Abstract
Background
Rapid weight gain in the first years of life is associated with adult obesity. Whether there are critical windows for this long term effect is unclear.
Objective
To study anthropometry in adolescence by gender according to weight and height growth velocities at different ages between birth and five years.
Design
Anthropometric parameters, including fat and fat-free mass by bipodal impedancemetry, were measured in 468 8–17 year old adolescents. We retrospectively collected early infancy data and individually estimated weight and height growth velocities in 69.4% of them using a mathematical model. Associations between birth parameters, growth velocities and anthropometric parameters in adolescence were studied.
Results
Weight growth velocity at three months was associated with overweight (OR for a 1 SD increase [95% CI]=1.52[1.04–2.22]), fat mass and waist circumference in adolescence in both genders, and with fat-free mass only in boys (r=0.29, P<0.001 versus r=−0.01, ns in girls). Weight growth velocities after 2 years were associated with all anthropometric parameters in adolescence, in both genders. Between 6 months and 2 years, weight growth velocities were significantly associated only with adolescent height in boys; in girls, associations with fat mass in adolescence were weaker.
Discussion
Our results support the hypothesis of two critical windows in early childhood associated with the later risk of obesity: up to 6 months and from 2 years onwards. The study of the determinants of growth during these two periods is of major importance for the prevention of obesity in adolescence.
Introduction
There is more and more evidence that children who gain weight rapidly during early life are at higher risk of obesity in later life (1), although definitions of “weight gain” and “later life” are very different between the studies. The early-life periods considered also vary between studies. Both the first months or years of life (2–4) and the period of the adiposity rebound (5–7) have been associated with later obesity, but it is difficult from available data to assess the relative contribution of each period. This is important to better understand the link between early weight and height growth and obesity later in life, so that the appropriate timing of potential preventive measures can be defined.
Most of the studies approximate height or weight growth velocity by the difference between two height or two weight measures (1, 8–11) and they use the average growth over the period. The contribution of different sub-periods of age can therefore not be determined. Obesity is mostly assessed by the body mass index (BMI), but this index does not distinguish between fat and fat-free mass. Some studies have shown that rapid growth rates in infancy or in childhood are specifically associated with more fat mass later in life (10, 12). Gender differences have rarely been assessed, even though both body composition and weight and height growth in infancy are quite different between boys and girls (13).
Our aim was to study body composition in adolescence, between 8 and 17 years, according to weight and height growth velocities at different ages between 3 months and 5 years, separately in boys and girls. Velocities were estimated from a growth model which allowed us to smooth the data and to choose the periods of interest.
Subjects and Methods
Subjects
The children were recruited in Fleurbaix and Laventie, two neighbouring towns in northern France, with respectively 2488 and 4426 inhabitants in 1992, when the Fleurbaix-Laventie Ville Santé (FLVS) study started. The first part of the study (FLVS I), a five-year follow-up of children involved in a nutritional education program at school (14), included 579 families.
The second part, FLVS II, was an epidemiological study on the determinants of weight change in the population. This study was proposed to all families participating in the FLVS I study that were still living in the two towns and who could be contacted (i.e. 393 families). We recruited and examined 294 families between April and September 1999 (i.e. an acceptance rate of 75%). All family members aged 8 years and over (1113 subjects) were examined at home in 1999, and 255 boys and 251 girls aged 8 to 17 years were included. Fewer of the children studied were overweight than those not recruited (8.0 % vs 13.5%, P=0.008 by the IOTF definition (15)). Written consent was obtained from the children and from their parents. The FLVS II study was approved by the ethics committee of Lille (France) and the computer files were declared to the “Commission Nationale Informatique et Liberté”.
At the end of FLVS II study in 2003, we collected data from the ”Carnet de santé” of 227 boys and 241 girls (92%). The ”Carnet de santé” is a health booklet, given in France to all parents at their child’s birth, and filled in by health practitioners throughout childhood. These 468 children are the study population for this report.
Anthropometric data
All weight and height measurements in the “Carnet de santé” up until the age of 12 years were extracted for the present analysis, including measures at birth. Measurements at nine months and two years were available for most children because a medical examination is required by the French administration.
In addition, as part of the longitudinal follow-up of the children in the FLVS I and II studies, trained physicians collected anthropometric data on children on one or more occasions when they were between 5 and 17 years. Weight was measured in light clothes and without shoes to the nearest 0.1 kg, and height to the nearest cm. Waist circumference was measured to the nearest 0.5 cm, during expiration when breathing normally, at the smallest diameter between iliac crest and the lower rib. Arm circumference was measured to the nearest mm, using a tape measure, on a relaxed arm, at the midpoint between the acromion and the olecranon. The thickness of four skinfolds were measured to the nearest 0.1 mm, in duplicate, using Harpenden callipers, on the left side of the body: tricipital (posterior aspect of the arm, at the midpoint between the acromion and the olecranon), bicipital (anterior aspect of the arm, at the midpoint between the acromion and the olecranon), subscapular (1 cm below the inferior angle at the scapula) and supra-iliac (1 cm over the iliac crest, at the midaxillary line). The duplicate measures were averaged. The sum of skinfolds, sum of arm skinfolds (tricipital + bicipital) and body mass index (BMI=weight/height2, kg/m2), were calculated. Overweight and obesity were defined according to the IOTF gender- and age- specific BMI cut-offs (15). Fat mass and fat-free mass were obtained by a bipedal impedancemetry device (Tanita TBF310) as part of the FLVS II study. Pubertal stage was determined by the physicians according to Tanner’s classification.
Statistical analysis
The aim of the statistical analysis was to correlate weight and height growth velocities at different ages in early life with anthropometric parameters in adolescence. The first step of the analysis was to derive the weight and height growth velocities for each child at different ages, from a mathematical model of a child’s growth between 0 and 12 years (described below). The second step was to correlate the estimated weight and height growth velocities at different ages between 3 months and 5 years with the anthropometric measures at the last available examination of the children in 1999, the entry examination of the FLVS II Study: children aged 8 to 17 years are called “adolescents” for the sake of simplicity. Correlation coefficients were used instead of regression coefficients in order to insure the comparability between each period of early life and each anthropometric parameter.
Weight and height growth models
Measurements of weight and height from 0 to 12 years, obtained either from the “Carnet de santé” or the clinical examination, allowed us to model each child’s growth individually, using an adaptation of the Jenss growth curve model (16, 17), by including additional quadratic term in age (ci.t2). This model was validated in our population by an analysis of the residuals to confirm the goodness of fit. Mean growth velocities estimated by piecewise models are more accurate but require many more measurements; they gave similar results to those estimated from our model (results not shown).
Our model allowed us to estimate a child’s growth from 0 to 12 years instead of 0 to 8 years, as in the initial Jenss growth model (16). Thus, we were able to include more children since many of them were measured during FLVS I study and have then at least one measurement after 5 years. For their inclusion, children have to meet some conditions on the amount of measures: each child had to have at least six measures between 0 and 12 years, with at least two before 2 years and at least one after 5 years; 161 boys and 164 girls met these conditions for the weight growth model (69.4%) and 160 boys and 152 girls for the height growth model (66.7%). Boys who met the weight or the height model conditions were one year younger than the others (P=0.03), but after age adjustment, none of the anthropometric characteristic was significantly different. In girls, there were no significant differences. The number of weight measurements available for different age-periods between 0 and 12 years are presented in Table 1 for the children studied; the number and distribution of available height measurements were similar.
TABLE 1.
Information on the number of weight measurements by age intervals (in years) in boys and girls meeting the conditions1 of the model
| Age interval (y) | Measures (n) | Boys (N=161) | Girls (N=164) | Total (N=325) | %2 |
|---|---|---|---|---|---|
| ≤ 2 years | Median (min-max) | 6 (2–15) | 7 (2–14) | 7 (2–15) | |
|
| |||||
| 2 ≤ n ≤ 5 | 80 | 66 | 146 | 45 | |
| n ≥ 6 | 81 | 98 | 179 | 55 | |
|
| |||||
| > 2 & ≤ 5 years | Median (min-max) | 1 (0–11) | 1 (0–8) | 1 (0–11) | |
|
| |||||
| 0 | 75 | 59 | 134 | 41 | |
| 1 ≤ n ≤ 2 | 51 | 59 | 110 | 34 | |
| n ≥ 3 | 35 | 46 | 81 | 25 | |
|
| |||||
| > 5 & ≤ 12 years | Median (min-max) | 4 (1–7) | 4 (1–9) | 4 (1–9) | |
|
| |||||
| 1 ≤ n ≤ 2 | 20 | 23 | 43 | 13 | |
| n ≥ 3 | 141 | 141 | 282 | 87 | |
|
| |||||
| Total ≤ 12 years | Median (min-max) | 10 (6–27) | 14 (6–26) | 12 (6–27) | |
|
| |||||
| 6 ≤ n ≤ 9 | 76 | 61 | 137 | 42 | |
| n ≥ 10 | 85 | 103 | 188 | 58 | |
six or more measures in the age range 0 to 12 years & two or more measures before 2 years, at least one measure after 5 years
percentage compared to the population meeting the conditions of the model (both genders)
The same model was used to fit both the weight and the height growth:
where t is age (in months), yi weight (in kg) or height (in cm) of the ith child at age t and εi the residual error at age t. The least-squares estimates ai, bi, ci, di and ei were determined for each child with the PROC NLIN using Gauss-Newton iterative method in SAS.
This model is the sum of two components: firstly, ai+bi.t+ci.t2 which principally models growth after 3 years of age and, secondly, exp(di+ei.t) which models growth during the first three years, and is characterised by a sharp increase in the first months of life, followed by a slowly declining growth rate (18).
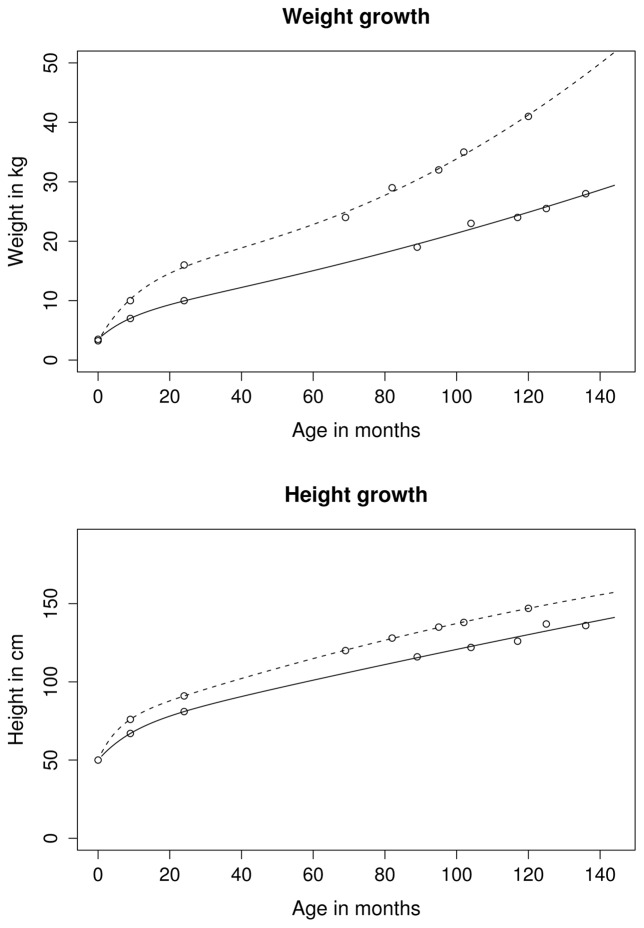
We constrained the parameters to obtain easier model convergence: for the height and the weight models, parameter bi was constrained to be positive (positive mean growth), ai>exp(di) (positive birth weight) and ei negative (declining growth rate during the first 3 years). For the weight model only, ci was additionally constrained to be positive. Indeed, weight growth is accelerating after eight years of age, whereas height growth is accelerating in some children, remaining stable or decelerating in others. Figure 1 presents the model and the observed data for two subjects having distinct growth velocities at 3 months.
FIGURE 1.
Fitted weight and height growth curve between 0 and 12 years in two selected subjects from the study with high and low growth velocities at three months (descriptive data without statistical test).
The first derivative of the equation of the individual models is the growth velocity at a given age (Figure 2). We used ages 3 and 6 months and 1, 2, 3 and 5 years as representative of different periods of early childhood, for the analysis of the association with body composition in adolescence. The growth velocity at 10 years was computed for the descriptive analysis only.
FIGURE 2.
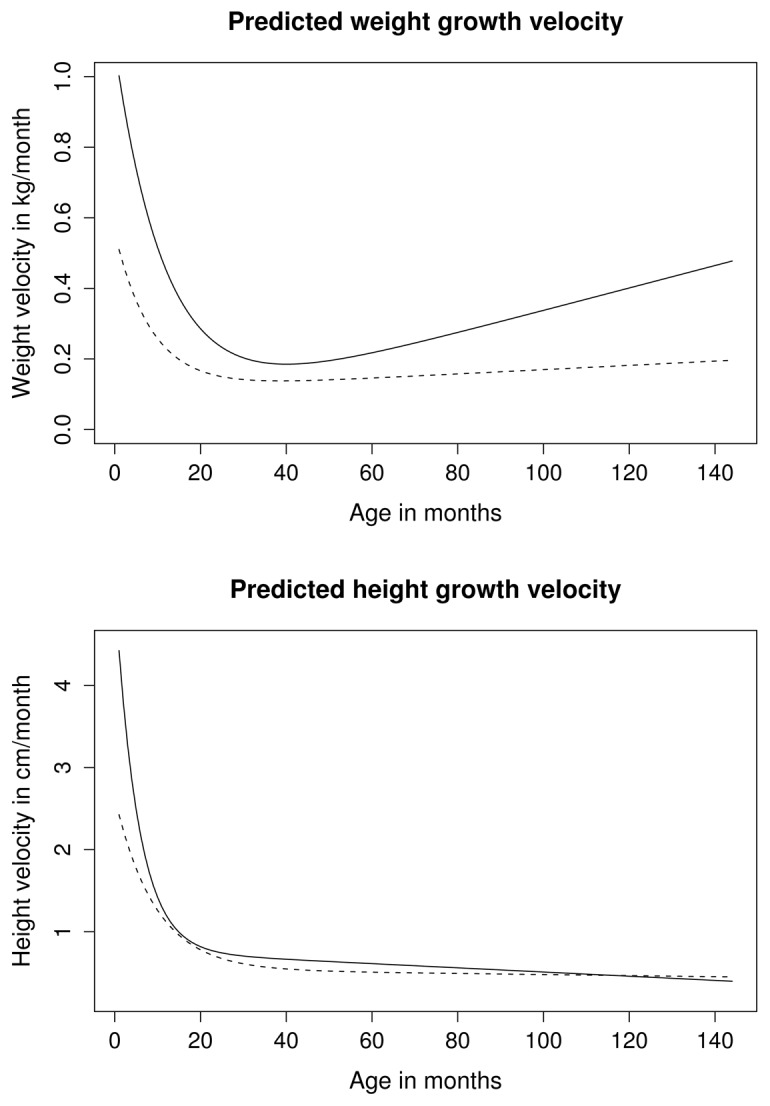
Weight and height growth velocity, predicted from the model, between 0 and 12 years in two selected subjects from the study with high and low growth velocities at three months (descriptive data without statistical test).
Analysis of the relationships between early childhood growth rates and body composition in adolescence
Results are given as means (SD) or geometric means [CI] for the log-transformed variables (total fat mass, sum of brachial and four skinfolds) or as percentages (number). All between gender comparisons were performed using Student’s tests or Chi-square tests.
Anthropometric measures in adolescence (X1 variables) were correlated with growth rates of height and weight estimated at different ages during childhood (X2 variables). Partial correlation coefficients between an X1 variable and an X2 variable were the Pearson correlation coefficients between the residuals of the regressions of X1 and X2 on their respective adjustment factors. X1 variables were systematically adjusted for age and Tanner stage and for height when X1 was associated with height. When X1 was the arm circumference, we additionally adjusted for the sum of arm skinfolds in order to obtain a brachial fat-free mass index. X2 variables were systematically adjusted for gestational age when it was a birth outcome. Because of the presence of siblings in our study population, analysis also took into account familial resemblance: in all regressions, we introduced a family variable as a random effect in a linear mixed model (19).
Differences in relationships between boys and girls were tested by including an interaction term with gender in linear regression models, with pre-adjusted X1 as the dependant variable and pre-adjusted X2 as the independent variable. As our sample of “adolescents” covered a wide age range (8–17 years), we also tested interactions with an age class variable (defined by the median age of the population, 13.5 years), in order to evaluate whether relationships were consistent across age categories.
The relationships between weight and height growth velocities at different ages between 3 months and 5 years and the risk of being overweight in adolescence were assessed from a logistic model adjusted for age, Tanner stage and gender. Power was lacking to stratify this analysis by gender.
The quality of fit of the individual growth models could have been taken into account by an intra-subject variance parameter in the subsequent analyses; however, as this variance was at least 6 times smaller than the inter-subject variance at all ages (except 3 months), we decided to ignore this parameter. At 3 months, taking this variance component into account did not change our conclusions (results not shown).
SAS Software version 9.1 was used for all other analyses. All significance tests were two-sided, a probability value greater than 0.05 was noted as non significant.
Results
Characteristics of the children (Table 2)
TABLE 2.
| N | Boys | Girls | P | ||
|---|---|---|---|---|---|
|
| |||||
| Gender (%) | 468 | 48.5 (227) | 51.5 (241) | 0.52 | |
| Birth and infancy: | |||||
| Gestational age (wks of pregnancy) | 449 | 38.7 (2.0) | 38.9 (1.6) | 0.19 | |
|
| |||||
| Birth weight (kg) | 463 | 3.33 (0.56) | 3.30 (0.47) | 0.58 | |
| Birth length (cm) | 455 | 50.2 (2.1) | 49.4 (2.0) | 0.005 | |
| Birth BMI (kg/m2) | 452 | 13.2 (1.4) | 13.4 (1.3) | 0.31 | |
| Birth ponderal index (kg/m3) | 452 | 26.4 (2.7) | 26.9 (2.6) | 0.02 | |
|
| |||||
| 9 month weight (kg) | 437 | 8.91 (1.0) | 8.49 (1.0) | <0.0001 | |
| 9 month height (cm) | 416 | 71.9 (2.8) | 70.7 (3.0) | <0.0001 | |
| 9 month BMI (kg/m2) | 411 | 17.2 (1.6) | 17.0 (1.7) | 0.22 | |
|
| |||||
| 2 year weight (kg) | 425 | 12.5 (1.6) | 12.0 (1.5) | 0.001 | |
| 2 year height (cm) | 411 | 87.3 (3.5) | 86.0 (3.6) | 0.0002 | |
| 2 year BMI (kg/m2) | 406 | 16.4 (1.7) | 16.3 (1.7) | 0.57 | |
|
| |||||
| Adolescence: | |||||
| Age (y) | 468 | 13.4 (2.4) | 13.5 (2.7) | 0.81 | |
|
| |||||
| Tanner stage | 468 | 1 | 22.0 (50) | 19.5 (47) | 0.02 |
| 2 | 20.7 (47) | 12.0 (29) | |||
| 3 | 19.4 (44) | 19.9 (48) | |||
| 4 | 26.0 (59) | 27.4 (66) | |||
| 5 | 11.9 (27) | 21.2 (51) | |||
|
| |||||
| Height (m) | 467 | 1,59 (0.15) | 1,56 (0.13) | 0.004 | |
| Weight (kg) | 468 | 47.9 (14.6) | 47.1 (12.8) | 0.51 | |
| BMI (kg/m2) | 467 | 18.4 (3.2) | 19.1 (3.3) | 0.03 | |
| Fat mass (kg) | 462 | 5.5 [5.1 – 6.0] | 9.7 [8.9 – 10.6] | <0.0001 | |
| Fat-free mass (kg) | 462 | 41.1 (11.7) | 35.3 (7.1) | <0.0001 | |
| Sum of total skinfolds (mm) | 463 | 32.8 [30.8 – 35.0] | 45.6 [43.0 – 48.3] | <0.0001 | |
| Arm circumference (cm) | 464 | 22.8 (3.5) | 23.0 (3.2) | 0.48 | |
| Sum of brachial skinfolds (mm) | 465 | 16.1 [15.1 – 17.0] | 22.2 [21.0 – 23.4] | <0.0001 | |
| Overweight (%) | 467 | 8.9 (20) | 10.0 (24) | 0.45 | |
| Obese (%) | 1.3 (3) | 2.9 (7) | |||
mean (SD) or geometric mean [95% CI] or percentage (subject number)
p-value of Student t-test or Chi-squared test.
At birth, 9 months and 2 years (Table 2, part 1)
In our population, for a similar gestational age, birth weight was not significantly different between boys and girls, but boys were significantly longer than girls. Therefore, ponderal index at birth was higher in girls. At 9 months and 2 years, boys were heavier and taller than girls, but the mean BMI were not significantly different.
In adolescence (Table 2, part 2)
At examination, the adolescents had a mean age of 13.5 years. Girls were more mature and boys were taller than girls. Girls had higher mean BMI, fat mass and sum of skinfold thicknesses than boys. Boys had more fat-free mass than girls. Arm circumference was not significantly different between boys and girls. The percentages of overweight or obesity were not significantly different between boys (10.2%) and girls (12.9%).
Growth velocity from 3 months to 10 years (Table 3)
TABLE 3.
Mean weight and height growth velocity estimations1 (in kg/mo and in cm/mo respectively) between 3 months and 10 years calculated from the model
| Weight velocities (kg/month): | |||
|---|---|---|---|
|
| |||
| Boys | Girls | P2 | |
| N | 161 | 164 | |
| 3 months | 0.70 (0.13) | 0.65 (0.12) | 0.0003 |
| 6 months | 0.48 (0.11) | 0.46 (0.09) | 0.10 |
| 1 year | 0.29 (0.08) | 0.28 (0.07) | 0.55 |
| 2 years | 0.18 (0.05) | 0.18 (0.05) | 0.40 |
| 3 years | 0.16 (0.04) | 0.16 (0.05) | 0.49 |
| 5 years | 0.19 (0.05) | 0.19 (0.05) | 0.54 |
| 10 years | 0.27 (0.10) | 0.30 (0.10) | 0.04 |
| Height velocities (cm/month): | |||
|---|---|---|---|
|
| |||
| Boys | Girls | p2 | |
| N | 160 | 152 | |
| 3 months | 2.67 (0.38) | 2.56 (0.36) | 0.01 |
| 6 months | 1.91 (0.27) | 1.87 (0.27) | 0.18 |
| 1 year | 1.21 (0.21) | 1.21 (0.21) | 0.81 |
| 2 years | 0.78 (0.11) | 0.77 (0.11) | 0.63 |
| 3 years | 0.65 (0.08) | 0.64 (0.09) | 0.43 |
| 5 years | 0.55 (0.06) | 0.56 (0.08) | 0.39 |
| 10 years | 0.42 (0.16) | 0.48 (0.20) | 0.005 |
mean (SD)
Student test
Weight and height growth at 3 months were faster in boys than in girls: for weight, 0.70 ± 0.13 kg/month in boys versus 0.65 ± 0.12 kg/month in girls, P=0.0003. Thereafter, the growth velocities were not significantly different until 10 years of age, when they were higher for girls than for boys: for weight, 0.27 ± 0.10 kg/month in boys versus 0.30 ± 0.10 kg/month in girls, P=0.04.
Relationships between weight growth velocities in early childhood and overweight in adolescence (Figure 3)
FIGURE 3.
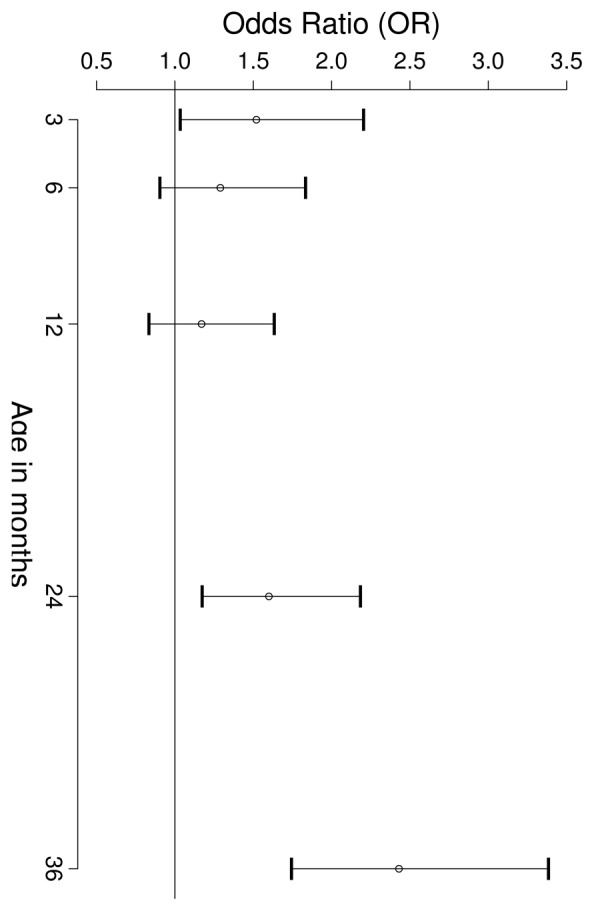
OR and 95% CI of being overweight (IOTF definition) in 325 adolescent boys and girls for a 1 SD increase in weight growth velocity at different ages between 3 and 36 months (logistic model adjusted for gender).
At 3 months, a 1 SD increase in weight growth velocity (143 g/month) increased the risk of being overweight or obese in adolescence (OR=1.52 [1.04 – 2.22]). A 1 SD increase in weight growth velocity at 3 and 5 years (corresponding to 50 and 43 g/month respectively) increased the risk of being overweight or obese in adolescence (OR=2.43 [1.75 – 3.39] and 5.08 [3.19 – 8.09] respectively). For the weight growth velocity at one year, the increased risk was not significant (OR=1.17 [0.84, 1.64]). Overweight in both adolescent boys and girls were not significantly associated with any of the height growth velocities.
Relationships between birth length and height growth velocities in early childhood with anthropometric measures in adolescence (results not shown)
Birth length and height growth velocities at all ages were positively correlated with the height in adolescence, the smallest correlations were for boys at 3 months (r=0.17, P=0.04) and for girls at 12 months (r=0.19, P=0.02). All other correlation coefficients were greater than 0.26 (P=0.0008).
In both genders, birth length and height growth velocities at all ages in early childhood were not significantly associated with any of the other anthropometric measures in adolescence.
Relationships between birth weight and weight growth velocities in early childhood with anthropometric measures in adolescence (Table 4)
TABLE 4.
Partial correlations between birth weight or weight growth velocities in infancy and anthropometric parameters, adjusted for confounders, in adolescent boys and girls1.
| Boys | Weight (N=214) at birth2 | Weight growth velocities (N=161) at: | |||||
|---|---|---|---|---|---|---|---|
| 3 mo | 6 mo | 1 y | 2 y | 3 y | 5 y | ||
|
|
|
|
|||||
| Height3 | 0.39*** | 0.19* | 0.27** | 0.30** | 0.32*** | 0.32*** | 0.39*** |
|
|
|
|
|||||
| BMI3 | 0.13 | 0.30** | 0.18* | 0.09 | 0.14 | 0.35*** | 0.65*** |
|
|
|
|
|||||
| Fat mass4 | 0.10 | 0.26** | 0.16* | 0.07 | 0.12 | 0.31*** | 0.57*** |
|
|
|
|
|||||
| Sum of skinfolds4 | 0.06 | 0.21** | 0.12 | 0.04 | 0.09 | 0.27** | 0.53*** |
|
|
|
|
|||||
| Waist circumference4 | 0.09 | 0.23** | 0.13 | 0.07 | 0.11 | 0.28** | 0.56*** |
|
|
|
|
|||||
| Fat-free mass4 | 0.12 | 0.29** | 0.14 | 0.04 | 0.03 | 0.18* | 0.44*** |
|
|
|
|
|||||
| Brachial fat free mass index5 | 0.09 | 0.26** | 0.09 | −0.03 | 0.01 | 0.17* | 0.41*** |
| Girls | Weight (N=231) at birth2 | Weight growth velocities (N=164) at: | |||||
|---|---|---|---|---|---|---|---|
| 3 mo | 6 mo | 1 y | 2 y | 3 y | 5 y | ||
|
|
|
|
|||||
| Height3 | 0.13 | 0.30** | 0.34*** | 0.43*** | 0.46*** | 0.41*** | 0.37*** |
|
|
|
|
|||||
| BMI3 | 0.03 | 0.19* | 0.23** | 0.19* | 0.23** | 0.36*** | 0.59*** |
|
|
|
|
|||||
| Fat mass4 | −0.02 | 0.24** | 0.21** | 0.11 | 0.13 | 0.27** | 0.51*** |
|
|
|
|
|||||
| Sum of skinfolds4 | −0.09 | 0.17* | 0.17* | 0.10 | 0.20** | 0.33*** | 0.53*** |
|
|
|
|
|||||
| Waist circumference4 | −0.05 | 0.18* | 0.20** | 0.18* | 0.19* | 0.28** | 0.45*** |
|
|
|
|
|||||
| Fat-free mass4 | 0.10 | −0.01 | 0.10 | 0.16* | 0.18* | 0.25** | 0.34*** |
|
|
|
|
|||||
| Brachial fat free mass index5 | −0.01 | −0.01 | 0.10 | 0.14 | 0.07 | 0.12 | 0.25** |
Maximum 5 missing values
Residuals of variable adjusted for gestational age
Residuals of variable adjusted for age and Tanner stage
Residuals of variable adjusted for age, Tanner stage and height
Residuals of arm circumference adjusted for age, Tanner stage, height and sum of arm skinfolds
P <0.05;
P <0.01;
P <0.001
Birth weight and weight growth velocities between 3 months and 5 years were significantly correlated with adolescent height in boys and girls (range=0.19–0.46; P<0.02), except birth weight in girls (r=0.13).
Birth weight was not significantly associated with adolescent BMI, either in boys or in girls. In contrast, correlations between weight growth velocity at 3 and 6 months and adolescent BMI were significant (r values: 0.18–0.30). In boys, correlations between 1 and 2 years decreased to low levels (r=0.09 at 1 year, ns). Thereafter, they started increasing again. In girls, correlations between weight growth velocity and adolescent BMI increased more regularly with age.
Birth weight was not significantly associated with the amount of adolescent fat mass (adjusted for adolescent height, age and Tanner stage). Weight growth velocities at 3 and 6 months but not at 1 year were positively correlated with total fat mass and sum of skinfold thicknesses (same adjustments) in boys as in girls, with comparable coefficients for both parameters and for both genders. In girls only, there was a significant interaction with age for some correlations between weight growth and fat mass in adolescence (results not shown): correlations were only significant for the younger girls (age≤13.5 years) compared to the others (e.g. r=0.38, P=0.0003 vs r=−0.03, P=0.80 between weight growth velocity at 3 months and fat mass in adolescence).
There was a positive correlation between birth weight and adolescent fat-free body mass when genders were pooled (r=0.12, P=0.01). In boys, a faster weight growth velocity at 3 months was associated with a higher adolescent fat-free mass (r=0.29, P <0.001) and with a higher brachial fat-free mass index (r=0.26, P <0.001). There was also a relationship, albeit weaker, between weight growth velocity at 6 months and fat-free mass (r=0.16, P =0.05). However, in girls, rapid weight growth velocity at 3 months was not associated with a higher amount of adolescent fat-free mass; the interaction term with gender was statistically significant (P=0.002). Correlations between 6 month, 1 and 2 year weight growth velocities and adolescent fat-free mass were low and not significantly different from zero for boys but increased progressively for girls.
Weight growth velocities at 3 and 5 years were correlated with all anthropometric variables in adolescence. The correlation coefficients were high (range at 60 months: 0.25–0.65; P<0.002). The anthropometric variable most associated with growth velocity at 60 months was BMI (r=0.65 in boys and 0.59 in girls).
Discussion
Our results suggest that two periods in early childhood are associated with body composition within the adolescent 8 to 17 year age range studied: the first 6 months, and period from 2 years onwards. In between, differences in weight growth contributed more to height than weight differences in adolescence. The relationship between weight growth velocities and adolescent anthropometric measures other than height, were not confounded by height growth since height growth velocities were not significantly associated with any of the adolescent measures.
The first critical period: before 6 months
From our results and other studies, we can conclude that during this first period, weight growth appears to be associated, rather consistently, with body composition in later life (8, 10, 12, 20, 21). This association might be stronger with fat-free mass than fat mass, especially in developing countries (8, 10). As in our study, the association between weight growth and fat-free mass was significantly stronger in males compared to females in the only other study that has examined gender differences (20).
These relationships are not unexpected when one considers that it is the period of fastest growth in the entire life span. Any variation in this process may therefore have long lasting consequences. Several determinants of growth and energy metabolism commence in this 6 month period (22), among which is oral nutrition (23). In this period of intense development of adipose tissue (24), different foods have been shown to lead to different adipocyte development, in both size and in number (25) and this period is potentially determinant for the constitution of the pool of fat cells, even though fat cells can be recruited over the entire life span (26). The offspring’s growth is also, for the first time, free of maternal intrauterine constraints and genetic factors, especially paternal, may be expressed (27). Some biological processes such as maturation of the gastrointestinal tract (24) or development of major hypothalamic regulatory functions (28) take place in early life. Finally, the early life period is characterized by transient hormonal secretions. In particular, we hypothesize that the physiological peak of plasma testosterone at three months in boys (29) could have anabolic effects stimulating the development of fat-free mass and explaining the stronger association between weight growth velocity at 3 months and fat-free mass in males than in females.
The second critical period: from 2 years onward
Previous studies showed that either greater BMI gain or weight gain from about two or three years of age were associated with adult adiposity, central obesity and, to a lesser degree, a higher fat-free mass in later life has been shown (8, 10, 12, 21).
As suggested by Ekelund et al. (12), the mechanisms leading to these relationships may be different from those during the first critical period. Indeed in our study, weight growth at 3 months was not correlated with weight growth at 3 years, neither in boys nor in girls (results not shown). This second period is that of the adiposity rebound, which occurs earlier in fat children (29). Fat gain may therefore explain much of the variability in weight gain differences between 3 and 5 years. Again, nutrition could probably be a key factor in this period, and this is influenced by parental eating habits (30) and is the period during which taste is developing (31, 32). It is also a period during which physical activity habits start, even if tracking through childhood is not clear (33). Finally, specific genetic factors that would express preferentially from 2 years could be involved.
The gap: from 6 months to 2 years
Correlations between weight gain velocities and adolescent anthropometric measures (except height) decreased in boys, as in girls, during this period. Several studies reported this pattern (8, 10, 21, 34), and it has been commented on in the two more recent ones (21, 34).
During this period, height growth seems to preferentially drive weight gain as previously reported (34). This period has also been noted as a period of critical height development, since nutritional intervention (drinks rich in high quality protein) has been shown to improve growth rates (35). Height growth rather than fat storage may be stimulated preferentially during this period (21), during which there is an increase in the ratio of protein to lipids in macronutrient intake (36). Mechanisms have been described that may allow a trade-off and change of energy allocation from fat mass to fat-free mass and in particular to bone growth. For example, PPAR-gamma is implicated as a transcription factor in the differentiation from the common bone marrow stromal cells in adipocytes or osteoblasts (37, 38). However, it might still be possible that this period is characterised by the silent (as far as weight is concerned) recruitment of preadipocytes in preparation for the adiposity rebound (26).
Strength and Weaknesses
Our growth model was able to handle the heterogeneity of weight and height measurements between 0 and 12 years in quality (no standardisation for the measures of the “Carnet de santé”) and in quantity, because the model minimized the measurement errors (at least 6 measures for 5 parameters). It also allowed us to take into account the non-linearity of growth during infancy (Figure 1), and to investigate different periods of infancy and early childhood, between 0 and 5 years. Our model showed the known trends: faster growth of boys in the first six months after birth and faster growth of girls after approximately 9 years, when they begin their pubertal growth spurt (13). Another strength of our study is the use of different indicators of fat mass and fat-free mass obtained by both anthropometry and bio-impedancemetry.
However, we may discuss the generalisability of our results as our population is not representative of French adolescents and have a relatively low prevalence of obesity compared to contemporary studies of French children (39, 40). This may have reduced the variability and thus the power of the study, and the main consequence would be an underestimation of the associations with fat mass in adolescence. The concordance of the results with that of the other studies is the most convincing argument that our results are not biased or chance findings. Moreover, because of the constraints of the model in terms of number of weight and height measurements required per child, we only used 66% of our initial population. Nevertheless, anthropometric parameters for children whose weight growth velocities could or could not be used in the models, were not statistically different, except for age in boys. Another weakness is the absence of accurate measures to estimate fat and fat-free mass, measures such as DEXA or air-displacement plethysmography. However, the few results in common with a study using air-displacement plethysmography (12) were similar to our results. Lastly, our “adolescent” children were cross-sectionally examined at different ages between 8 and 17 years, a wide period of ages. We adjusted all the adolescent measures for age, Tanner stage and, when it could be a supplementary confounding factor, for height. We also tested the interaction between correlated variables and age period, and such an interaction was only significant with weight growth velocities in the relationship with fat mass parameters in girls, with correlations lower in the older than in the younger girls (i.e. p for interaction < 0.05). We postulate that the changes in fat mass induced by puberty, and by age, may overcome the early childhood effect in the older girls.
Conclusion
In conclusion, our study supports the hypothesis that there are two critical periods in early childhood which are particularly associated with the later risk of overweight or obesity and not determined by the same factors since weight growth during these two periods are not significantly correlated (our results and (11)). The first months of life could be more critical for girls than for boys, as in girls this period of growth is significantly correlated with fat mass only, not with fat-free mass. The second period, may be the most critical in the ages studied (our results and (21)), for both genders, since higher weight growth velocities at 3 and 5 years appear more strongly related with gain in fat mass than in fat-free mass. In between, we suggest a period of preferential height growth. Before more knowledge is accumulated on that later period, our finding encourages to carefulness for any intervention during this “plateau” period: limiting energy intake could impact on height development rather than on overweight. For now, public health measures should probably focused on the two critical periods.
Acknowledgments
Supports:
Laboratoires Knoll, CEDUS, Groupe Fournier, Lesieur, Nestlé France, Produits Roche and TEPRAL support the Fleurbaix Laventie Ville Santé Studies.
Marie Aline Charles received grants from the “Association de Langue Française pour l’étude des diabètes et du métabolisme” (ALFEDIAM) and from the “Mutuelle Generale de l’Education Nationale” (MGEN)
We thank directors and teachers of the schools who made the study possible, the parents and their children who agreed to participate into the study, Laboratoires Knoll, CEDUS, Groupe Fournier, Lesieur, Nestlé France, Produits Roche and TEPRAL for their support of the Fleurbaix Laventie Ville Santé Studies.
Marie-Aline Charles received grants from the “Association de Langue Française pour l’étude des diabètes et du métabolisme” (ALFEDIAM) and from the “Mutuelle Generale de l’Education Nationale” (MGEN)
Members of the Fleurbaix Laventie Ville Santé Group:
Arnaud Basdevant, Jean-Michel Borys, Jean-Louis Bresson, Marie-Aline Charles, Pierre Ducimetière, Philippe Froguel, Barbara Heude, Agnes Lommez, Jean-Michel Oppert, Monique Romon.
Footnotes
JB conducted the data analyses and drafted the manuscript. BH and JM provided statistical expertise and JM contributed more particularly to the growth model. MAC supervised the study. JB, BH and MAC participated in the interpretation of the results and the writing of the manuscript. BH, PD and MAC provided critical input and advised on the analyses. All authors contributed to the revision of the manuscript.
None of the authors had any conflicts of interest.
References
- 1.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life-a systematic review. Obes Rev. 2005;6:143–54. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 2.Kinra S, Baumer JH, Smith GD. Early growth and childhood obesity: a historical cohort study. Arch Dis Child. 2005;90:1122–7. doi: 10.1136/adc.2004.066712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennison BA, Edmunds LS, Stratton HH, et al. Rapid infant weight gain predicts childhood overweight. Obesity (Silver Spring) 2006;14:491–9. doi: 10.1038/oby.2006.64. [DOI] [PubMed] [Google Scholar]
- 4.Stettler N, Zemel BS, Kumanyika S, et al. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109:194–9. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 5.Dietz WH. Periods of risk in childhood for the development of adult obesity-what do we need to learn? J Nutr. 1997;127:1884S–1886S. doi: 10.1093/jn/127.9.1884S. [DOI] [PubMed] [Google Scholar]
- 6.Reilly JJ, Armstrong J, Dorosty AR, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skinner JD, Bounds W, Carruth BR, et al. Predictors of children’s body mass index: a longitudinal study of diet and growth in children aged 2–8 y. Int J Obes Relat Metab Disord. 2004;28:476–82. doi: 10.1038/sj.ijo.0802405. [DOI] [PubMed] [Google Scholar]
- 8.Sachdev HS, Fall CHD, Osmond C, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–66. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 9.Kuh D, Hardy R, Chaturvedi N, et al. Birth weight, childhood growth and abdominal obesity in adult life. Int J Obes Relat Metab Disord. 2002;26:40–7. doi: 10.1038/sj.ijo.0801861. [DOI] [PubMed] [Google Scholar]
- 10.Wells JC, Hallal PC, Wright A, et al. Fetal, infant and childhood growth: relationships with body composition in Brazilian boys aged 9 years. Int J Obes (Lond) 2005;29:1192–8. doi: 10.1038/sj.ijo.0803054. [DOI] [PubMed] [Google Scholar]
- 11.Ekelund U, Ong KK, Linné Y, et al. Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab. 2007;92:98–103. doi: 10.1210/jc.2006-1071. [DOI] [PubMed] [Google Scholar]
- 12.Ekelund U, Ong K, Linné Y, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES) Am J Clin Nutr. 2006;83:324–30. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 13.Veldhuis JD, Roemmich JN, Richmond EJ, et al. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005;26:114–46. doi: 10.1210/er.2003-0038. [DOI] [PubMed] [Google Scholar]
- 14.Maillard G, Charles MA, Lafay L, et al. Macronutrient energy intake and adiposity in non obese prepubertal children aged 5–11 y (the Fleurbaix Laventie Ville Santé Study) Int J Obes Relat Metab Disord. 2000;24:1608–17. doi: 10.1038/sj.ijo.0801446. [DOI] [PubMed] [Google Scholar]
- 15.Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauspie RC. Mathematical models for the study of individual growth patterns. Rev Epidemiol Sante Publique. 1989;37:461–76. [PubMed] [Google Scholar]
- 17.Berkey CS. Comparison of two longitudinal growth models for preschool children. Biometrics. 1982;38:221–34. [PubMed] [Google Scholar]
- 18.van Dommelen P, van Buuren S, Zandwijken GRJ, et al. Individual growth curve models for assessing evidence-based referral criteria in growth monitoring. Stat Med. 2005;24:3663–74. doi: 10.1002/sim.2234. [DOI] [PubMed] [Google Scholar]
- 19.Brown H, Prescott R. Applied Mixed Models in Medicine. Chichester (UK): John Wiley and Sons Ltd; 1999. [Google Scholar]
- 20.Li H, Stein AD, Barnhart HX, et al. Associations between prenatal and postnatal growth and adult body size and composition. Am J Clin Nutr. 2003;77:1498–505. doi: 10.1093/ajcn/77.6.1498. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy A, Hughes R, Tilling K, et al. Birth weight; postnatal, infant, and childhood growth; and obesity in young adulthood: evidence from the Barry Caerphilly Growth Study. Am J Clin Nutr. 2007;86:907–13. doi: 10.1093/ajcn/86.4.907. [DOI] [PubMed] [Google Scholar]
- 22.Chanoine J. Ghrelin in growth and development. Horm Res. 2005;63:129–38. doi: 10.1159/000084688. [DOI] [PubMed] [Google Scholar]
- 23.Koletzko B, Aggett PJ, Bindels JG, et al. Growth, development and differentiation: a functional food science approach. Br J Nutr. 1998;80(Suppl 1):S5–45. doi: 10.1079/bjn19980104. [DOI] [PubMed] [Google Scholar]
- 24.Kuzawa CW. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am J Phys Anthropol. 1998;(Suppl 27):177–209. doi: 10.1002/(sici)1096-8644(1998)107:27+<177::aid-ajpa7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 25.Soriguer Escofet FJ, Esteva de Antonio I, Tinahones FJ, et al. Adipose tissue fatty acids and size and number of fat cells from birth to 9 years of age--a cross-sectional study in 96 boys. Metabolism. 1996;45:1395–401. doi: 10.1016/s0026-0495(96)90121-3. [DOI] [PubMed] [Google Scholar]
- 26.Ailhaud G. Le tissu adipeux au cours du développement: quelles conséquences sur la prévention de l’obésité infantile? French J Pediatr Puériculture. 2001;14:457–60. [Google Scholar]
- 27.Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr. 2004;134:205–10. doi: 10.1093/jn/134.1.205. [DOI] [PubMed] [Google Scholar]
- 28.Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–80. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- 29.Forest MG, Sizonenko PC, Cathiard AM, et al. Hypophyso-gonadal function in humans during the first year of life. 1. Evidence for testicular activity in early infancy. J Clin Invest. 1974;53:819–28. doi: 10.1172/JCI107621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101:539–49. [PubMed] [Google Scholar]
- 31.Fisher JO, Birch LL. Fat preferences and fat consumption of 3- to 5-year-old children are related to parental adiposity. J Am Diet Assoc. 1995;95:759–64. doi: 10.1016/S0002-8223(95)00212-X. [DOI] [PubMed] [Google Scholar]
- 32.Birch LL. Development of food acceptance patterns in the first years of life. Proc Nutr Soc. 1998;57:617–24. doi: 10.1079/pns19980090. [DOI] [PubMed] [Google Scholar]
- 33.Jackson DM, Reilly JJ, Kelly LA, et al. Objectively measured physical activity in a representative sample of 3- to 4-year-old children. Obes Res. 2003;11:420–5. doi: 10.1038/oby.2003.57. [DOI] [PubMed] [Google Scholar]
- 34.Corvalán C, Gregory CO, Ramirez-Zea M, et al. Size at birth, infant, early and later childhood growth and adult body composition: a prospective study in a stunted population. Int J Epidemiol. 2007;36:550–7. doi: 10.1093/ije/dym010. [DOI] [PubMed] [Google Scholar]
- 35.Martorell R. Results and implications of the INCAP follow-up study. J Nutr. 1995;125:1127S–1138S. doi: 10.1093/jn/125.suppl_4.1127S. [DOI] [PubMed] [Google Scholar]
- 36.Boggio V, Grossiord A, Guyon S, et al. Consommation alimentaire des nourrissons et des enfants en bas âge en France en 1997 (Food consumption of infants and young children in France in 1997) French. Arch Pediatr. 1999;6:740–7. doi: 10.1016/s0929-693x(99)80356-x. [DOI] [PubMed] [Google Scholar]
- 37.David V, Martin A, Lafage-Proust M, et al. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–62. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- 38.Lecka-Czernik B, Suva L. Resolving the Two “Bony” Faces of PPAR-gamma. PPAR Res. 2006;2006:27489. doi: 10.1155/PPAR/2006/27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Peretti C, Castetbon K. Surpoids et obésité chez les adolescents scolarisés en classe de troisième. French DREES: Etudes et Résultats. 2004;283:1–8. [Google Scholar]
- 40.Rolland-Cachera M, Castetbon K, Arnault N, et al. Body mass index in 7–9-y-old French children: frequency of obesity, overweight and thinness. Int J Obes Relat Metab Disord. 2002;26:1610–6. doi: 10.1038/sj.ijo.0802146. [DOI] [PubMed] [Google Scholar]