Abstract
The excretion of tetracycline HCl in sputum was studied in 42 hospital patients suffering from various respiratory disorders who were receiving oral tetracycline therapy of 1·0 gram daily in divided doses.
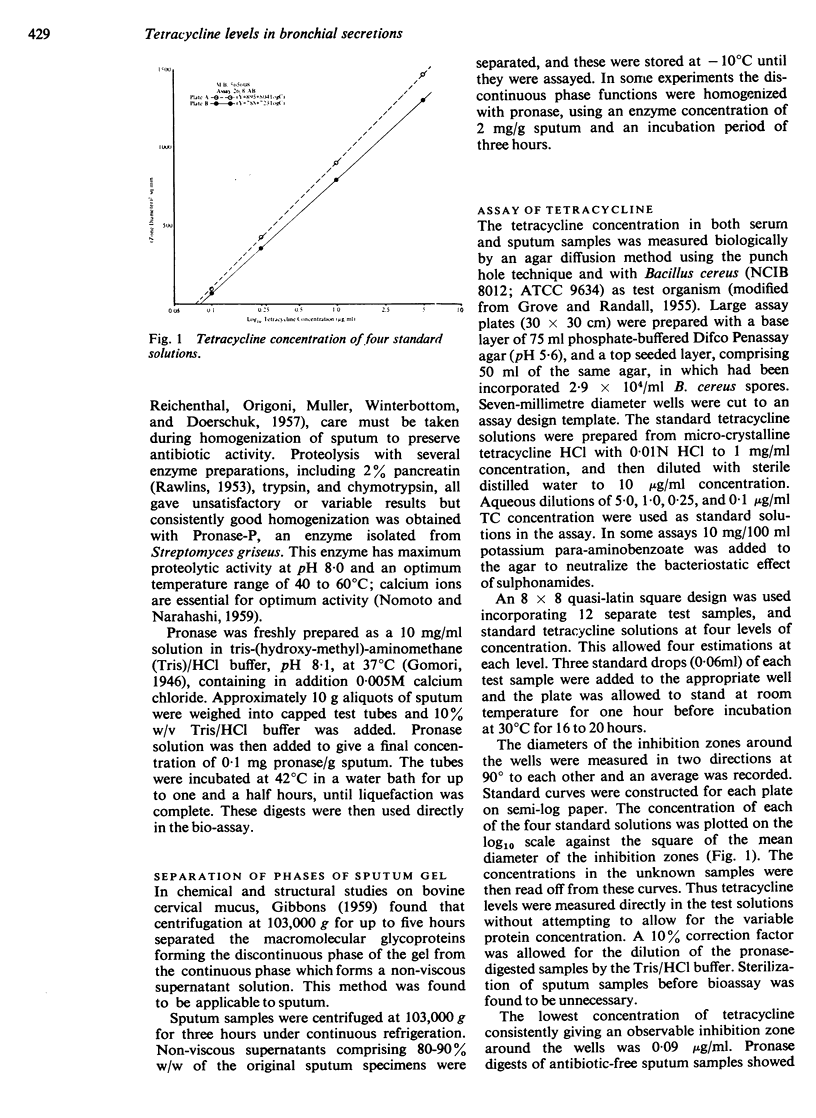
The tetracycline levels were estimated biologically by a cup-plate agar diffusion method using B. cereus. Two separate methods of sputum homogenization are described: enzymic liquefaction with pronase, a powerful proteolytic enzyme, and ultracentrifugation at 103,000 g for three hours which sediments the viscous mucoids leaving 80-90% as a clear, non-viscous supernatant. The two methods allowed duplicate assays on sputum samples and generally showed close agreement. The ultracentrifugation technique is favoured for normal purposes because of its simplicity and the avoidance of any biochemical interference.
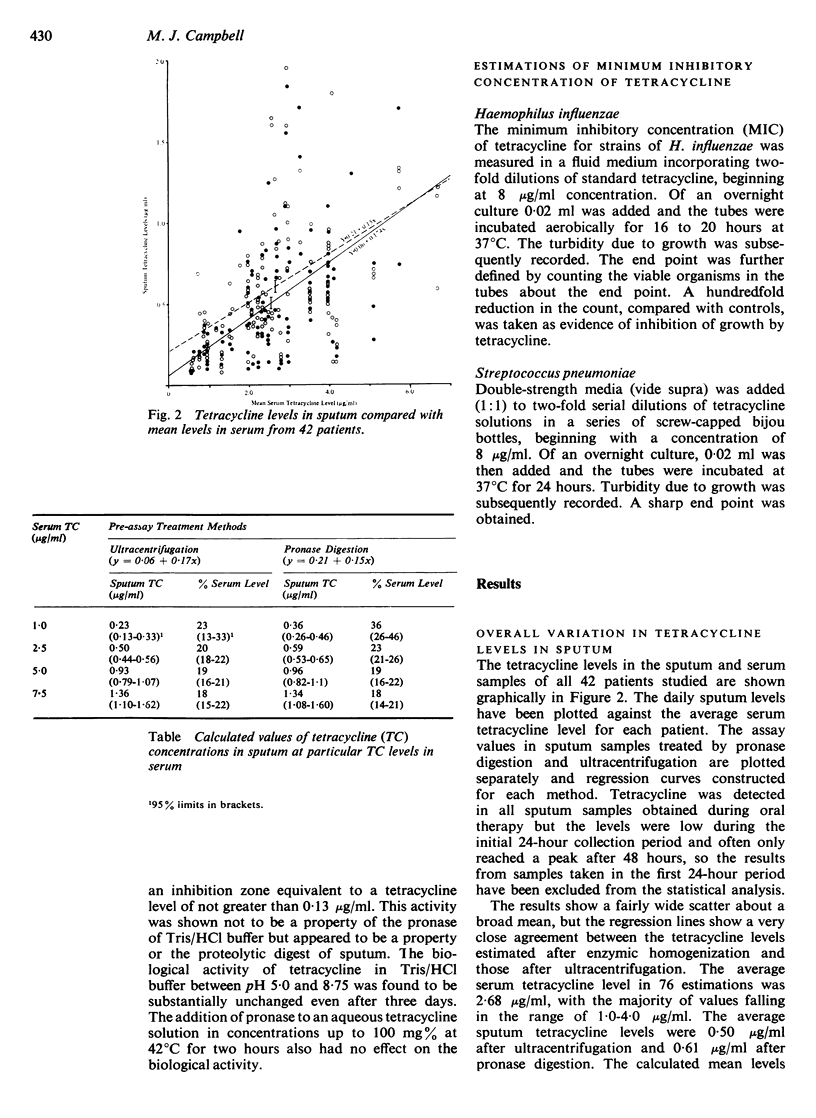
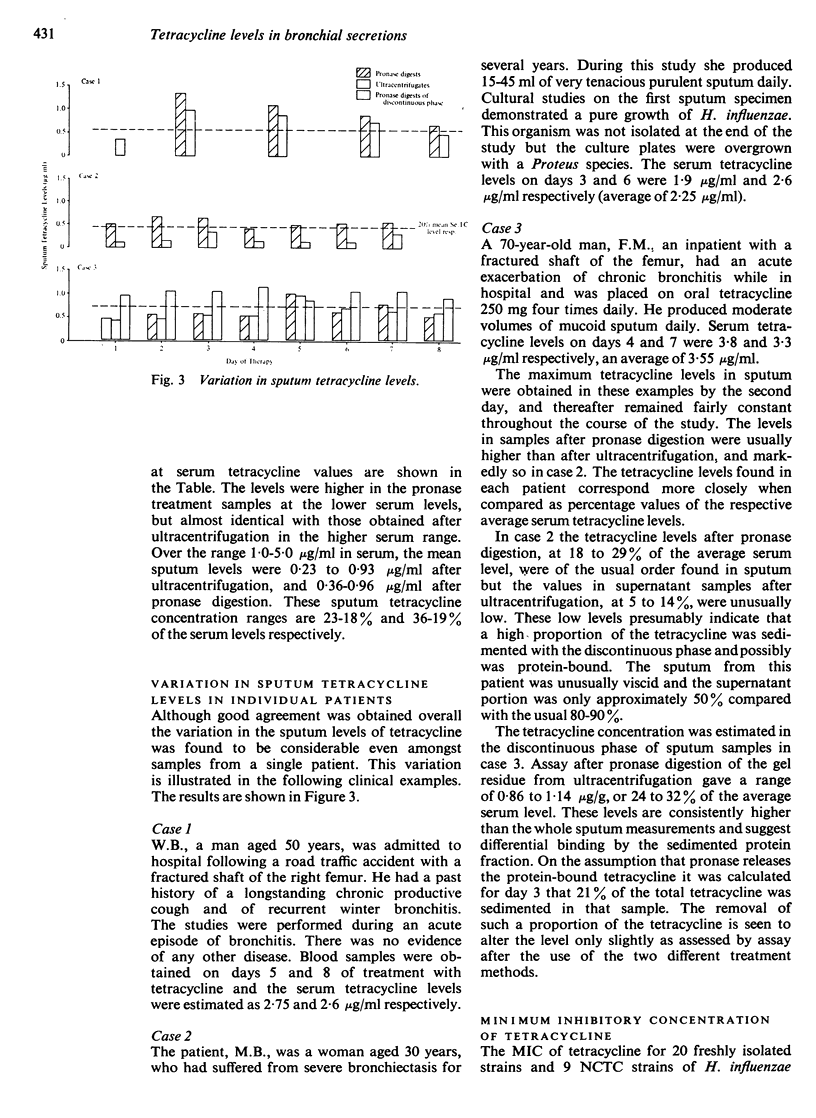
A close correlation was shown between the tetracycline levels in sputum samples and the average serum level for each patient. Over the serum tetracycline range 1·0-5·0 μg/ml, the mean sputum tetracycline levels after centrifugation were 23-18%, and the average level was 0·50 μg/ml.
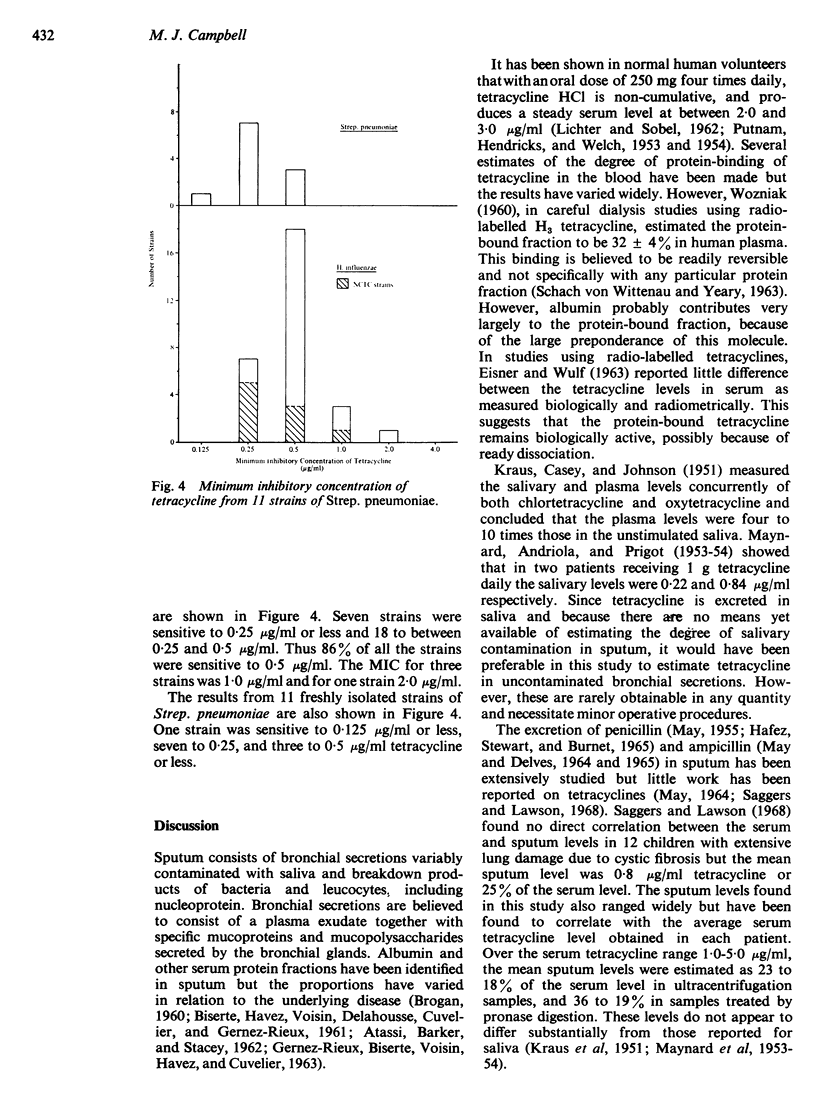
The levels found in sputum were compared with the minimum inhibitory concentration of tetracycline for strains of H. influenzae and Strep. pneumoniae. Eighty-six percent of 29 H. influenzae strains tested and all Strep. pneumoniae strains tested were sensitive to 0·5 μg/ml tetracycline. The remaining H. influenzae strains had a minimum inhibitory concentration of 1·0 μg/ml or higher, a tetracycline level in sputum which appeared to be outside the mean range at the oral dosage of 1·0 gram daily.
Hence it is suggested that a small proportion of patients with lower respiratory tract infections due to H. influenzae would be unresponsive, or only partially responsive, to this usual therapeutic range of tetracycline.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATASSI M. Z., BARKER S. A., STACEY M. Neuraminic acid and its relation to chronic bronchitis. V. Glass column electrophoresis of sputum. Clin Chim Acta. 1962 Sep;7:706–709. doi: 10.1016/0009-8981(62)90154-7. [DOI] [PubMed] [Google Scholar]
- BROGAN T. D. The high molecular weight components of sputum. Br J Exp Pathol. 1960 Jun;41:288–297. [PMC free article] [PubMed] [Google Scholar]
- DAVIS A. L., GROBOW E. J., TOMPSET R., McCLEMENT J. H. Bacterial infection and some effects of chemoprophylaxis in chronic pulmonary emphysema. I. Chemoprophylaxis with intermittent tetracycline. Am J Med. 1961 Sep;31:365–381. doi: 10.1016/0002-9343(61)90125-5. [DOI] [PubMed] [Google Scholar]
- EISNER H. J., WULF R. J. THE METABOLIC FATE OF CHLORTETRACYCLINE AND SOME COMPARISONS WITH OTHER TETRACYCLINES. J Pharmacol Exp Ther. 1963 Oct;142:122–131. [PubMed] [Google Scholar]
- FLETCHER C. M. Chronic bronchitis. Its prevalence, nature, and pathogenesis. Am Rev Respir Dis. 1959 Oct;80:483–494. doi: 10.1164/arrd.1959.80.4P1.483. [DOI] [PubMed] [Google Scholar]
- FRANKLIN A. W., GARROD L. P. Chloramphenicol treatment of bronchiectasis in children. Br Med J. 1953 Nov 14;2(4845):1067–1069. doi: 10.1136/bmj.2.4845.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher C. M. Some recent advances in the prevention and treatment of chronic bronchitis and related disorders with special reference to the effects of cigarette smoking. Proc R Soc Med. 1965 Nov;58(11 Pt 1):918–928. [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. A. Chemical properties of two mucoids from bovine cervical mucin. Biochem J. 1959 Oct;73:209–217. doi: 10.1042/bj0730209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. A. The biochemical and physical properties of epithelial mucus. Am Rev Respir Dis. 1961 Apr;83:568–569. [PubMed] [Google Scholar]
- HAFEZ F. F., STEWART S. M., BURNET M. E. PENICILLIN LEVELS IN SPUTUM. Thorax. 1965 May;20:219–225. doi: 10.1136/thx.20.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERS J. F., MULDER J. The mucosal epithelium of the respiratory tract in muco-purulent bronchitis caused by Haemophilus influenzae. J Pathol Bacteriol. 1953 Jul;66(1):103–108. doi: 10.1002/path.1700660114. [DOI] [PubMed] [Google Scholar]
- HIRSCH H. A., FINLAND M. Susceptibility of Hemophilus influenzae to 21 antibiotics in vitro. Am J Med Sci. 1960 Jan;239:33–40. doi: 10.1097/00000441-196001000-00005. [DOI] [PubMed] [Google Scholar]
- HOLT L. B. The growth-factor requirements of Haemopiius influenzae. J Gen Microbiol. 1962 Feb;27:317–322. doi: 10.1099/00221287-27-2-317. [DOI] [PubMed] [Google Scholar]
- KRAUS F. W., CASEY D. W., JOHNSON V. Aureomycin and terramycin in human saliva. Proc Soc Exp Biol Med. 1951 Nov;78(2):554–558. doi: 10.3181/00379727-78-19138. [DOI] [PubMed] [Google Scholar]
- Kislak J. W., Razavi L. M., Daly A. K., Finland M. Susceptibility of pneumococci to nine antibiotics. Am J Med Sci. 1965 Sep;250(3):261–268. doi: 10.1097/00000441-196509000-00003. [DOI] [PubMed] [Google Scholar]
- LICHTER E. A., SOBEL S. Variable sensitivity of assay methods of tetracycline and demethylchlortetracycline in human volunteers. Clin Pharmacol Ther. 1962 Sep-Oct;3:580–592. doi: 10.1002/cpt196235580. [DOI] [PubMed] [Google Scholar]
- MAY J. R., DELVES D. M. AMPICILLIN IN THE TREATMENT OF HAEMOPHILUS INFLUENZAE INFECTIONS OF THE RESPIRATORY TRACT. Thorax. 1964 Jul;19:298–305. doi: 10.1136/thx.19.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY J. R., DELVES D. M. TREATMENT OF CHRONIC BRONCHITIS WITH AMPICILLIN: SOME PHARMACOLOGICAL OBSERVATION. Lancet. 1965 May 1;1(7392):929–933. doi: 10.1016/s0140-6736(65)91253-5. [DOI] [PubMed] [Google Scholar]
- MAY J. R. The bacteriology of chronic bronchitis. Lancet. 1953 Sep 12;265(6785):534–537. doi: 10.1016/s0140-6736(53)90274-8. [DOI] [PubMed] [Google Scholar]
- MULDER J., GOSLINGS W. R. O., VAN DER PLAS M. C., CARDOZO P. L. Studies on the treatment with antibacterial drugs of acute and chronic mucopurulent bronchitis caused by Hemophilus influenzae. Acta Med Scand. 1952 May 10;143(1):32–49. doi: 10.1111/j.0954-6820.1952.tb14249.x. [DOI] [PubMed] [Google Scholar]
- RAWLINS G. A. Liquefaction of sputum for bacteriological examination. Lancet. 1953 Sep 12;265(6785):538–539. doi: 10.1016/s0140-6736(53)90275-x. [DOI] [PubMed] [Google Scholar]
- REID L. M. Pathology of chronic bronchitis. Lancet. 1954 Feb 6;266(6806):274–278. [PubMed] [Google Scholar]
- ROLINSON G. N., STEVENS S. Microbiological studies on a new broad-spectrum penicilin, "Penbritin". Br Med J. 1961 Jul 22;2(5246):191–196. doi: 10.1136/bmj.2.5246.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggers B. A., Lawson D. In vivo penetration of antibiotics into sputum in cystic fibrosis. Arch Dis Child. 1968 Aug;43(230):404–409. doi: 10.1136/adc.43.230.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von WITTENAU M., YEARY R. The excretion and distribution in body fluids of tetracyclines after intravenous administration to dogs. J Pharmacol Exp Ther. 1963 May;140:258–266. [PubMed] [Google Scholar]


