Abstract
Background
This retrospective study aimed to evaluate clinicopathological findings of remnant pancreatic cancers in survivors of invasive ductal adenocarcinomas of the pancreas (PDAC).
Methods
A group of 23 patients out of 826 who had curative resections for PDAC between 1980 and 2011 was identified and treated for metachronous pancreatic cancer.
Results
The following tubular adenocarcinomas were found at the first surgery: 3 well differentiated, 17 moderately differentiated, 1 papillary, and 1 poorly differentiated. Treatments for the remnant pancreas consisted of remnant pancreatectomy in 12 patients, chemotherapy in 6, and the best supportive care in 5. The mean time to treatment was 74.2 months. The 12 patients who received remnant resections had 10 PDACs and 2 intraductal papillary mucinous carcinomas. The median survival time was 31.6 months, and 8 patients are still alive.
Conclusions
Long-term survivors after curative resection for pancreatic cancer should receive follow-up for remnant pancreatic cancer, and aggressive resection should be considered for more favorable prognosis of PDAC.
Introduction
Pancreatic cancer is known to have a high frequency of postoperative recurrences and a poor prognosis. Pancreatic cancer is now the fifth leading cause of cancer-related deaths in Japan [1], and most patients develop a recurrence of the same cancer within 1 or 2 years after tumor removal [2]. The most frequent recurrence patterns for pancreatic cancer after resection are local recurrence, hepatic metastasis, and peritoneal dissemination [3–6]. The high frequency of reported recurrences has been attributed to malignant cells remaining on resected margins in up to 50 % of cases even after macroscopically curative resection [7]. This R1-like situation could explain the high local recurrence rate [7]. Another reason is the presence of systemic occult disease at the time of operation in most of the patients, which could lead to distant metastasis in the liver (50 % of resected patients) or peritoneum (25 %) [5, 7–9].
Remnant pancreatic cancer sometimes appears in survivors after curative resection for invasive ductal adenocarcinoma of the pancreas (PDAC). However, there are few reports on the development of pancreatic cancer in the remnant pancreas after pancreatectomy [1], [10]. Consequently, the clinical characteristics of remnant pancreatic cancer after curative resection for PDAC are not well known. The aim of this study was to evaluate clinicopathological findings regarding remnant pancreatic cancer in PDAC survivors.
Materials and methods
Between 1980 and 2011 at the Department of Gastroenterological Surgery of Tokyo Women’s Medical University, 826 patients who had curative resections for PDAC were studied retrospectively. The remnant pancreatic cancer was detected by means of the serum levels of carcinoembryonic antigen and CA19-9 as well as computed tomography (CT), abdominal ultrasonography, and magnetic resonance imaging (MRI).
The patients were followed up in the outpatient clinic of our hospital or a related hospital and clinic every 3–6 months, and most of the follow-up data collected from these clinical hospital files were studied. (They were evaluated by examination every 3–4 months in the outpatient clinic of our hospital and every 3–6 months in the related hospital and clinic.)
A remnant pancreatic cancer was defined by the following criteria: (1) pathologically negative surgical margins at the first surgery (R0 operation) without M1 but with extra regional lymph nodes according to the UICC. (2) No recurrent tumor detected by CT or MRI examination within 1 year after the first operation. (3) The tumor appeared to develop and exist in the remnant pancreas. Twenty-three patients (2.8 %) fulfilling these criteria were ultimately employed for this retrospective study (Table 1).
Table 1.
Characteristics of primary pancreatic cancer
| Primary operation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Age | Gender (M/F) | Location | Operation | Differentiation | Tumor size(mm) | UICC | Stage | Pancreatic duct margin | Preoperative CA19-9 levels(U/ml) | Postoperative adjuvant chemotherapy |
| 1 | 67 | M | Head | PD | Moderately | 25 | T3N1M0 | IIB | (−) | Not described | (−) |
| 2 | 66 | F | Body | DP | Moderately | 30 | T2N0M0 | IB | (−) | 56 | (−) |
| 3 | 54 | M | Body | DP | Moderately | 17 | T3N0M0 | IIA | (−) | Not described | (−) |
| 4 | 53 | M | Head | PPPD+PV | Well | 15 | T3N0M0 | IIA | (−) | 1 | (−) |
| 5 | 63 | M | Head | PPPD | Moderately | 13 | T1N0M0 | IA | (−) | Not described | (−) |
| 6 | 72 | M | Head | PD+PV | Moderately | 30 | T3N0M0 | IIA | dysplasia | Not described | (−) |
| 7 | 57 | M | Head | PD | Moderately | 15 | T3N1M0 | IIB | (−) | 19 | (−) |
| 8 | 60 | M | Body | DP | Well | 33 | T3N0M0 | IIA | (−) | 179 | GEM |
| 9 | 56 | M | Head | PPPD | Moderately | 45 | T3N1M0 | IIB | (+) | 1910 | S-1 |
| 10 | 59 | F | Head | PPPD+PV | moderately | 37 | T3N1M1 | IV | (−) | 420 | GEM |
| 11 | 55 | M | Head | PPPD+PV | Moderately | 45 | T3N0M0 | IIA | (+) | 784 | GEM |
| 12 | 58 | F | Body | DP | Moderately | 30 | T3N0M0 | IIA | (+) | Not described | (−) |
| 13 | 82 | M | Body | DP+PV | Moderately | 20 | T3N0M0 | IIA | PanIN-1B | 8 | (−) |
| 14 | 69 | M | Body | DP | Moderately | 15 | T3N0M0 | IIA | (−) | 113 | (−) |
| 15 | 59 | F | Head | DPPHR | Poorly | 15 | T3N0M0 | IIA | (−) | 64 | (−) |
| 16 | 55 | F | Body | DP+Ace | Moderately | 80 | T3N1M1 | IV | (−) | 362 | immunotherapy |
| 17 | 62 | M | Head | PPPD+PV | Moderately | 30 | T3N1M0 | IIB | (−) | 138 | GEM |
| 18 | 65 | F | Body | DP | Moderately | 15 | T1N0M0 | IA | (−) | 15 | (−) |
| 19 | 70 | F | Body | Dorsal pancreas resection+DP | Moderately | 13 | T2N0M0 | IB | (−) | 14 | GEM |
| 20 | 57 | M | Head | PPPD+PV | Moderately | 15 | T3N1M0 | IIB | (−) | 19 | GEM |
| 21 | 48 | M | Body | DP | Papillary | 25 | T3N0M0 | IIA | (−) | 24 | GEM+immunotherpy |
| 22 | 82 | F | Body | DP | Moderately | 15 | T3N1M0 | IIB | (−) | 9 | GEM+immunotherpy |
| 23 | 63 | F | Head | PPPD | Well | 20 | T3N1M0 | IIB | (−) | 84 | S-1 |
PD pancreaticoduodenectomy, DP distal pancreatectomy, PPPD pylorus preserving PD, PV portal vein resection and reconstruction, DPPHR duodenum preserving pancreas head resection, y year, m month, IOR intraoperative radiation, BSC best supportive care, SSPPD subtotal stomach preserving pancreaticoduodenectomy, IPMC intraductal papillary mucinous carcinoma GEM gemcitabine
All of these 23 cases were pathologically confirmed by histological information to be PDAC with R0 treatment at the first operation. Clinicopathological features including sex, age, primary and secondary tumor characteristics, and long-term survival data were collected from hospital records. These factors related to the primary operation including sex, age, location, operation, degree of differentiation, tumor size, N of UICC (lymph node state), pancreatic duct margin, preoperative CA19-9 levels, perineural invasion, and postoperative adjuvant chemotherapy were analyzed using both univariate and multivariate analyses.
Statistical analysis
The survival times of unresected and resected patients were estimated using the Kaplan–Meier method and compared using the log-rank test. A univariate and multivariate Cox proportional hazards model was used to evaluate which factors demonstrated an independent effect on disease-free intervals (DFIs). P values less than 0.05 were considered statistically significant. Analysis was performed using SPSS Statistics 22.0 (IBM Corp., Chicago, IL).
Results
At the first surgery, the tumor was located in the pancreatic head in 12 patients and in the pancreatic body in 11 patients. Pylorus-preserving pancreatoduodenectomy (PPPD) was performed in 8 patients, pancreatoduodenectomy (PD) in 3 patients, duodenum-preserving pancreatic head resection (DPPHR) in 1 patient, and distal pancreatectomy (DP) in 11 patients (Tables 1, 2). A well-differentiated tubular adenocarcinoma was detected in 3 patients, a moderately differentiated tubular adenocarcinoma in 18 patients, a papillary adenocarcinoma in 1 patient, and a poorly differentiated adenocarcinoma in 1 patient. These patients were classified into stages according to UICC classification as follows: 2 in IA, 2 in IB, 10 in IIA, 7 in IIB, and 2 in IV. All patients underwent curative resection. The median and mean DFIs for these patients were 53.6 and 74.2 months (range, 15–240), respectively. Regarding treatment procedures, 12 patients underwent total excision of the remnant pancreas, 6 received chemotherapy, and 5 received the best supportive care (BSC). The six patients treated with chemotherapy consisted of four who received chemotherapy after refusing surgery despite resectable PDAC and two with unresectable PDAC due to locally advanced tumors. The five BSC patients consisted of three with unresectable PDAC (2 with locally advanced tumor and 1 with liver metastasis) and two with resectable PDAC who refused surgery.
Table 2.
Characteristics of remnant pancreatic cancer
| Remnant operation | |||||||
|---|---|---|---|---|---|---|---|
| No | DFI | Treatment | Differentiation | UICC | Stage | Prognosis(after 2nd operation) | Recurrence form |
| 1 | 20 years | BSC(resection impossible) | (−) | Death(6 m) | Peritoneum | ||
| 2 | 11 year 11 month | Remnant pancreas resection+PV | Moderately | T3N1M0 | IIB | Death(11 month) | Peritoneum |
| 3 | 9 year 5 month | Chemotherapy(resection possible) | (−) | Death(3 year) | Liver, peritoneum | ||
| 4 | 10 year 1 month | Chemotherapy(resection possible) | (−) | Death(1 year 4 month) | Liver, peritoneum | ||
| 5 | 10 year 2 month | BSC(resection impossible) | (−) | Death(6 month) | Liver, peritoneum | ||
| 6 | 9 year 9 month | BSC(resection impossible) | (−) | Death(2 month) | Liver, peritoneum, lung | ||
| 7 | 9 year 2 month | Remnant pancreas resection+remnant stomach resection+IOR | Moderately | T3N1M0 | IIB | Death(4 month) | Pleura |
| 8 | 5 year 8 month | Remnant pancreas resection | Well | T1N0M0 | IA | Alive(7 year) | (−) |
| 9 | 6 year 3 month | Remnant pancreas resection | Moderately | T3N0M0 | IIA | Alive(1 year 3 month) | (−) |
| 10 | 4 year 5 month | Chemotherapy(resection possible) | (−) | Death(1 year 6 month) | Liver, peritoneum | ||
| 11 | 2 year 5 month | Chemotherapy(resection possible) | (−) | Death(9 month) | Liver, peritoneum | ||
| 12 | 2 year 5 month | Remnant pancreas resection | Moderately | T3N0M0 | IIA | Death(2 year 1 month) | Liver |
| 13 | 3 year5 month | BSC(resection possible) | (−) | Death(10 month) | Liver, peritoneum | ||
| 14 | 3 year 3 month | Remnant pancreas resection+PV | Well | T3N1M0 | IIB | Death(2 year 6 month) | Liver, lung |
| 15 | 2 year 9 month | Remnant pancreas resection | Poorly | T3N0M0 | IIA | Alive(7 year 6 month) | (−) |
| 16 | 2 year 6 month | Remnant pancreas resection+remnant stomach resection | IPMC | TisN0M0 | 0 | Alive(8 month) | (−) |
| 17 | 1year 3month | DP | Moderately | T3N1M0 | IIB | Alive(3 year) | Peritoneum |
| 18 | 2 year 7 month | DPPHR | IPMC | TisN0M0 | 0 | Alive(3 year 8 month) | (−) |
| 19 | 2year 9 month | Chemoradiotherapy(resection impossible) | (−) | Death(12 month) | Peritoneum | ||
| 20 | 2 year 8 month | Remnant pancreas resection | Moderately | T3N1M0 | IIB | Alive(6 month) | (−) |
| 21 | 13 year 1 month | Chemoradiotherapy(resection impossible) | (−) | Dead(4 year 5 month) | Peritoneum | ||
| 22 | 1 year 6 month | BSC(resection possible) | (−) | Dead(2 year 2 month) | Peritoneum | ||
| 23 | 5 year 2 month | Remnant pancreas resection | Moderately | T3N1M0 | IIB | Alive(1 month) | (−) |
DFI disease free interval PD pancreaticoduodenectomy, DP distal pancreatectomy, PPPD pylorus preserving PD, PV portal vein resection and reconstruction, DPPHR duodenum preserving pancreas head resection, y year, m month, IOR intraoperative radiation, BSC best supportive care, SSPPD subtotal stomach preserving pancreaticoduodenectomy, IPMC intraductal papillary mucinous carcinoma IOR intraoperative radiation therapy
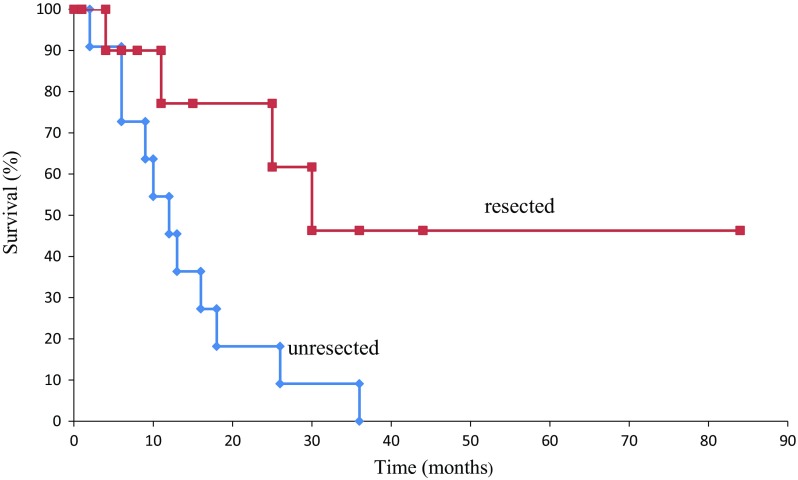
The pathological findings of the remnant pancreas in the 12 resected cases were PDAC in 10 patients and intraductal papillary mucinous carcinoma with an associated invasive carcinoma in 2 patients. The 12 patients were found to be in the following stages (UICC classification): 2 in 0, 1 in IA, 3 in IIA, and 6 in IIB. The same histopathological features in specimens from the first and second operations were recognized in 8 of the 12 (67 %) cases. The mortality and morbidity rates of resected patients were 0 and 41.6 %, respectively. Complications were observed in five patients: 2 with delayed gastric emptying, 2 with intra-abdominal abscess, and 1 with sepsis. The average length of hospital stay was 34 days (13–70). No unresected patients had survived, and eight patients who underwent total excision of the remnant pancreas survived. The 5-year survival rate for all the 826 studied resected pancreatic cancer patients was 21.3 %. The mean and median survival times after the second operation were 23.7 (range, 1–90) and 31.6 months, respectively. The 1-year and 3-year survival rates and the median survival time were 45.5, 9.1 %, and 12 months for unresected patients, respectively, contrasting with 79.6, 53 %, and not obtained for resected patients, respectively (P = 0.0049 by log-rank test) (Fig. 1).
Fig. 1.
Kaplan-Meier curve for overall survival between resected and unresected patients for the remnant pancreatic cancer
Recurrences were observed in 16 patients (13 with peritoneal dissemination [56.5 %], 9 with liver metastasis [39.1 %], 2 with lung metastasis [8.7 %], and 1 with pleural metastasis [4.3 %]). A univariate analysis for DFI showed no significant difference by any of clinicopathological features. None of the lymph node state, degree of differentiation, and perineural invasion could predict survival. Therefore, multivariate analysis for DFI could not have been investigated.
Discussion
The 5-year survival rate reported after surgical resection of PDAC is approximately 20 % [11]. Most patients develop recurrence within 1 or 2 years after tumor removal [3, 12, 13]. The surgical mortality rate associated with pancreatectomy has decreased to less than 5 % despite high morbidity rates and recent improvements in operative technique and perioperative management that have resulted in an increase in the number of long-term survivors after pancreatectomy [14–20]. Thus, there have been some recent reports regarding cases of remnant pancreatic cancer after pancreatectomy [21–24]. We evaluated clinicopathological findings of the remnant pancreatic cancer in survivors of PDAC.
It is difficult to define whether a pancreatic carcinoma developing in the remnant pancreas after a pancreatectomy for PDA is a local recurrence or a newly developed primary cancer [2]. The remnant pancreatic cancer was defined in this study according to those criteria mentioned earlier. In the literature, the time interval from primary PDA to remnant pancreatic cancer ranges from 12 to 89 months (mean 37.6 months), using our definition (Table 3) [1, 2, 21–30]. On the other hand, the time interval in our studied cases ranged from 15 to 240 months (mean 74.2 months) (Table 2), which is much longer than the previous reports. This result suggests that long-term follow-up after surgery for PDAC may be needed even beyond 5 years to monitor for remnant pancreatic cancer. In Japan, postoperative follow-up of patients with resected pancreatic cancer by means of CT or MRI examination every 3–6 months is generally warranted and covered by insurance. Because most patients with resected pancreatic cancer are likely to develop recurrence soon after resection, an intensive follow-up schedule is considered necessary to find recurrence in a remnant pancreas. Whether such intensive follow-up could be performed for the long term may be debatable when considering cost-effectiveness. The results of this study indicated that a newly developed cancer in the remnant pancreas could be detected by performing CT or MRI examination every 6–12 months for patients having survived for 5 years. Such long-term survivors could be few, but it is important to implement intensive follow-up beyond 5 years for such patients.
Table 3.
Reported cases of resected remnant pancreatic cancer
| First operation | Remnant pancreeatic cancer | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Age | Gender (M/F) | Location | Operation | Differentiation | UICC | Stage | Term to diagnosis | Treatment | Differentiation | UICC | Stage | Prognosis | Recurrence form | Author |
| 1 | 52 | F | Head | PPPD | Pap | T3N1M0 | IIB | 1 year10 month | DP+GR | Pap | Not described | Not described | (−) | Wada [24] | |
| 2 | 67 | F | Body/tail | DP | Well | T1N0M0 | IA | 7 year4 month | PPPD | Moderately | T1N1M0 | IB | Alive(8 month) | (−) | Eriguchi [25] |
| 3 | 58 | M | Head | PPPD | Pap/well | T2N0M0 | IB | 3 year | DP | Pap | T2N0M0 | IB | Alive(6 year3 month) | Liver | Tajima [2] |
| 4 | 63 | M | Head | PD | Well to moderately | T3N0M0 | IIA | 3 year7 m | DP | Well | not described | Alive(10 m) | (−) | Takamatsu [22] | |
| 5 | 65 | M | Head | PPPD | Well | T1N0M0 | IA | 7 year | DP | Well | T3N1M0 | IIB | Alive(10 m) | (−) | Koizumi [26] |
| 6 | 67 | M | Body | DP | Well | T1N0M0 | IA | 2 year9 month | PPPD | Moderate to poorly | T3N1M0 | IIB | Alive(8 month) | Liver | Koizumi |
| 7 | 63 | M | Head | PD+PV | Moderately | T3N0MX | IIA | 1 year | DP+GR+C(T)R | Moderately | not described | Alive(2 year) | LN | Valle [21] | |
| 8 | 60 | M | Body | DP | Moderately | T3N0M0 | IIA | 2 year2 month | PPPD | Poorly | T2N0M0 | IB | Dead(7 month) | peritoneum | Doi [27] |
| 9 | 44 | M | Head | PPPD | Not described | T2N1MX | IIB | 3 year 4 month | DP | Not described | T2NXM0 | Alive(22 month) | (−) | D’Amato [28] | |
| 10 | 72 | F | Head | PPPD | Moderately | T3N0M0 | IIA | 2 year5 month | DP+lateral segmentectomy | Moderately | not described | Dead(5 month) | Liver | Miura [23] | |
| 11 | 52 | F | Head | PPPD | Pap | T3N1M0 | IIB | 1y10 month | DP | Pap | not described | Dead(44 month) | Not described | Miura | |
| 12 | 67 | M | Head | PD | Well | T2N1M0 | IIB | 5y8 month | DP | Well | not described | Alive(2 month) | (−) | Kinoshita [29] | |
| 13 | 55 | F | Head | SSPPD+PV | Anaplastic | T1N0M0 | IA | 2 year | DP | Well | T2N0M0 | IB | Alive(5 year4 8 month) | (−) | Hashimoto [1] |
| 14 | 69 | F | Head | SSPPD | Pap | T1N0M0 | IA | 3 year 2 mmonth | DP | Well | T1N1M0 | IB | Alive(10 month) | (−) | Hashimoto |
| 15 | 80 | M | Head | PPPD | Moderately | T3N0M0 | IIA | 1 year 9 month | DP | Moderately | T3N1M0 | IIB | Dead(1 year 6 month) | Not described | Hashimoto |
| 16 | 60 | M | Tail | DP | Pap | T2N1M0 | IIB | 2 year 9 month | TP | Pap | T1N0M0 | IA | Alive(7 year 1 month) | (−) | Hashimoto |
| 17 | 75 | F | Tail | DP | Well | T3N1M0 | IIB | 3 year 3 month | TP | Well | T1N0M0 | IA | Alive(1 year 1 month) | (−) | Hashimoto |
| 18 | 76 | M | Body | DP | Moderately | T1N0M0 | IA | 1 year 11 month | TP | Moderately | T3N1M0 | IIB | Dead(1 year 1 month) | (−) | Hashimoto |
| 19 | 62 | F | Head | PPPD+PV | Well | T3N1M0 | IIB | 1 year 5 month | chemotherapy | Not described | not described | Dead(11 month) | Liver | Hashimoto | |
| 20 | 66 | M | Head | PD | Moderately | T3N0M0 | IIA | 1y4 m | TP | Moderately | T3N0M0 | IIA | Alive(61 month) | (−) | Miyazaki [30] |
| 21 | 68 | F | Head | PD+PV | Moderately | T3N1M0 | IIB | 3 year 5 month | TP | Moderately | T3N0M0 | IIA | Alive(61 month) | (−) | Miyazaki |
| 22 | 62 | F | Head | PD+PV | Moderately | T3N1M0 | IIB | 2 year 8 month | TP | Moderately | T3N1M0 | IIB | Dead(15 month) | Peritoneum | Miyazaki |
| 23 | 76 | F | Head | PD | Well | T3N0M0 | IIA | 3 year 5 month | TP | Moderately | T3N1M0 | IIB | Dead(25 month) | Peritoneum,liver | Miyazaki |
| 24 | 80 | M | Body | DP | Moderately | T3N0M0 | IIA | 2 year | TP | Well | T3N0M0 | IIA | Alive(28 monthm) | (−) | Miyazaki |
| 25 | 67 | M | Head | PD | Moderately | T2N0M0 | IB | 7 year 5 month | TP | Poorly | T1N0M0 | IA | Alive(18 month) | (−) | Miyazaki |
PD pancreaticoduodenectomy, DP distal pancreatectomy, PPPD pylorus preserving PD, SSPPD subtotal stomach preserving pancreaticoduodenectomy, PV portal vein resection and reconstruction, y year, m month, GR distal gastrectomy, C(T)R toransvers colon resection
The same pathological differentiation of tumors between primary and second operation in our study was recognized in 8 (67 %) of the 12 resected cases, consistent with a previous report indicating 16 (69.6 %) of the 23 described cases as listed in Table 2. Launois et al. [31] observed 32 % of multifocal carcinomas of the pancreas in a series of 47 total pancreatectomies for patients with PDAC. Likewise, a considerably high incidence of multicentric precancerous foci in the pancreas has been documented in patients with PDAC [2]. In PDAC, multicentric lesions were found in 16–34 % of cases [32–35], implying that a minute cancerous focus is likely to exist in the remnant pancreas at the time of the initial surgery [22].
These results suggest that multicentric metachronous pancreatic cancers may develop in the remnant pancreas. Kleff et al. [10] reported that 7 (3.1 %) of the 227 patients who underwent initial pancreatic resection for pancreatic cancer developed remnant pancreatic cancer, consistent with the incidence of remnant pancreatic cancer in our results. Therefore, multicentric foci of PDAC may develop metachronously not only after a short interval but also after a longer interval as a new primary cancer.
Miyazaki et al. [30]. reported that repetitive pancreatectomy may be beneficial for the prognosis in selected patients with isolated local recurrence in the remnant pancreas after primary pancreatectomy for pancreatic cancer without increased operative morbidity or mortality. They recognized 11 of 67 patients with isolated local recurrences only in the remnant pancreas who underwent repetitive pancreatectomies. Moreover, at the primary operation, 6 (67 %) of the 9 patients with R0 resection had the same pathological features as in our study.
In this study, we showed that patients with remnant pancreatic cancers who were resected had a better prognosis than those that were unresected. This suggests that remnant pancreatectomy for pancreatic cancer is feasible and may prolong survival. Although early discovery of remnant pancreatic cancer would be difficult, adequate interval follow-up with imaging examinations is important. Further studies are required to elucidate the carcinogenic mechanism in the remnant pancreas.
In conclusion, survivors after curative resection for pancreatic cancer should receive follow-up for the remnant pancreas for an extended period. Aggressive resection of the remnant pancreas should be considered for a more favorable prognosis of patients with PDAC.
Acknowledgements
No financial support was received for this study.
Compliance with ethical standards
Conflicts of Interest
The authors declare no conflicts of interest associated with this study.
References
- 1.Hashimoto D, Chikamoto A, Ohmuraya M, et al. Pancreatic cancer in the remnant pancreas following primary pancreatic resection. Surg Today. 2014;44:1313–1320. doi: 10.1007/s00595-013-0708-0. [DOI] [PubMed] [Google Scholar]
- 2.Tajima Y, Kuroki T, Ohno T, et al. Resectable carcinoma developing in the remnant pancreas 3 years after pylorus-preserving pancreaticoduodenectomy for invasive ductal carcinoma of the pancreas. Pancreas. 2008;36:324–327. doi: 10.1097/MPA.0b013e318156adae. [DOI] [PubMed] [Google Scholar]
- 3.Sperti C, Pasquali C, Piccoli A, et al. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21:195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- 4.Kayahara M, Nagakawa T, Ueno K, et al. An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging. Cancer. 1993;72:2118–2123. doi: 10.1002/1097-0142(19931001)72:7<2118::AID-CNCR2820720710>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Westerdahl J, Andren-Sandberg A, Ihse I. Recurrence of exocrine pancreatic cancer—Local or hepatic? Hepatogastroenterology. 1993;40:384–387. [PubMed] [Google Scholar]
- 6.Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. doi: 10.1002/1097-0142(19900701)66:1<56::AID-CNCR2820660112>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Smeenk HG, Incrocci L, Kazemier G, et al. Adjuvant 5-FU-based chemoradiotherapy for patients undergoing R-1/R-2 resections for pancreatic cancer. Dig Surg. 2005;22:321–328. doi: 10.1159/000089250. [DOI] [PubMed] [Google Scholar]
- 8.Smeenk HG, Tran TC, Erdmann J, et al. Survival after surgical management of pancreatic adenocarcinoma: does curative and radical surgery truly exist? Langenbecks Arch Surg. 2005;390:94–103. doi: 10.1007/s00423-004-0476-9. [DOI] [PubMed] [Google Scholar]
- 9.Tepper J, Nardi G, Sutt H. Carcinoma of the pancreas: review of MGH experience from 1963 to 1973: analysis of surgical failure and implications for radiation therapy. Cancer. 1976;37:1519–1524. doi: 10.1002/1097-0142(197603)37:3<1519::AID-CNCR2820370340>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 10.Kleeff J, Reiser C, Hinz U, et al. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. 2007;245:566–572. doi: 10.1097/01.sla.0000245845.06772.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas 201 Patients. Ann Surg. 1995;221:721–731. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa O, Wada H, Ohigashi H, et al. Postoperative cytology for drained fluid from the pancreatic bed after “curative” resection of pancreatic cancers: does it predict both the patient’s prognosis and the site of cancer recurrence? Ann Surg. 2003;238:103–110. doi: 10.1097/01.SLA.0000074982.51763.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi T, Sato Y, Hirukawa H, et al. Total pancreatectomy combined with partial pancreas autotransplantation for recurrent pancreatic cancer: a case report. Transplant Proc. 2012;44:1176–1179. doi: 10.1016/j.transproceed.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Balcom JH, 4th, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections. Changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–398. doi: 10.1001/archsurg.136.4.391. [DOI] [PubMed] [Google Scholar]
- 15.Butturini G, Marcucci S, Morinari E, et al. Complications after pancreaticoduodenectomy: the problem of current definitions. J Hepatobiliary Pancreat Surg. 2006;13:207–211. doi: 10.1007/s00534-005-1035-7. [DOI] [PubMed] [Google Scholar]
- 16.Trede M, Saeger HD, Schwall G, et al. Resection of pancreatic cancer: surgical achievements. Langenbecks Arch Surg. 1998;383:121–128. doi: 10.1007/PL00008074. [DOI] [PubMed] [Google Scholar]
- 17.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klinkenbijl JH, van der Schelling GP, Hop WC, et al. The advantages of pylorus-preserving pancreatoduodenectomy in malignant disease of the pancreas and periampullary region. Ann Surg. 1992;216:142–145. doi: 10.1097/00000658-199208000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeo CJ, Cameron JL, Maher MM, et al. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998;227:821–831. doi: 10.1097/00000658-199806000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez RE, Fernandez-del Castillo C, Rattner DW, et al. Outcome of pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg. 2000;231:293–300. doi: 10.1097/00000658-200003000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalla Valle R, Mancini C, Crafa P, et al. Pancreatic carcinoma recurrence in the remnant pancreas after a pancreaticoduodenectomy. JOP. 2006;7:473–477. [PubMed] [Google Scholar]
- 22.Takamatsu S, Ban D, Irie T, et al. Resection of a cancer developing in the remnant pancreas after a pancreaticoduodenectomy for pancreas head cancer. J Gastrointest Surg. 2005;9:263–269. doi: 10.1016/j.gassur.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Miura F, Takada T, Amano H, et al. Repeated pancreatectomy after pancreatoduodenectomy. J Gastrointest Surg. 2007;11:179–186. doi: 10.1007/s11605-006-0026-6. [DOI] [PubMed] [Google Scholar]
- 24.Wada K, Takada T, Yasuda H, et al. A repeated pancreatectomy in the remnant pancreas 22 months after pylorus-preserving pancreatoduodenectomy for pancreatic adenocarcinoma. J Hepatobiliary Pancreat Surg. 2001;8:174–178. doi: 10.1007/s005340170043. [DOI] [PubMed] [Google Scholar]
- 25.Eriguchi N, Aoyagi S, Imayama H, et al. Resectable carcinoma of the pancreatic head developing 7 years and 4 months after distal pancreatectomy for carcinoma of the pancreatic tail. J Hepatobiliary Pancreat Surg. 2000;7:316–320. doi: 10.1007/s005340070055. [DOI] [PubMed] [Google Scholar]
- 26.Koizumi M, Sata N, Kasahara N, et al. Remnant pancreatectomy for recurrent or metachronous pancreatic carcinoma detected by FDG-PET: two case reports. JOP. 2010;11:36–40. [PubMed] [Google Scholar]
- 27.Doi R, Ikeda H, Kobayashi H, et al. Carcinoma in the remnant pancreas after distal pancreatectomy for carcinoma. Eur J Surg Suppl. 2002;588:62–65. [PubMed] [Google Scholar]
- 28.D’Amato A, Gentili V, Santella S, et al. Carcinoma of the pancreatic remnant developing after pancreaticoduodenectomy for adenocarcinoma of the head of pancreas. Chir Ital. 2002;54:539–544. [PubMed] [Google Scholar]
- 29.Kinoshita H, Yamade N, Nakai H, et al. Successful resection of pancreatic carcinoma recurrence in the remnant pancreas after a pancreaticoduodenectomy. Hepatogastroenterology. 2011;58:1406–1408. doi: 10.5754/hge09366. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki M, Yoshitomi H, Shimizu H, et al. Repeat pancreatectomy for pancreatic ductal cancer recurrence in the remnant pancreas after initial pancreatectomy: is it worthwhile. Surgery. 2014;155:58–66. doi: 10.1016/j.surg.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 31.Launois B, Franci J, Bardaxoglou E, et al. Total pancreatectomy for ductal adenocarcinoma of the pancreas with special reference to resection of the portal vein and multicentric cancer. World J Surg. 1993;17:122–126. doi: 10.1007/BF01655724. [DOI] [PubMed] [Google Scholar]
- 32.Ihse I, Lilja P, Arnesjo B, et al. Total pancreatectomy for cancer. An appraisal of 65 cases. Ann Surg. 1977;186:675–680. doi: 10.1097/00000658-197712000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tryka AF, Brooks JR. Histopathology in the evaluation of total pancreatectomy for ductal carcinoma. Ann Surg. 1979;190:373–381. doi: 10.1097/00000658-197909000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Heerden JA, ReMine WH, Weiland LH, et al. Total pancreatectomy for ductal adenocarcinoma of the pancreas. Mayo Clinic experience. Am J Surg. 1981;142:308–311. doi: 10.1016/0002-9610(81)90336-6. [DOI] [PubMed] [Google Scholar]
- 35.SarrMG Behrns KE, van Heerden JA. Total pancreatectomy.An objective analysis of its use in pancreatic cancer. Hepatogastroenterology. 1993;40:418–421. [PubMed] [Google Scholar]