Abstract
Summary
Knowledge of risk factors for hip fracture among very old people is limited. Walking indoors with help from ≤1 person, Parkinson’s disease, currently smoking, delirium in the previous month, underweight, and age were associated with increased risk of hip fracture and could be important for preventive strategy development.
Introduction
The purpose of this study is to investigate risk factors for hip fracture among a representative sample of very old people.
Methods
In total, 953 participants from the Umeå 85+/Gerontological Regional Database population-based cohort study were interviewed and assessed during home visits. Associations of baseline characteristics with hip fracture during the maximum 5-year follow-up period were analyzed using Cox proportional hazards regression.
Results
Participants had a mean age of 89.3 ± 4.7 years; 65.8 % were women, 36.8 % lived in residential care facilities, 33.6 % had dementia, and 20.4 % had histories of hip fracture. During a mean follow-up period of 2.7 years, 96 (10.1 %) individuals sustained hip fracture. Walking indoors with help from no more than one person (hazard ratio [HR] = 8.57; 95 % confidence interval [CI], 1.90–38.71), Parkinson’s disease (HR = 5.12; 95 % CI, 1.82–14.44), currently smoking (HR = 4.38; 95 % CI 2.06–9.33), delirium in the previous month (HR = 2.01; 95 % CI, 1.15–3.49), underweight (body mass index <22; HR = 1.74, 95 % CI, 1.09–2.77), and age (HR = 1.09; 95 % CI, 1.04–1.14) were associated independently with an increased risk of hip fracture. Hip prosthesis at baseline decreased the risk of hip fracture (HR = 0.37; 95 % CI, 0.15–0.91), but only for those with bilateral hip prostheses.
Conclusions
Seven factors were associated independently with incident hip fracture during follow-up in this sample of very old people. These factors could have important clinical implications in identifying persons at high risk of hip fracture, as well as in the development of effective preventive strategies.
Keywords: Dementia, Hip fracture, Independent living, Residential facility, Risk factor, Very old
Introduction
The risk of hip fracture increases exponentially with advancing age in both women and men [1]. The annual incidence of hip fracture worldwide is estimated to increase from 1.6 million in 2000 [2] to at least 4.5 million by 2050 [3], due primarily to population aging. The population aged ≥60 years is expected to more than double during the next four decades, with those aged ≥80 years forming the most rapidly growing age group [4]. As a consequence of population aging, several studies have reported an increase in the mean age at which hip fracture occurs [5].
In older people, hip fracture leads to considerable risks of dependence in activities of daily living (ADL), institutionalization, and mortality [6]. The risks of negative consequences associated with hip fracture seem to increase further with advancing age [7]. Furthermore, risk factors for hip fracture might change with age; for instance, the predictive roles of lower body weight, previous osteoporotic fracture, and hip fracture in first-degree relatives appear to lose significance after the age of 80 years [8]. One reason for the potential difference in factors related to hip fracture between the very old (>80 years) population and younger people is the age-related increase in the prevalence of diseases and conditions such as dementia, stroke, delirium, multimorbidity, and physical impairment.
Although the incidence of hip fracture is known to increase with age in most regions of the world [1], present knowledge about risk factors for hip fracture among very old people is limited. To our knowledge, no previous population-based cohort study has examined these risk factors in a representative sample of very old people. The majority of cohort studies conducted in samples with a mean age ≥80 years and those involving subgroup analyses of individuals aged ≥80 years have included only community-dwelling, ambulatory, or women [9–16]. In addition, data concerning dementia disorders and levels of cognitive function are usually absent [10–18] or limited [19, 20]. Thus, expansion of our knowledge about factors associated with hip fracture in the very old population is important to identify high-risk individuals in this age group and develop effective strategies for prevention. Consequently, the aim of the present study was to investigate risk factors for hip fracture among very old people, including individuals with dementia and persons living in residential care facilities.
Methods
Procedure
This analysis employed data from the Umeå 85+/Gerontological Regional Database (GERDA) study, a population-based cohort study conducted by Umeå University, Sweden, in 2000–2002, 2005–2007, and 2010–2012. From a randomized starting point, every other 85-year-old and every 90- and ≥95-year-old inhabitant of one urban and five rural municipalities in the county of Västerbotten was selected from National Tax Agency registers. Written information was first sent to all eligible participants by mail, and oral informed consent to participate was obtained during telephone calls placed shortly thereafter. When appropriate due to cognitive impairment, relatives or otherwise authorized representatives provided informed consent. The Regional Ethics Review Board of Umeå approved the study (§ 99–326, § 05-063 M, § 09-178 M, § 13-432-32 M).
Trained assessors with prior medical knowledge (physicians, medical students, nurses, and physiotherapists) performed the interviews and assessments in the participants’ homes to enable individuals with, for example, severe dementia and multimorbidity to participate. Care personnel and/or relatives were also interviewed when the participants lived in residential care facilities or when required due to cognitive impairment. Medical records were reviewed to confirm diagnoses and medications. For participants who took part in more than one data collection period, data from the earliest occasion including home visitation and review of medical records were used in the present analyses.
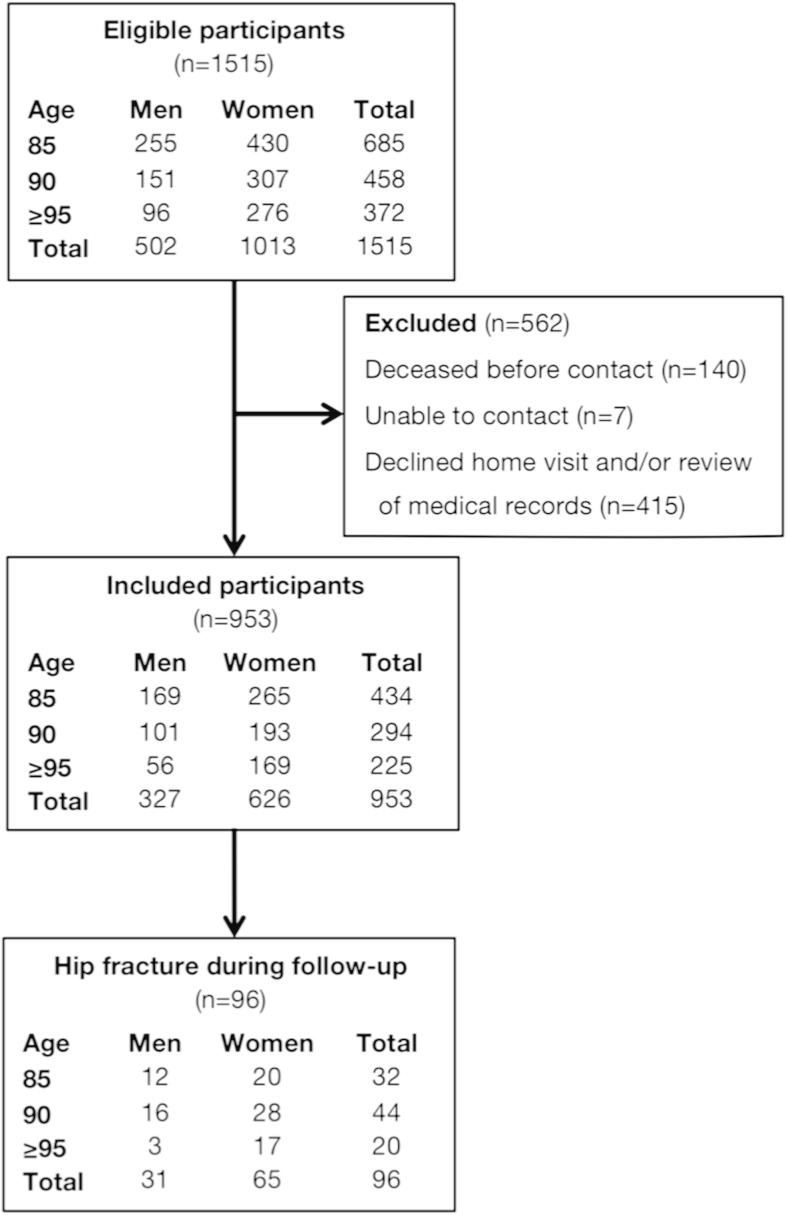
Participants
From the 1368 persons invited to participate between 2000 and 2012 (see Fig. 1), the present study included the 953 individuals consenting to home visitation and review of medical records (participation rate 69.7 %; Fig. 1). Age did not differ between the 415 individuals who declined to participate in this study and the 953 individuals who consented (P = .812), but a higher proportion of women than men declined to participate (32.3 % vs. 26.4 %, P = 0.028).
Fig. 1.
Flow chart of study sample
Hip fracture
Data on occurrence and type of femur fracture between 1 January 1980 and 31 October 2013 were collected through review of medical records and discharge registers from the three local hospitals (Umeå, Skellefteå, and Lycksele), maintained by the County Council of Västerbotten. Hip fractures were categorized as cervical (femoral neck) or trochanteric (inter- and sub-trochanteric regions), based on International Classification of Diseases (ICD) codes. Hip fractures occurring before baseline were classified according to ICD-8 (820.00/01, 820.10/11, 820.90/91), ICD-9 (820A–D), and ICD-10 (S72.00–S72.21) codes. Hip fracture incidence was followed for each participant and hip fracture type, categorized using ICD-10 codes. Follow-up started at the date of study inclusion and ended at the first occurrence of one of the following events: hip fracture, death, the last day of the maximum 5-year follow-up period, or—for individuals included in 2010–2012—the last date of data collection (31 October 2013). Dates of death were collected from death certificates, electronic medical records, and population registers.
Factors potentially associated with hip fracture
Variables assessed at baseline were chosen based on associations with hip fracture, falls, or osteoporosis in previous studies of older people (Table 1). Body weight and height were measured, and body mass index (BMI, kg/m2) was calculated. Vision was rated as impaired when a participant was unable to read a sentence printed in 4-mm-high capital letters, with or without glasses. Hearing was rated as impaired when a participant was unable to hear a conversation at normal speaking volume from a 1-m distance, with or without a hearing aid. Cognitive function was assessed using the Mini-Mental State Examination (MMSE) [21]. MMSE scores range from 0 to 30, with scores ≤23 considered to indicate impaired cognition. The Barthel ADL Index (scores, 0–20) was used to measure dependence in personal activities of daily living (P-ADL) [22], with a score of 20 indicating total independence. The Barthel ADL Index “mobility on level surface” item was singled out to describe participants’ ability to move indoors. The ADL staircase, a development of the Katz ADL Index [23], was used to assess dependence in instrumental ADL and P-ADL. ADL staircase scores were dichotomized at 0 (independence in all ten activities) to compare independent with dependent individuals. To measure functional capacity, participants’ ability to rise once from a chair and then sit down independently, without using the hands, was tested. Nutritional status was evaluated using the Mini-Nutritional Assessment (MNA) Scale [24]. MNA Scale scores range from 0 to 30, with a score <17 indicating malnutrition, scores of 17–23.5 indicating risk of malnourishment, and scores ≥24 indicating good nutritional status. Socio-demographic information and data on falls in the previous year were collected during interviews, as were medical history and current use of medication, which were verified later by reviewing medical records. A fall was defined as an event in which the individual involuntarily ended up on the floor/ground. A specialist in geriatric medicine either confirmed pre-existing diagnoses of osteoporosis or set new clinical diagnoses of osteoporosis, mainly based on low-energy fractures and/or dual-energy X-ray absorption (DXA) assessment. The same specialist also confirmed diagnoses of dementia, depression, and delirium according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria [25] by review of medical records, current medical treatment, and baseline assessments, including the 15-item Geriatric Depression Scale [26] and the Organic Brain Syndrome scale [27].
Table 1.
Baseline characteristics for the total sample and participants with hip fracture during follow-up, as well as hazard ratios (HR) for hip fracture during follow-up
| Characteristic | Total sample (n = 953) | Hip fracture cases (n = 96) | HR (95 % CI) | P value |
|---|---|---|---|---|
| Age (years) | 89.3 ± 4.7 | 89.8 (4.3) | 1.07 (1.02–1.11) | 0.004 |
| Age group (years) | 0.001 | |||
| 85 | 434 (45.5) | 32 (33.3) | ||
| 90 | 293 (30.8) | 44 (45.8) | 2.31 (1.47–3.65) | <0.001 |
| ≥95 | 225 (23.6) | 20 (20.8) | 2.01 (1.14–3.55) | 0.016 |
| Women | 627 (65.8) | 65 (67.7) | 0.99 (0.64–1.52) | 0.957 |
| Living in residential care facility | 351 (36.8) | 38 (39.6) | 1.81 (1.20–2.74) | 0.005 |
| Living alone | 757 (79.4) | 78 (81.3) | 1.27 (0.76–2.12) | 0.357 |
| Currently smoking (n = 942) | 29 (3.1) | 6 (6.3) | 2.88 (1.40–5.96) | 0.004 |
| History of smoking (n = 941) | 330 (35.0) | 33 (35.9) | 1.02 (0.67–1.57) | 0.912 |
| History of ≥1 fall previous year (n = 901) | 423 (46.9) | 53 (56.4) | 1.58 (1.05–2.37) | 0.029 |
| Medical diagnoses and conditions | ||||
| History of any fracture | 501 (52.6) | 53 (55.2) | 1.22 (0.82–1.82) | 0.333 |
| History of hip fracture | 194 (20.4) | 21 (21.9) | 1.37 (0.85–2.23) | 0.200 |
| Osteoporosis | 309 (32.4) | 32 (33.3) | 1.14 (0.75–1.75) | 0.533 |
| Hip prosthesisa | 114 (12.0) | 6 (6.3) | 0.43 (0.19–0.99) | 0.048 |
| Dementia | 320 (33.6) | 28 (29.2) | 1.33 (0.85–2.08) | 0.213 |
| Delirium in the previous month | 206 (21.6) | 21 (21.9) | 1.84 (1.12–3.00) | 0.016 |
| Depressive disorder | 335 (35.2) | 33 (34.4) | 1.20 (0.78–1.82) | 0.407 |
| Parkinson’s disease | 15 (1.6) | 4 (4.2) | 3.35 (1.23–9.13) | 0.018 |
| Cerebrovascular disease | 206 (21.6) | 13 (13.5) | 0.60 (0.33–1.07) | 0.082 |
| Heart failure | 272 (28.5) | 21 (21.9) | 0.94 (0.58–1.53) | 0.796 |
| Diabetes | 148 (15.5) | 15 (15.6) | 1.12 (0.64–1.94) | 0.693 |
| Rheumatoid arthritis | 118 (12.4) | 10 (10.4) | 0.79 (0.41–1.53) | 0.486 |
| Osteoarthritis—lower extremities (n = 946) | 317 (33.5) | 21 (22.1) | 0.57 (0.35–0.93) | 0.023 |
| Malignancy in the previous 5 years | 116 (12.2) | 13 (13.5) | 1.42 (0.79–2.56) | 0.239 |
| Thyroid disease | 137 (14.4) | 13 (13.5) | 0.87 (0.49–1.57) | 0.649 |
| ≥1 urinary infection in previous year | 234 (24.6) | 16 (16.7) | 0.68 (0.40–1.16) | 0.156 |
| Routine prescription medications | ||||
| Benzodiazepines | 231 (24.2) | 23 (24.0) | 0.98 (0.61–1.56) | 0.927 |
| Beta-blockers | 303 (31.8) | 28 (29.2) | 0.91 (0.58–1.41) | 0.662 |
| Selective serotonin reuptake inhibitors | 123 (12.9) | 18 (18.8) | 1.77 (1.06–2.96) | 0.029 |
| Diuretics | 480 (50.4) | 35 (36.5) | 0.63 (0.41–0.95) | 0.028 |
| Analgesics | 416 (43.7) | 43 (44.8) | 1.24 (0.83–1.85) | 0.299 |
| Paracetamol | 346 (36.3) | 37 (38.5) | 1.37 (0.91–2.07) | 0.133 |
| Non-steroidal anti-inflammatory drugs | 61 (6.4) | 5 (5.2) | 0.68 (0.28–1.68) | 0.407 |
| Opioids | 141 (14.8) | 17 (17.7) | 1.38 (0.82–2.33) | 0.228 |
| Levothyroxine sodium | 103 (10.8) | 12 (12.5) | 1.15 (0.63–2.10) | 0.657 |
| Neuroleptics | 110 (11.6) | 10 (10.4) | 1.05 (0.55–2.03) | 0.874 |
| Cortisone (oral) | 121 (12.7) | 7 (7.3) | 0.55 (0.26–1.19) | 0.129 |
| Systemic estrogen treatment | 143 (15.0) | 12 (12.5) | 0.77 (0.42–1.41) | 0.401 |
| Number of routine prescription medications | 6.6 ± 4.1 | 5.9 ± 3.7 | 0.98 (0.93–1.03) | 0.474 |
| Assessments | ||||
| Vision impairment (n = 922) | 174 (18.9) | 19 (20.2) | 1.48 (0.89–2.45) | 0.130 |
| Barthel ADL Index (0–20; n = 947) | 16.4 ± 5.5 | 17.6 ± 3.5 | 1.00 (0.96–1.05) | 0.962 |
| Walking indoors with help of no more than one person (n = 947)b | 838 (88.5) | 92 (96.8) | 2.48 (0.78–7.84) | 0.123 |
| Independent in instrumental ADL (n = 946)c | 218 (23.0) | 21 (22.1) | 0.66 (0.40–1.07) | 0.092 |
| Body mass index (BMI), mean ± SD (n = 906) | 25.2 ± 4.4 | 25.0 ± 4.8 | 0.97 (0.93–1.02) | 0.263 |
| Underweight (BMI < 22; n = 906) | 200 (22.1) | 26 (28.0) | 1.72 (1.09–2.70) | 0.020 |
| Mini-Mental State Examination (0–30; n = 926) | 21.2 (7.6) | 21.4 ± 7.3 | 0.97 (0.94–1.00) | 0.022 |
| Mini-Nutritional Assessment Scale (0–30; n = 890)d | 23.7 (4.2) | 24.1 (3.2) | 0.92 (0.86–0.98) | 0.007 |
| Able to rise from a chair independently without using hands (n = 939) | 575 (61.2) | 59 (64.8) | 0.74 (0.48–1.15) | 0.182 |
Data are presented as mean ± standard deviation or n (%). Univariate Cox proportional hazard regression was used to analyze associations between baseline characteristics and time to first hip fracture during follow-up
CI confidence interval, ADL activities of daily living, BMI body mass index
aFor the 114 participants with unilateral (n = 82) or bilateral (n = 32) hip prostheses
bAccording to Barthel ADL Index item 7
cAccording to ADL staircase
dHazard ratio during the first 500 days of follow-up only since the assumption of time independency was not fulfilled in total follow-up of 1827 days
Statistical analysis
Differences between women and men, as well as those between individuals who agreed and declined to participate, were tested using Pearson’s chi-squared test and Student’s t test. Univariate Cox proportional hazards regression models were used to analyze associations between baseline characteristics and time to hip fracture during follow-up. The Barthel ADL Index “mobility on level surface” item was dichotomized, as the increased risk of incident hip fracture was similar in participants who were able to walk independently or with help and those who were independently mobile in wheelchairs or immobile (data not shown). Non-linear associations between incident hip fracture and continuous or ordinal baseline variables were analyzed according to established cutoff scores or quartiles. As a result, BMI was dichotomized at 22.0, which indicates underweight in people aged >70 years [28]. The proportionality of hazards was tested using Schoenfeld residuals.
Baseline variables associated with risk of incident hip fracture at P < 0.15 in univariate Cox analyses were included in a multivariate Cox regression model, with the exception of the MNA variable. MNA was excluded due to singularity since it includes data on BMI, type of residence and indoor mobility, which are included as separate variables in the multivariate model. The correlations between all variables in the multivariate model were tested using Pearson and Spearman coefficients, and no strong correlations (r > 0.6) were found. Step-wise backward deletion was performed manually, with the least-significant variable eliminated until only significant variables remained. These variables, adjusted for sex, formed the final model and were re-tested using Schoenfeld residuals.
Individuals with unilateral and bilateral hip prostheses were included in the baseline hip prosthesis variable. Sensitivity analyses excluded individuals with bilateral hip prostheses, which greatly reduce the probability of future hip fracture.
The IBM SPSS software (version 22) was used for statistical calculations. All analyses were two tailed, and P < 0.05 was considered statistically significant.
Results
Table 1 shows participants’ baseline characteristics and hazard ratios (HRs) for hip fracture. The 953 participants had a mean (±standard deviation) age of 89.3 ± 4.7 years and mean BMI of 25.2 ± 4.4 kg/m2; 623 (65.4 %) lived in the urban municipality of Umeå, 627 (65.8 %) were women, and 351 (36.8 %) lived in residential care facilities. The mean number of prescribed drugs was 6.6 ± 4.1. Of 320 (33.6 %) participants diagnosed with a dementia disorder, 27 (8.4 %) were prescribed anti-dementia drugs. Of the 206 individuals who had experienced delirium in the previous month, 168 (81.6 %) were diagnosed with a dementia disorder. At baseline, 423 (46.9 %) participants had fallen at least once during the previous year. One hundred ninety-four (20.4 %) participants had histories of hip fracture at baseline; 106 (54.6 %) fractures were cervical, 78 (39.8 %) were trochanteric, and 10 (5.2 %) were unspecified proximal femur fractures. Women sustained the majority (n = 153, 78.9 %) of the 194 previous hip fractures. Women also had a higher proportion of previous hip fractures compared with men (24.4 and 12.6 %, respectively; P < 0.001) and osteoporosis (40.5 and 16.9 %, P < 0.001) compared with men.
During a mean follow-up period of 2.7 years (996 days; range, 1–1827 days; 2599 person-years), 96 (10.1 %) participants sustained at least one hip fracture (48 [50.0 %] cervical, 48 [50.0 %] trochanteric). Out of the 96 participants who sustained a hip fracture during follow-up, 21 had a previous hip fracture at baseline. The second hip fracture was always situated on the contralateral side and was of the same type as the first hip fracture for 18 of these 21 individuals (85.9 %). All hip fractures during follow-up were low-trauma hip fractures (resulting from falls from standing height or less), and 90 (93.8 %) of the hip fractures were sustained indoors. The mean time to first incident hip fracture was 739 ± 533.5 days. Table 2 shows sex- and age-specific hip fracture incidence rates, types of hip fracture, and hip fractures per 100,000 person-years. Women sustained the majority (n = 65, 67.7 %) of hip fractures during follow-up, but the proportionate incidence of hip fracture did not differ between women and men (10.4 and 9.5 %, respectively; P = 0.734). Type of hip fracture differed between sexes (P = 0.008); women had a higher proportion of trochanteric hip fractures than men (60.0 and 29.0 %, respectively) and men had a higher proportion of cervical hip fractures (71.0 and 40.0 %, respectively). The overall incidence of hip fracture was 3694 per 100,000 person-years.
Table 2.
Sex- and age-specific hip fracture incidence rates
| Age group (years) | Participants with follow-up hip fracture (cervical/trochanteric) | Person-years | Hip fractures per 100,000 person-years |
|---|---|---|---|
| Women | 65 (26/39) | 1765 | 3683 |
| 85 | 20 (9/11) | 873 | 2291 |
| 90 | 28 (10/18) | 542 | 5166 |
| 95+ | 17 (7/10) | 351 | 4843 |
| Men | 31 (22/9) | 834 | 3717 |
| 85 | 12 (11/1) | 465 | 2581 |
| 90 | 16 (10/6) | 274 | 5839 |
| 95+ | 3 (1/2) | 96 | 3125 |
| Total | 96 (48/48) | 2599 | 3694 |
Seven of the 18 baseline variables included in the multivariate Cox proportional hazards regression analyses were associated significantly with incident hip fracture. Six of the seven variables included in the final multivariate Cox model were associated independently with an increased risk of hip fracture: walking indoors with help from no more than one person (HR = 8.57), currently smoking (HR = 4.38), Parkinson’s disease (HR = 5.12), delirium in the previous month (HR = 2.01), underweight (BMI < 22; HR = 1.74), and age (HR = 1.09; Table 3). Hip prosthesis (unilateral or bilateral) at baseline decreased the risk of hip fracture (HR = 0.37). A history of one or more falls in the previous year showed borderline significance in the multivariate analyses (HR = 1.53, 95 % confidence interval [CI], 1.00–2.34; P = 0.053).
Table 3.
Hazard ratios for hip fracture in the multivariate Cox proportional hazard regression model
| Characteristics | Hazard ratio | 95 % CI | P value |
|---|---|---|---|
| Walking indoors with help from no more than one person | 8.57 | 1.90–38.71 | 0.005 |
| Parkinson’s disease | 5.12 | 1.82–14.44 | 0.002 |
| Currently smoking | 4.38 | 2.06–9.33 | <0.001 |
| Delirium in previous month | 2.01 | 1.15–3.49 | 0.014 |
| Underweight (BMI <22.0) | 1.74 | 1.09–2.77 | 0.020 |
| Age | 1.09 | 1.04–1.14 | 0.001 |
| Hip prosthesis | 0.37 | 0.15–0.91 | 0.031 |
| Female sex | 0.875 | 0.56–1.37 | 0.557 |
CI confidence interval, BMI body mass index
Sensitivity analyses performed without the 32 individuals with bilateral hip prostheses at baseline, none of which experienced hip fracture during follow-up, showed that unilateral hip prosthesis was not significantly associated with a reduced risk of incident hip fracture (univariate HR = 0.63, 95 % CI, 0.28–1.44; P = 0.277).
Discussion
In this representative sample of people aged ≥85 years, 96 participants sustained a hip fracture during follow-up, resulting in a hip fracture incidence of 3694 per 100,000 person-years. Independent risk factors for hip fracture were walking indoors with the help of no more than one person, Parkinson’s disease, currently smoking, delirium in the previous month, underweight, and age. The presence of a hip prosthesis at baseline seemed to be a protective factor against hip fracture, but only for those with bilateral hip prostheses.
The incidence of hip fracture was higher in this study than among comparable age groups in most previous studies, but in line with the reported incidence among very old Norwegians [29]. Sweden has one of the highest incidences of hip fracture in the world [30], and older people in northern Sweden have been shown to have a high prevalence of medical diagnoses and conditions, as well as a large number of prescribed drugs [31], which may offer some explanation to the high incidence rates observed in the present study. An additional contributing factor might be insufficient UV-B radiation for cutaneous vitamin D synthesis during a major portion of the year in this northern latitude (64° N). Although women in the present study suffered more than twice as many hip fractures compared with men, the proportional incidence did not differ between the sexes. This result is in line with previous studies of very old populations in Sweden and other western countries that suggest that sex difference seems to diminish with advancing age [32–34]. In our study, the lack of difference between sexes in fracture incidence nevertheless seems counter-intuitive since osteoporosis and a history of hip fracture, which are established risk factors for hip fracture in younger populations, were found to be more common in women. However, in very old populations, the frequency of falls appears to be higher in men than in women [35], which may offer some explanation given that up to 98 % of hip fractures are reported to be the result of a fall [36]. In line with previous studies [37], the type of fracture sustained differed between the sexes; women suffered more trochanteric fractures while men more cervical fractures. In very old people, the structural geometry of the femoral neck and intertrochanteric regions associated with increased bone fragility seems to vary between sexes and may predispose to fracture type sustained [38, 39]. In our study, when a second fracture occurred, it was situated on the contralateral side and most was of the same type as the first fracture, which supports an inherent structural cause.
Perhaps due to the examination of a representative sample, this study of very old people identified two risk factors for hip fracture that were not previously identified in cohort studies: delirium and walking indoors with help from no more than one person. Previous studies of postoperative complications of hip fracture have found an association between delirium and falls [40], which supports the hypothesis that delirium could be an important risk factor for hip fracture. Furthermore, due to cognitive impairment, people with delirium may be less inclined to utilize appropriate safe-landing strategies to avoid impact to the hip, which may subsequently increase the risk for hip fracture when falling. Fortunately, delirium appears to be both preventable and treatable; previous studies have found that multifactorial interventions have positive effects in hospitalized people aged ≥70 years [41]. In addition, the risk of hip fracture was more than eight times greater among participants in our study who could walk indoors with the help of no more than one person, compared with those lacking this ability. This finding is plausible, as individuals who require support from at least two people to walk are likely to be unable to independently perform tasks that expose them to the risk of falling.
Four variables previously found to be associated with increased risk of hip fracture in younger elderly persons also proved to be independent risk factors in very old individuals: age, currently smoking, underweight, and Parkinson’s disease. Thus, our study found a total of six risk factors that may be used to identify individuals with high risk of sustaining a hip fracture in order to implement preventative management in very old people. With advancing age, the predictive role of osteoporosis seems to decrease, as shown in this study and others [42, 43], whereas risk factors such as falls and fall-related factors appears to mount in influence [8, 44]. Therefore, it seems important that drugs for fracture prevention is supplemented with non-pharmacological interventions. For individuals that are of high age, currently smoking, underweight, or with Parkinson’s disease, which are factors associated with deterioration in muscle strength and balance, exercise programs may be appropriate to reduce risk of falls and fall-related injuries [45]. In addition, some risk factors, e.g., smoking and underweight, may be directly modifiable through help to stop smoking or dietary management.
Conversely, several of the established risk factors among younger elderly persons, for example, osteoporosis, female sex, cerebrovascular disease, and previous hip fracture or any prior fracture, which often are used in risk evaluation tools [46], were not associated with an increased risk of hip fracture in this sample of very old people. Our results are supported by previous studies showing a reduced predictive ability of these factors in older populations [8, 32, 43, 44, 47]. Hence, it may not be appropriate to extrapolate results of studies of younger populations of older people to very old populations, where a high prevalence of multimorbidity and cognitive and physical impairment may influence the risk of hip fracture. However, although the risk of incident hip fracture seems to decrease with time after stroke or fracture [47, 48], we did not examine the impacts of intervals between the occurrence of medical conditions/diagnoses and incident hip fracture in the present study, which may have influenced the results. In addition, contrary to the results of previous large cohort studies among people with Alzheimer’s disease (AD) [49, 50], neither dementia nor level of cognitive function was found to be a risk factor for hip fracture among our very old participants. In comparison to those studies [49, 50], our sample also included persons with severe forms of dementia, types of dementia other than AD, and only a small proportion were prescribed anti-dementia drugs. The latter has been shown to be associated with the risks of hip fracture and falls in earlier cohort studies [51, 52]. Although dementia was not a risk factor for hip fracture, more than 80 % of participants with delirium in our sample were diagnosed with a dementia disorder, perhaps implying an indirect influence.
The comprehensive baseline assessment of risk factors and home visitations, which resulted in a representable sample, are strengths of the present study. Furthermore, the quality of hip fracture data was assured by review of medical records and discharge registers for regional hospitals. This study also has some limitations. Information on risk factors for hip fracture established among younger elderly people, such as low BMD (measured by DXA), parental history of hip fracture, postural hypotension, and excessive alcohol intake, were not available or had too many missing values to be included in the analysis. The limited DXA measures available may have caused an under-diagnosis of osteoporosis in our study. Previous falling, an established risk factor for hip fracture in younger elderly, showed only borderline significance in the multivariate analysis. However, data on falls in the previous year were obtained retrospectively, introducing the possibility of recall bias, particularly among those with cognitive impairment. More women than men declined to participate in the study, which could have affected the results; however, we found no difference in hip fracture risk between women and men. Nevertheless, future studies on risk factors for hip fracture stratified on sex are warranted given that type of fracture sustained differed between men and women in our study. The use of cross-sectional baseline data led to a lack of information about changes in medical conditions, diseases, drug prescriptions, and functional capacity during follow-up. No data on other types of fracture during follow-up were collected, which prevented analysis of associations, for example, between hip prosthesis presence and femoral shaft or pelvic fractures. The results of our study seem to be generalizable to individuals aged 85, 90, and ≥95 years living in the studied geographical area, but may not be applicable to other very old general populations.
In summary, walking indoors with help from no more than one person, Parkinson’s disease, currently smoking, delirium in the previous month, underweight, and age seem to be independently associated with a higher risk of incident hip fracture in people aged ≥85 years. Bilateral hip prostheses seem to be associated with a lower risk. The results of this study could have important clinical implications in identifying very old people at high risk of hip fracture, as well as in the development of effective preventive strategies. However, further research is needed to confirm these associations; randomized controlled trials should be conducted to evaluate the effectiveness of preventive measures that are implemented based on our results.
Acknowledgments
The study was financially supported by the Swedish Research Council (K2009-69P-21298-04-4, K2014-99X-22610-01-6); the Vårdal Research Foundation; the King Gustav V and Queen Viktoria Foundation; the Research Foundation of the Faculty of Medicine and Odontolgy at Umeå University; the Detlof Research Foundation; the Swedish Dementia Association; funding from the European Union and the Regional Development fund; the Interreg IIIA Mitt-Scandi and the Bothnia-Atlantica program; the Swedish Research Council for Health, Working Life and Welfare [2013-1512]; and The Strategic Research Program in Care Sciences. The sponsors had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
Compliance with ethical standards
Written information was first sent to all eligible participants by mail, and oral informed consent to participate was obtained during telephone calls placed shortly thereafter. When appropriate due to cognitive impairment, relatives or otherwise authorized representatives provided informed consent. This study was approved by the Regional Ethics Review Board of Umeå.
Conflicts of interest
The authors Robert Wiklund, Annika Toots, Mia Conradsson, Birgitta Olofsson, Henrik Holmberg, Erik Rosendahl, Yngve Gustafson, and Håkan Littbrand have no conflicts of interest to declare.
References
- 1.Wade SW, Strader C, Fitzpatrick LA, Anthony MS. Sex- and age-specific incidence of non-traumatic fractures in selected industrialized countries. Arch Osteoporos. 2012;7(1–2):219–227. doi: 10.1007/s11657-012-0100-5. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 3.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7(5):407–413. doi: 10.1007/PL00004148. [DOI] [PubMed] [Google Scholar]
- 4.United Nations (2013) World Population Ageing 2013. ST/ESA/SER.a/348 edn. Department of Economic and Social Affairs, Population Division, New York
- 5.Haleem S, Lutchman L, Mayahi R, Grice JE, Parker MJ. Mortality following hip fracture: trends and geographical variations over the last 40 years. Injury. 2008;39(10):1157–1163. doi: 10.1016/j.injury.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Marks R. Hip fracture epidemiological trends, outcomes, and risk factors, 1970–2009. Int J Gen Med. 2010;3:1–17. [PMC free article] [PubMed] [Google Scholar]
- 7.Diamantopoulos AP, Hoff M, Hochberg M, Haugeberg G. Predictors of short- and long-term mortality in males and females with hip fracture—a prospective observational cohort study. PLoS One. 2013;8(10):e78169. doi: 10.1371/journal.pone.0078169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anpalahan M, Morrison SG, Gibson SJ. Hip fracture risk factors and the discriminability of hip fracture risk vary by age: a case-control study. Geriatr Gerontol Int. 2014;14(2):413–419. doi: 10.1111/ggi.12117. [DOI] [PubMed] [Google Scholar]
- 9.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, Cauley JA, Tracy JK, Hochberg MC, Rodondi N, Cawthon PM, Study of Osteoporotic Fractures Research G Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62(7):744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 10.Hillier TA, Lui LY, Kado DM, LeBlanc ES, Vesco KK, Bauer DC, Cauley JA, Ensrud KE, Black DM, Hochberg MC, Cummings SR. Height loss in older women: risk of hip fracture and mortality independent of vertebral fractures. J Bone Miner Res. 2012;27(1):153–159. doi: 10.1002/jbmr.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dargent-Molina P, Favier F, Grandjean H, Baudoin C, Schott AM, Hausherr E, Meunier PJ, Breart G. Fall-related factors and risk of hip fracture: the EPIDOS prospective study. Lancet. 1996;348(9021):145–149. doi: 10.1016/S0140-6736(96)01440-7. [DOI] [PubMed] [Google Scholar]
- 12.Dargent-Molina P, Schott AM, Hans D, Favier F, Grandjean H, Baudoin C, Meunier PJ, Breart G. Separate and combined value of bone mass and gait speed measurements in screening for hip fracture risk: results from the EPIDOS study. Epidemiologie de l'Osteoporose. Osteoporos Int. 1999;9(2):188–192. doi: 10.1007/s001980050134. [DOI] [PubMed] [Google Scholar]
- 13.Dargent-Molina P, Douchin MN, Cormier C, Meunier PJ, Breart G, Group ES Use of clinical risk factors in elderly women with low bone mineral density to identify women at higher risk of hip fracture: the EPIDOS prospective study. Osteoporos Int. 2002;13(7):593–599. doi: 10.1007/s001980200078. [DOI] [PubMed] [Google Scholar]
- 14.Nevitt MC, Johnell O, Black DM, Ensrud K, Genant HK, Cummings SR. Bone mineral density predicts non-spine fractures in very elderly women. Study of Osteoporotic Fractures Research Group. Osteoporos Int. 1994;4(6):325–331. doi: 10.1007/BF01622192. [DOI] [PubMed] [Google Scholar]
- 15.Schott AM, Cormier C, Hans D, Favier F, Hausherr E, Dargent-Molina P, Delmas PD, Ribot C, Sebert JL, Breart G, Meunier PJ. How hip and whole-body bone mineral density predict hip fracture in elderly women: the EPIDOS Prospective Study. Osteoporos Int. 1998;8(3):247–254. doi: 10.1007/s001980050061. [DOI] [PubMed] [Google Scholar]
- 16.Grønskag AB, Forsmo S, Romundstad P, Langhammer A, Schei B. Incidence and seasonal variation in hip fracture incidence among elderly women in Norway. The HUNT Study. Bone. 2010;46(5):1294–1298. doi: 10.1016/j.bone.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Tromp AM, Ooms ME, Popp-Snijders C, Roos JC, Lips P. Predictors of fractures in elderly women. Osteoporos Int. 2000;11(2):134–140. doi: 10.1007/PL00004174. [DOI] [PubMed] [Google Scholar]
- 18.Thorell K, Ranstad K, Midlov P, Borgquist L, Halling A. Is use of fall risk-increasing drugs in an elderly population associated with an increased risk of hip fracture, after adjustment for multimorbidity level: a cohort study. BMC Geriatr. 2014;14:131. doi: 10.1186/1471-2318-14-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JS, Simpson JM, March LM, Cameron ID, Cumming RG, Lord SR, Seibel MJ, Sambrook PN. Risk factors for fracture following a fall among older people in residential care facilities in Australia. J Am Geriatr Soc. 2008;56(11):2020–2026. doi: 10.1111/j.1532-5415.2008.01954.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen JS, Sambrook PN, Simpson JM, Cameron ID, Cumming RG, Seibel MJ, Lord SR, March LM. Risk factors for hip fracture among institutionalised older people. Age Ageing. 2009;38(4):429–434. doi: 10.1093/ageing/afp051. [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10(2):61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 23.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The Index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 24.Guigoz Y, Vellas B, Garry PJ. Assessing the nutritional status of the elderly: the Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev. 1996;54(1 Pt 2):S59–65. doi: 10.1111/j.1753-4887.1996.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 25.The Diagnostic and Statistical Manual of Mental Disorders, Forth Edition: DSM-IV-Text Revision (2000). American Psychiatric Association, Washington, DC
- 26.Sheikh JI. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(00):165–173. [Google Scholar]
- 27.Björkelund KB, Larsson S, Gustafson L, Andersson E. The Organic Brain Syndrome (OBS) scale: a systematic review. Int J Geriatr Psychiatr. 2006;21(3):210–222. doi: 10.1002/gps.1449. [DOI] [PubMed] [Google Scholar]
- 28.The National Board of Health and Welfare (2011) Nutrition for good health and social care (In Swedish). ISBN 978-91-86885-39-7, 2011-9-2 edn.,
- 29.Omsland TK, Holvik K, Meyer HE, Center JR, Emaus N, Tell GS, Schei B, Tverdal A, Gjesdal CG, Grimnes G, Forsmo S, Eisman JA, Sogaard AJ. Hip fractures in Norway 1999–2008: time trends in total incidence and second hip fracture rates: a NOREPOS study. Eur J Epidemiol. 2012;27(10):807–814. doi: 10.1007/s10654-012-9711-9. [DOI] [PubMed] [Google Scholar]
- 30.Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C, Epidemiology IOFWGo, Quality of L (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 23 (9):2239–2256 [DOI] [PMC free article] [PubMed]
- 31.von Heideken WP, Gustavsson JM, Lundin-Olsson L, Kallin K, Nygren B, Lundman B, Norberg A, Gustafson Y. Health status in the oldest old. Age and sex differences in the Umea 85+ Study. Aging Clin Exp Res. 2006;18(2):116–126. doi: 10.1007/BF03327426. [DOI] [PubMed] [Google Scholar]
- 32.Prior JC, Langsetmo L, Lentle BC, Berger C, Goltzman D, Kovacs CS, Kaiser SM, Adachi JD, Papaioannou A, Anastassiades T, Towheed T, Josse RG, Brown JP, Leslie WD, Kreiger N, Ca MOSRG. Ten-year incident osteoporosis-related fractures in the population-based Canadian Multicentre Osteoporosis Study—comparing site and age-specific risks in women and men. Bone. 2015;71:237–243. doi: 10.1016/j.bone.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilson F, Moniruzzaman S, Gustavsson J, Andersson R. Trends in hip fracture incidence rates among the elderly in Sweden 1987–2009. J Public Health (Oxf) 2013;35(1):125–131. doi: 10.1093/pubmed/fds053. [DOI] [PubMed] [Google Scholar]
- 34.Requena G, Abbing-Karahagopian V, Huerta C, De Bruin ML, Alvarez Y, Miret M, Hesse U, Gardarsdottir H, Souverein PC, Slattery J, Schneider C, Rottenkolber M, Schmiedl S, Gil M, De Groot MC, Bate A, Ruigomez A, Garcia Rodriguez LA, Johansson S, de Vries F, Montero D, Schlienger R, Reynolds R, Klungel OH, de Abajo FJ. Incidence rates and trends of hip/femur fractures in five European countries: comparison using e-healthcare records databases. Calcif Tissue Int. 2014;94(6):580–589. doi: 10.1007/s00223-014-9850-y. [DOI] [PubMed] [Google Scholar]
- 35.von Heideken WP, Gustafson Y, Kallin K, Jensen J, Lundin-Olsson L. Falls in very old people: the population-based Umea 85+ study in Sweden. Arch Gerontol Geriatr. 2009;49(3):390–396. doi: 10.1016/j.archger.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Parkkari J, Kannus P, Palvanen M, Natri A, Vainio J, Aho H, Vuori I, Jarvinen M. Majority of hip fractures occur as a result of a fall and impact on the greater trochanter of the femur: a prospective controlled hip fracture study with 206 consecutive patients. Calcif Tissue Int. 1999;65(3):183–187. doi: 10.1007/s002239900679. [DOI] [PubMed] [Google Scholar]
- 37.Tanner DA, Kloseck M, Crilly RG, Chesworth B, Gilliland J. Hip fracture types in men and women change differently with age. BMC Geriatr. 2010;10:12. doi: 10.1186/1471-2318-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yates LB, Karasik D, Beck TJ, Cupples LA, Kiel DP. Hip structural geometry in old and old-old age: similarities and differences between men and women. Bone. 2007;41(4):722–732. doi: 10.1016/j.bone.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pulkkinen P, Gluer CC, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy for improved risk assessment and fracture prevention. Bone. 2011;49(4):600–604. doi: 10.1016/j.bone.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 40.Stenvall M, Olofsson B, Lundström M, Svensson O, Nyberg L, Gustafson Y. Inpatient falls and injuries in older patients treated for femoral neck fracture. Arch Gerontol Geriatr. 2006;43(3):389–399. doi: 10.1016/j.archger.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Lundström M, Olofsson B, Stenvall M, Karlsson S, Nyberg L, Englund U, Borssén B, Svensson O, Gustafson Y. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res. 2007;19(3):178–186. doi: 10.1007/BF03324687. [DOI] [PubMed] [Google Scholar]
- 42.Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, Burckhardt P, Cooper C, Christiansen C, Cummings S, Eisman JA, Fujiwara S, Gluer C, Goltzman D, Hans D, Krieg MA, La Croix A, McCloskey E, Mellstrom D, Melton LJ, 3rd, Pols H, Reeve J, Sanders K, Schott AM, Silman A, Torgerson D, van Staa T, Watts NB, Yoshimura N. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18(8):1033–1046. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 43.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ, 3rd, O'Neill T, Pols H, Reeve J, Silman A, Tenenhouse A. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 44.Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35(2):375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 45.El-Khoury F, Cassou B, Charles MA, Dargent-Molina P. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f6234. doi: 10.1136/bmj.f6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanis J, Oden A, Johnell O. Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke. 2001;32(3):702–706. doi: 10.1161/01.STR.32.3.702. [DOI] [PubMed] [Google Scholar]
- 48.Clinton J, Franta A, Polissar NL, Neradilek B, Mounce D, Fink HA, Schousboe JT, Matsen FA., 3rd Proximal humeral fracture as a risk factor for subsequent hip fractures. J Bone Joint Surg Am. 2009;91(3):503–511. doi: 10.2106/JBJS.G.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker NL, Cook MN, Arrighi HM, Bullock R. Hip fracture risk and subsequent mortality among Alzheimer's disease patients in the United Kingdom, 1988–2007. Age Ageing. 2011;40(1):49–54. doi: 10.1093/ageing/afq146. [DOI] [PubMed] [Google Scholar]
- 50.Tolppanen AM, Lavikainen P, Soininen H, Hartikainen S. Incident hip fractures among community dwelling persons with Alzheimer's disease in a Finnish nationwide register-based cohort. PLoS One. 2013;8(3):e59124. doi: 10.1371/journal.pone.0059124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gill SS, Anderson GM, Fischer HD, Bell CM, Li P, Normand SL, Rochon PA. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med. 2009;169(9):867–873. doi: 10.1001/archinternmed.2009.43. [DOI] [PubMed] [Google Scholar]
- 52.Olazaran J, Valle D, Serra JA, Cano P, Muniz R. Psychotropic medications and falls in nursing homes: a cross-sectional study. J Am Med Dir Assoc. 2013;14(3):213–217. doi: 10.1016/j.jamda.2012.10.020. [DOI] [PubMed] [Google Scholar]