Abstract
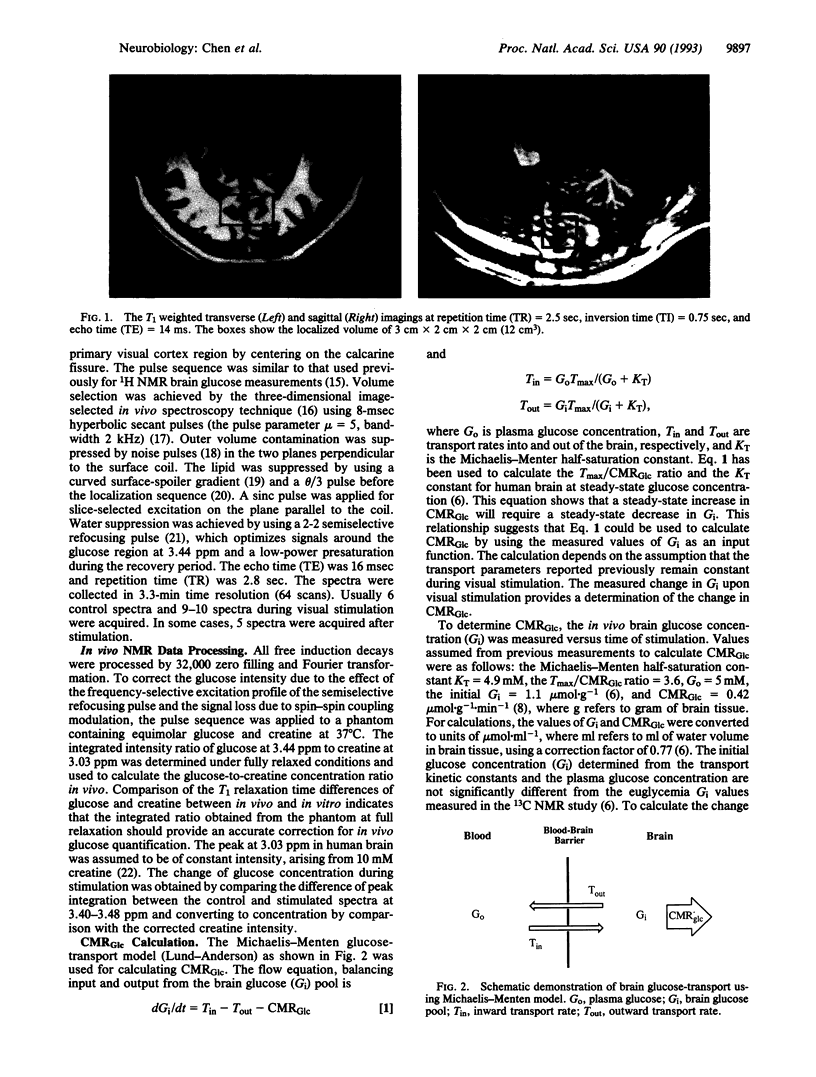
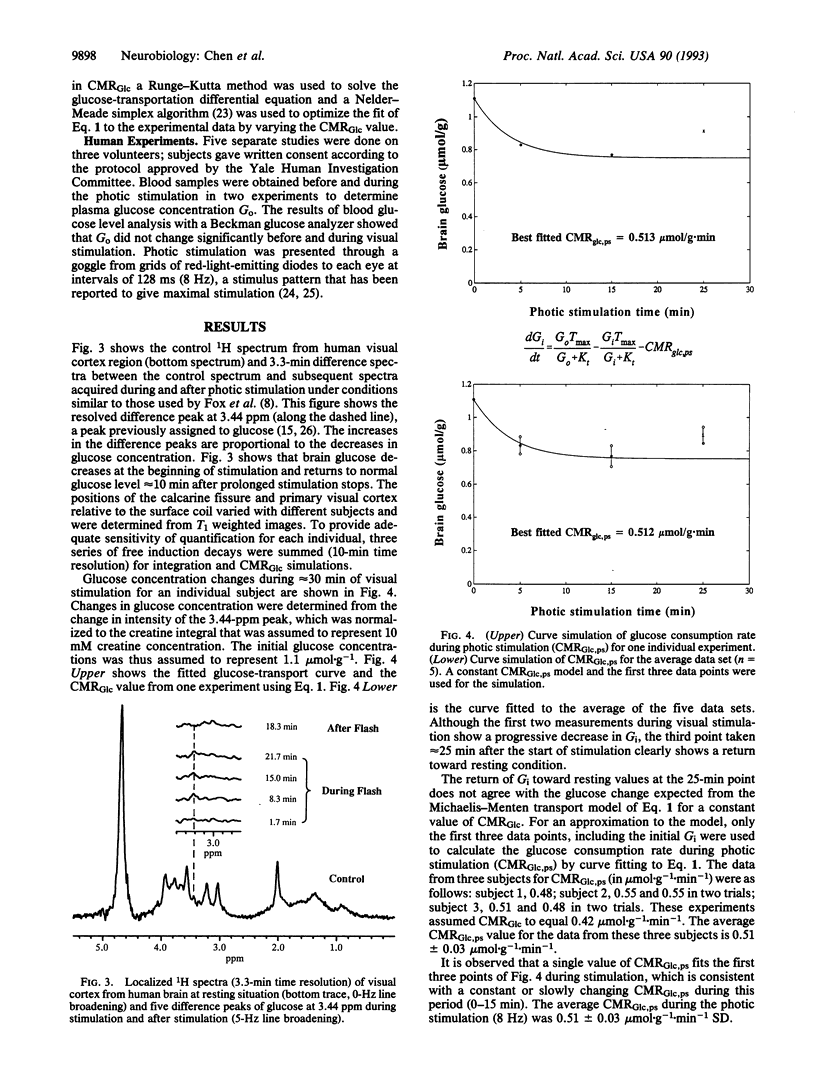
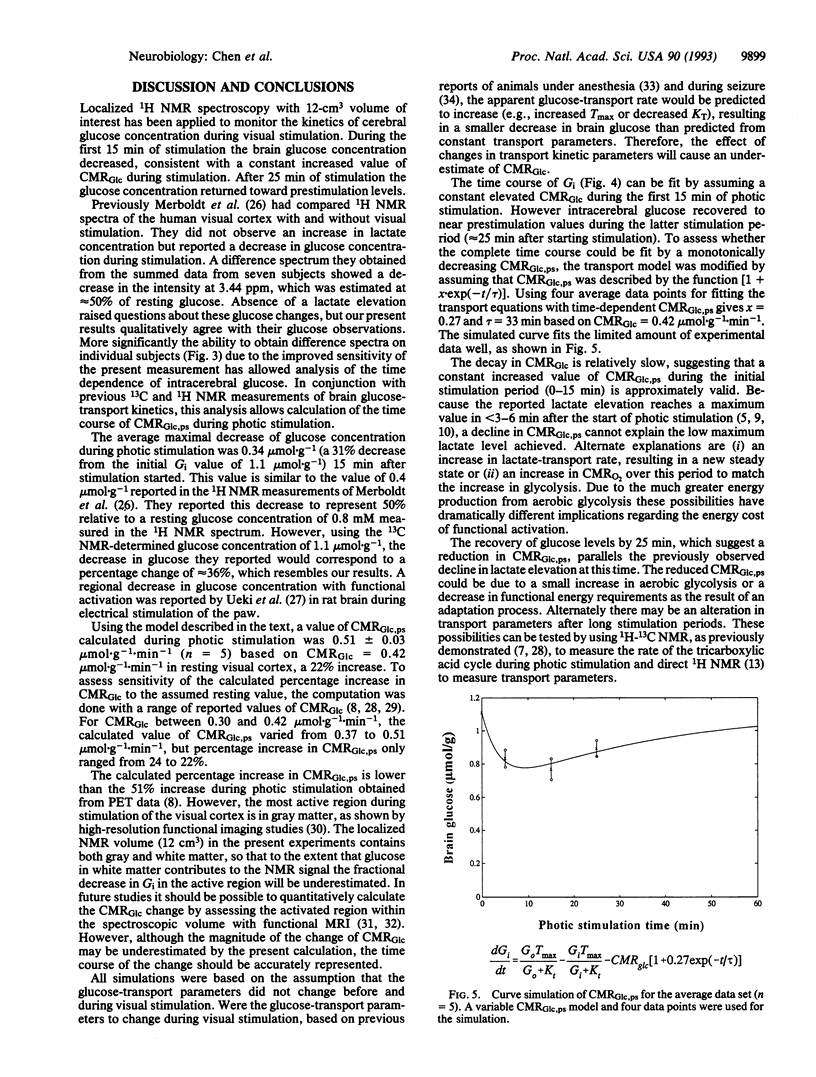
Spatially localized 1H NMR spectroscopy has been applied to measure changes in brain glucose concentration during 8-Hz photic stimulation. NMR spectroscopic measurements were made in a 12-cm3 volume centered on the calcarine fissure and encompassing the primary visual cortex. The average maximum change in glucose levels was 0.34 mumol.g-1 (n = 5) at 15 min; glucose level had turned toward resting level at 25 min. The glucose change was used to calculate the increase of glucose cerebral metabolic rate in the visual cortex region for individual subjects by using the Michaelis-Menten model of glucose transport on the assumption of constant transport kinetics. The glucose cerebral metabolic rate was calculated to increase over the nonstimulated rate by 22% during the first 15 min of photic stimulation. A model in which the glucose metabolic rate gradually decreases during stimulation was proposed as a possible explanation for the recovery of brain glucose and previously measured lactate concentrations to prestimulus values after 15 min.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belliveau J. W., Kennedy D. N., Jr, McKinstry R. C., Buchbinder B. R., Weisskoff R. M., Cohen M. S., Vevea J. M., Brady T. J., Rosen B. R. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991 Nov 1;254(5032):716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Blamire A. M., Ogawa S., Ugurbil K., Rothman D., McCarthy G., Ellermann J. M., Hyder F., Rattner Z., Shulman R. G. Dynamic mapping of the human visual cortex by high-speed magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11069–11073. doi: 10.1073/pnas.89.22.11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgström L., Chapman A. G., Siesjö B. K. Glucose consumption in the cerebral cortex of rat during bicuculline-induced status epilipticus. J Neurochem. 1976 Oct;27(4):971–973. doi: 10.1111/j.1471-4159.1976.tb05165.x. [DOI] [PubMed] [Google Scholar]
- Chen W., Ackerman J. J. Surface coil single-pulse localization in vivo via inhomogeneous surface spoiling magnetic gradient. NMR Biomed. 1989 Apr;1(4):205–207. doi: 10.1002/nbm.1940010409. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E., Mintun M. A., Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988 Jul 22;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Stimulus rate dependence of regional cerebral blood flow in human striate cortex, demonstrated by positron emission tomography. J Neurophysiol. 1984 May;51(5):1109–1120. doi: 10.1152/jn.1984.51.5.1109. [DOI] [PubMed] [Google Scholar]
- Gjedde A., Rasmussen M. Pentobarbital anesthesia reduces blood-brain glucose transfer in the rat. J Neurochem. 1980 Dec;35(6):1382–1387. doi: 10.1111/j.1471-4159.1980.tb09013.x. [DOI] [PubMed] [Google Scholar]
- Gruetter R., Novotny E. J., Boulware S. D., Rothman D. L., Mason G. F., Shulman G. I., Shulman R. G., Tamborlane W. V. Direct measurement of brain glucose concentrations in humans by 13C NMR spectroscopy. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1109–1112. doi: 10.1073/pnas.89.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter R., Rothman D. L., Novotny E. J., Shulman G. I., Prichard J. W., Shulman R. G. Detection and assignment of the glucose signal in 1H NMR difference spectra of the human brain. Magn Reson Med. 1992 Sep;27(1):183–188. doi: 10.1002/mrm.1910270118. [DOI] [PubMed] [Google Scholar]
- Heiss W. D., Pawlik G., Herholz K., Wagner R., Göldner H., Wienhard K. Regional kinetic constants and cerebral metabolic rate for glucose in normal human volunteers determined by dynamic positron emission tomography of [18F]-2-fluoro-2-deoxy-D-glucose. J Cereb Blood Flow Metab. 1984 Jun;4(2):212–223. doi: 10.1038/jcbfm.1984.30. [DOI] [PubMed] [Google Scholar]
- Kwong K. K., Belliveau J. W., Chesler D. A., Goldberg I. E., Weisskoff R. M., Poncelet B. P., Kennedy D. N., Hoppel B. E., Cohen M. S., Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund-Andersen H. Transport of glucose from blood to brain. Physiol Rev. 1979 Apr;59(2):305–352. doi: 10.1152/physrev.1979.59.2.305. [DOI] [PubMed] [Google Scholar]
- Mason G. F., Behar K. L., Rothman D. L., Shulman R. G. NMR determination of intracerebral glucose concentration and transport kinetics in rat brain. J Cereb Blood Flow Metab. 1992 May;12(3):448–455. doi: 10.1038/jcbfm.1992.62. [DOI] [PubMed] [Google Scholar]
- Merboldt K. D., Bruhn H., Hänicke W., Michaelis T., Frahm J. Decrease of glucose in the human visual cortex during photic stimulation. Magn Reson Med. 1992 May;25(1):187–194. doi: 10.1002/mrm.1910250119. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Tank D. W., Menon R., Ellermann J. M., Kim S. G., Merkle H., Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordidge R. J. Random noise selective excitation pulses. Magn Reson Med. 1987 Jul;5(1):93–98. doi: 10.1002/mrm.1910050113. [DOI] [PubMed] [Google Scholar]
- Petroff O. A., Spencer D. D., Alger J. R., Prichard J. W. High-field proton magnetic resonance spectroscopy of human cerebrum obtained during surgery for epilepsy. Neurology. 1989 Sep;39(9):1197–1202. doi: 10.1212/wnl.39.9.1197. [DOI] [PubMed] [Google Scholar]
- Prichard J., Rothman D., Novotny E., Petroff O., Kuwabara T., Avison M., Howseman A., Hanstock C., Shulman R. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D. L., Novotny E. J., Shulman G. I., Howseman A. M., Petroff O. A., Mason G., Nixon T., Hanstock C. C., Prichard J. W., Shulman R. G. 1H-[13C] NMR measurements of [4-13C]glutamate turnover in human brain. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9603–9606. doi: 10.1073/pnas.89.20.9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappey-Marinier D., Calabrese G., Fein G., Hugg J. W., Biggins C., Weiner M. W. Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1992 Jul;12(4):584–592. doi: 10.1038/jcbfm.1992.82. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Duara R., Haxby J., Grady C., White B. J., Kessler R. M., Kay A. D., Cutler N. R., Rapoport S. I. Down's syndrome in adults: brain metabolism. Science. 1983 Aug 19;221(4612):781–783. doi: 10.1126/science.6224294. [DOI] [PubMed] [Google Scholar]
- Silver M. S., Joseph R. I., Chen C. N., Sank V. J., Hoult D. I. Selective population inversion in NMR. Nature. 1984 Aug 23;310(5979):681–683. doi: 10.1038/310681a0. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. Localization of functional activity in the central nervous system by measurement of glucose utilization with radioactive deoxyglucose. J Cereb Blood Flow Metab. 1981;1(1):7–36. doi: 10.1038/jcbfm.1981.4. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Ueki M., Linn F., Hossmann K. A. Functional activation of cerebral blood flow and metabolism before and after global ischemia of rat brain. J Cereb Blood Flow Metab. 1988 Aug;8(4):486–494. doi: 10.1038/jcbfm.1988.89. [DOI] [PubMed] [Google Scholar]




