biological Perspectives). [Google Scholar]
- 6.Van-Ofwegen L. “Xeniidae” World register of marine species. Retrieved January 29, 2012.
- 7.Borneman EH. Aquarium corals. Selection, husbandry and natural history 2001–2009. TFH Publications; 2009. p. 145–53.
- 8.Van-Ofwegen L. Cespitularia Milne-Edwards & Haime. 2013:1850. [Google Scholar]
- 9.Duh C.H., El-Gamal A.A., Chiang C.Y., Chu C.J., Wang S.K., Dai C.F. Novel terpenoids from the formosan soft coral Cespitularia hypotentaculata. J Nat Prod. 2002;65:1429–1433. doi: 10.1021/np020077w. [DOI] [PubMed] [Google Scholar]
- 10.Cheng J.Y., El-Razek M.H.A., Shen Y.C. Verticillane and norverticillane diterpenoids from the formosan soft coral Cespitularia hypotentaculata. Helv Chim Acta. 2009;92:2146–2154. [Google Scholar]
- 11.Duh C.Y., Li C.H., Wang S.K., Dai C.F. Diterpenoids, Norditerpenoids, and secosteroids from the Formosan soft coral Cespitularia hypotentaculata. J Nat Prod. 2006;69:1188–1192. doi: 10.1021/np0505465. [DOI] [PubMed] [Google Scholar]
- 12.Shen Y.C., Cheng Y., Kobayashi J., Kubota T., Takahashi Y., Mikami Y., et al. Nitrogen-containing verticillene diterpenoids from the Taiwanese soft coral Cespitularia taeniata. J Nat Prod. 2007;70:1961–1965. doi: 10.1021/np078011u. [DOI] [PubMed] [Google Scholar]
- 13.Bowden B.F., Coll J.C., Gulbis J.M., Mackay M.F., Willis R.H. Studies of Australian soft corals. XXXVIII. Structure determination of several diterpenes derived from a Cespitularia species (Coelenterata, Octocorallia, Xeniidae) Aust J Chem. 1986;39:803–812. [Google Scholar]
- 14.Cheng Y., Chen C., Kuo Y., Shen Y. New nitrogen-containing sesquiterpenoids from the Taiwanese soft coral cespitularia taeniata MAY. Chem Biodiversity. 2009;6:1266–1272. doi: 10.1002/cbdv.200800195. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y., Wang S., Chen C., Kuo Y., Shen Y. Cespitulones A and B, cytotoxic diterpenoids of a new structure class from the soft coral Cespitularia taeniata. Marine Drugs. 2014;12:3477–3486. doi: 10.3390/md12063477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen Y., Ho C., Kuo Y., Lin Y. Cespitulactones A and B, new diterpenoids from Cespitularia taeniata. Bioorg Med Chem Lett. 2005;16(9):2369–2372. doi: 10.1016/j.bmcl.2006.01.118. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Z.L., Cao W.Y., Zhou G.X., Wichtl M. A sesquiterpene lactam from Artractylodes macrocephala. Phytochemistry. 1997;45(4):765–767. [Google Scholar]
- 18.Shen Y.C., Lin Y., Kuo Y.S., Cheng Y. Cespitulactams A, B, and C, three new nitrogen-containing diterpenes from Cespitularia taeniata May. Tetrahedron Lett. 2005;46:7893–7897. [Google Scholar]
- 19.Shen Y.C., Lo K.L., Kuo Y.S., Kuo Y.C., Chen Y.C., Khalil A.T. Cespihypotin Q-V, verticillene diterpenoids from Cespitularia hypotentaculata. J Nat Prod. 2008;71:1993–1997. doi: 10.1021/np8005327. [DOI] [PubMed] [Google Scholar]
- 20.Shen Y.C., Wu Y.R., Lin J.J., Lo K.L., Kuo Y.C., Khalil A.T. Eight new diterpenoids from soft coral Cespitularia hypotentaculata. Tetrahedron. 2007;63:10914–10920. [Google Scholar]
- 21.Roy K.P., Maarisit W., Roy M.C., Taira J., Ueda K. Five new diterpenoids from an Okinawan soft coral, Cespitularia sp. Marine Drugs. 2012;10:2741–2748. doi: 10.3390/md10122741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng S., Lin E., Wen Z., Chiang M., Duh C. Two new verticillane-type diterpenoids from the formosan soft coral Cespitularia hypotentaculata. Chem Pharm Bull. 2010;47:6651–6655. doi: 10.1248/cpb.58.848. [DOI] [PubMed] [Google Scholar]
- 23.Braekmann J.C., Daloze D., Ottinger R., Tursch B. Chemical studies of marine invertebrates. XXVII. On the absolute configuration of aromadendrane sesquiterpenes from the soft coral Cespitularia aff. Subviridis. Experientia. 1977;33(8):993. doi: 10.1007/BF01945925. [DOI] [PubMed] [Google Scholar]
- 24.Shen Y., Lin J., Wu Y., Cheng J., Duh C., Lo K.L. New norditerpenoids from Cespitularia hypotentaculata. Tetrahedron Lett. 2006;47:6651–6655. [Google Scholar]
- 25.Lin Y., Fazary A.E., Shen Y. Cespitulins A–D, novel diterpenoids from Taiwanese Cespitularia taeniata. Tetrahedron Lett. 2010;51:6654–6657. [Google Scholar]
- 26.Chang J., Fazary A.E., Lin Y., Hwang T., Shen Y. New verticillane diterpenoids from Cespitularia taeniata. Chem Biodivers. 2012;9:654–661. doi: 10.1002/cbdv.201100122. [DOI] [PubMed] [Google Scholar]
- 27.Konig G., Wright A. A new caryophyllene-based diterpene from the soft coral, Cespitularia sp. J Nat Prod. 1993;56(12):2198–2200. [Google Scholar]
- 28.Janairo J.R., Janairo G.C., Ragasa C.Y., Bowden B.F. A marine verticillane diterpenoid from Cespitularia erecta. Nat Prod Res. 2008;22(1):48–52. doi: 10.1080/14786410701589725. [DOI] [PubMed] [Google Scholar]
- 29.Roy P.K., Roy M.C., Taira J., Ueda K. Structure and bioactivity of a trisnorditerpenoid and a diterpenoid from an Okinawan soft coral, Cespitularia sp. Tetrahedron Lett. 2014;55(8):1421–1423. [Google Scholar]
- 30.Bowden B.F., Coll J.C., Tapiolas D.M. Studies of Australian soft corals. XXX. A novel trisnorsesquiterpene from a Cespitularia species and the isolation of guaiazulene from a small blue Alcyonium species. Aust J Chem. 1983;36:211–214. [Google Scholar]
- 31.Tung N.H., Minh C.V., Ha T.T., Kiem P.V., Huong H.T., Dat N.T., et al. C29 sterols with a cyclopropane ring at C-25 and 26 from the Vietnamese marine sponge Ianthella sp. and their anticancer properties. Bioorg Med Chem Lett. 2009;19:4584–4588. doi: 10.1016/j.bmcl.2009.06.097. [DOI] [PubMed] [Google Scholar]
- 32.Elshamy A.I., Abdel-Razik A.F., Nassar M.I., Mohamed T.K., Ibrahim M.A., El-Kousy S.M. A new gorgostane derivative from the Egyptian Red Sea soft coral Heteroxenia ghardaqensis. Nat Prod Res. 2013;27(14):1250–1254. doi: 10.1080/14786419.2012.724417. [DOI] [PubMed] [Google Scholar]
- 33.Baker B.J., Kerr R.G. Biosynthesis of marine sterols. Top Curr Chem. 1993;167:1–31. [Google Scholar]
- 34.Sjöstrand U., Bohlin L., Fisher L., Colin M., Djerassi C. Minor and trace sterols from marine invertebrates 28. A novel polyhydroxylated sterol from the soft coral Anthelia glauca. Steroids. 1981;38(3):347–354. doi: 10.1016/0039-128x(81)90069-6. [DOI] [PubMed] [Google Scholar]
- 35.Sica D., Musumeci D. Secosteroids of marine origin. Steroids. 2004;69:743–756. doi: 10.1016/j.steroids.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Enwall E.L., van der Helm D., Hsu I.N., Pattabhiraman T., Schmitz F.J., Spraggins R.L., Weinhrimer A.J. Crystal structure and absolute configuration of two cyclopropane containing marine steroids. J Chem Soc Chem Commun. 1972;4:215–216. [Google Scholar]
- 37.Hegazy M.F., Mohamed T.A., Alhammady M.A., Shaheen A.M., Reda E.R., Elshamy A.I., et al. Molecular architecture and biomedical leads of terpenes from red sea marine invertebrates. Mar Drugs. 2015;13:3154–3181. doi: 10.3390/md13053154. [DOI] [PMC free article] [PubMed] [Google Scholar]