Abstract
Echinococcosis is one of the 17 neglected tropical diseases (NTDs) recognized by the World Health Organization. The two major species of medical importance are Echinococcus granulosus and Echinococcus multilocularis. E. granulosus affects over 1 million people and is responsible for over $3 billion in expenses every year. In this minireview, we discuss aspects of the epidemiology, clinical manifestations, and diagnosis of cystic echinococcosis or cystic hydatid disease caused by E. granulosus.
INTRODUCTION
Echinococcosis is a zoonotic infection caused by the larval stage of cestode species belonging to the genus Echinococcus. Although E. granulosus was initially regarded as the only causative agent of cystic echinococcosis (CE), it was clear that there were different taxa with differences in adult morphology, host specificity, and pathogenicity (1). Different strains of E. granulosus were identified to precisely portray their specificity for intermediate hosts (e.g., sheep, buffalo, horses, cattle, pigs, camels, and cervids). The lion strain, which was defined based on the definite host, was the exception. Recent advances in phylogenetic systematics have resulted in the recognition of nine species of Echinococcus: E. granulosus sensu stricto (G1 to G3), E. equinus (G4), E. ortleppi (G5), E. canadensis (G6 to G10), E. multilocularis, E. vogeli, E. oligarthrus, E. felidis, and E. shiquicus (1–3). The taxonomy of cystic echinococcosis continues to be under discussion and is far from being completed. For example, the taxonomic status of genotypes G6, G7, G8, and G10 of E. granulosus has not been solved (4).
Different species of Echinococcus cause different diseases in humans. Cystic echinococcosis (CE) is caused by E. granulosus sensu stricto, E. equinus, E. ortleppi, and E. canadensis. Alveolar echinococcosis is caused by E. multilocularis and polycystic echinococcosis by E. vogeli and E. oligarthrus. The most recently described species, E. shiquicus, is found in the Qinghai-Tibet plateau. It inhabits the small intestine of the Tibetan fox (Vulpes ferrilata), and the larval stage is found in the plateau black-lipped pika (Ochotona curzoniae). To date, no human cases of infection by E. shiquicus have been described, but its recent isolation from dogs in that region of China emphasizes the need to conduct studies to examine the possibility of human infections (5).
The parasites are maintained in nature by carnivores, which act as definitive hosts (a canine, felid, or hyenid), and by intermediate hosts, which are usually herbivores (e.g., sheep, goats, cattle, camels, and cervids) that harbor the larval stage of the parasite (metacestode). The adult egg-producing stage of the cestode inhabits the small intestine of the carnivore. The definitive host can be infected with hundreds of worms, with each worm producing thousands of eggs each day. The eggs, which are shed in the stool of the definitive host, are infective upon release. The eggs can remain infective for months or up to a year depending on environmental conditions. The eggs are sensitive to desiccation and heat but can survive freezing temperatures (6).
Once ingested by the intermediate host, the oncosphere hatches from the egg, penetrates the intestinal mucosa, and migrates through the bloodstream to internal organs such as the liver. A fluid-filled cyst (metacestode or hydatid cyst) develops in the affected organ after a period of time that can vary. Protoscolices bud from the germinal layer (see below) and develop within the cyst and, when ingested by a definitive host, evaginate and attach to the intestinal mucosa, developing into sexually mature adults in a period averaging 4 to 7 weeks. A single cyst can have thousands of protoscolices, and each protoscolex is capable of developing into an adult worm if ingested by the definitive host or, if the cystic fluid is spilled in a cavity such as the peritoneum, into a new cyst (secondary echinococcosis) (6). All mammals in which metacestodes develop act as intermediate hosts, but not all intermediate hosts perpetuate the life cycle. For example, humans are considered accidental or aberrant hosts as they are highly unlikely to be involved in disease transmission. Human-to-human transmission does not occur (6).
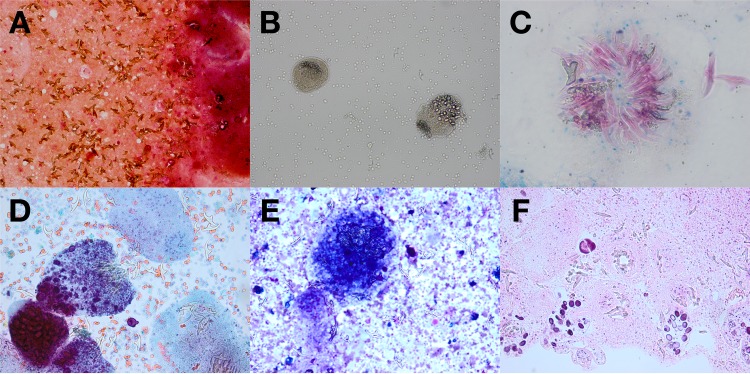
Cysts in each anatomic site, with the exception of bone, are composed of the periparasitic host tissue (pericyst), which encompasses the endocyst of larval origin. The endocyst has an outer, acellular laminated layer and an inner, or germinal, layer that gives rise to brood capsules and protoscolices. The cyst is filled with clear fluid and, when fertile, with numerous brood capsules and protoscolices. In some stages of cyst development, daughter vesicles of various sizes are present (6, 7). In cysts that are degenerating, one might see abundant free-floating hooklets. These structures represent the “hydatid sand” that sometimes can be seen during imaging procedures when the patient is asked to shift positions (see Fig. 1A).
FIG 1.
(A) Gram stain of cyst fluid (magnification, ×200). (B) Wet-mount preparation of cyst fluid (magnification, ×200). (C) Ziehl-Neelsen stain of cyst fluid (oil immersion; magnification, ×1,000). (D) Papanicolaou stain of cyst fluid (magnification, ×400; polarized). (E) Diff-Quick stain of cyst fluid (magnification, ×400; polarized). (F) Hematoxylin and eosin stain of formalin-fixed paraffin-embedded cell block of cyst fluid (magnification, ×200). (Images courtesy of Diane Hensel [panels A and B], Cindy McCloskey [panel C], and Rachel Conrad [panels D to F], University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA; reproduced with permission.)
The minimum time for development of protoscolices is unknown, but, on the basis of animal studies, it is estimated to be 10 months or longer after infection. The growth rate of the echinococcal cyst is poorly understood. In a few studies using ultrasound, such as an observational study carried out in Kenya, 43% of the cysts grew at a rate of 6 to 15 mm per year and 30% grew 1 to 5 mm per year, while approximately 16% of the cysts showed no growth or showed collapse. Cysts range in size from those that measure a few centimeters in diameter to much larger cysts containing up to 48 liters of fluid, also called giant cysts (6). It has been suggested that the size of a hydatid cyst can be related to the genotype. For example, a recent study showed that in infected patients, the average diameter of E. canadensis G7 liver cysts was 5.9 cm (range, 3 to 10 cm) compared to 10.7 cm (range, 5 to 21 cm) for E. granulosus (8).
EPIDEMIOLOGY
Cystic echinococcosis has a cosmopolitan distribution and represents a major public health problem in some regions (9). It is considered endemic in areas such as Peru, Chile, Argentina, Uruguay, southern Brazil, the Mediterranean region, central Asia, western China, and East Africa. Cystic echinococcosis is not found in Antarctica and has been eliminated through comprehensive control programs in Iceland, New Zealand, Tasmania, Falkland Islands, and Cyprus (10).
The G1 genotype of Echinococcus granulosus sensu stricto is responsible for the vast majority (88%) of human cases worldwide. It has a cosmopolitan distribution and is associated with transmission from sheep as an intermediate host (11). E. canadensis G6 and G7 are responsible for 7.3% and 3.7% of infections worldwide, respectively. There have been no human cases of E. equinus described in the literature (11).
The human incidence can exceed 50 per 100,000 person-years in areas of endemicity, and prevalence rates as high as 5% to 10% can be found in parts of Peru, Argentina, east Africa, and China (10). Every year, echinococcosis is responsible for the loss of at least 1 million disability-adjusted life years (DALYs) and of $3 billion dollars in expenses, including treatment and livestock losses (9).
Cystic echinococcosis typically occurs in poor pastoral regions in which sheep or other livestock are raised and in which dogs are kept, for herding or property guarding, in close proximity to households. Dogs in such regions are frequently fed offal, and, for religious and other reasons, their populations might not be curtailed (12). The prevalence of cystic echinococcosis increases with age, and women are affected more frequently than men; this might be related to domestic activities that bring them in closer contact with dogs through feeding, herding, or milking livestock (12).
CLINICAL MANIFESTATIONS
The incubation period and the clinical presentation of CE are highly variable. The latter is dependent upon several features such as the involved organ, the location of the cyst within the organ, and its relation with surrounding structures, its size, and the integrity of the wall. Other factors, such as the genotype, have been suggested to have a role. For example, cysts belonging to the cervid genotype (G8) have been reported to most frequently localize in the lung, tend to grow slowly, and are less likely to cause complications (13). A recent case series suggested that E. canadensis G6 might have a higher affinity for the human brain (14).
Cystic echinococcosis is usually asymptomatic unless complications occur. Rupture with resultant infection or anaphylaxis, fistula development with adjacent structures (e.g., in the biliary tract, intestine, and bronchus) or mass effect on neighboring structures are the major mechanisms by which a cyst usually becomes symptomatic (15). It is not uncommon for cysts to be discovered incidentally by imaging studies done for other reasons.
Most patients (40% to 80% of cases) have a single cystic lesion located in a single organ. The liver is affected in 70% of the cases, the right lobe more commonly than the left. The lung is the next most frequently affected organ and is affected in about 20% of the cases (6, 15). Cysts can localize in virtually any organ and structure, such as abdominal or pleural cavities, kidney, spleen, bone, brain, eye, ovary, testis, and pancreas (6, 15). Rare immune-mediated reactions such as urticaria, asthma, membranous nephropathy, and anaphylaxis have also been well described (15).
A proportion of patients present with cyst-related complications that require medical attention in a timely manner. For cysts located in the liver, a cysto-biliary fistula is the most common complication (13% to 37% of cases) (16). The high complication rate has been attributed to the increased intracystic pressure (30 to 80 cm H2O) compared to the low intracystic pressure present in nonparasitic hepatic cysts (17). Cysto-biliary fistulas can be classified as frank or occult. A frank cysto-biliary fistula is generally easy to diagnose, both clinically and radiographically. An occult cysto-biliary fistula is difficult to detect; if not identified preoperatively, the postoperative course is usually complicated. Several scoring systems have been used to predict its presence preoperatively (16, 18).
DIAGNOSIS
The diagnosis of cystic echinococcosis rests mainly on imaging. The World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) published in 2003 a standardized classification of ultrasound images based on the first classification developed by H. A. Gharbi, W. Hassine, M. W. Brauner, and K. Dupuch in 1981 (19). This classification is intended to be used both in the epidemiological field and in clinical settings to dictate stage-specific management.
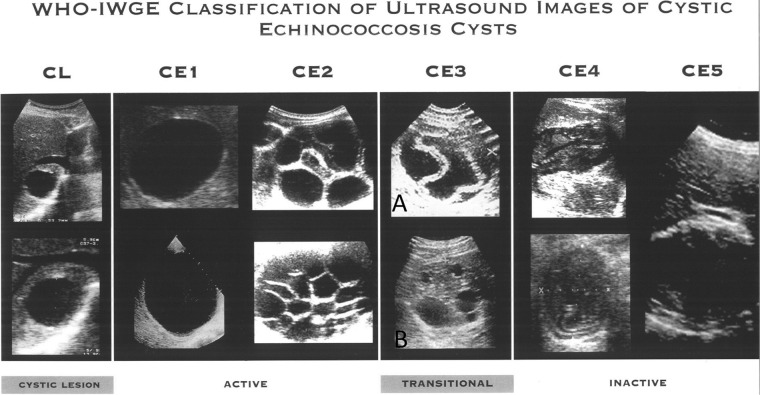
The classification allocates cystic echinococcosis cysts into three relevant groups: active (CE1 and CE2), transitional (CE3), and inactive (CE4 and CE5) (see Fig. 2). The cystic lesion (CL) stage consists of unilocular cystic lesions without pathognomonic signs on ultrasound and whose parasitic nature needs to be confirmed with further investigations. The group consisting of CE3 (transitional) cysts has recently been separated into CE3a (with detached endocyst) and CE3b (predominantly solid, with daughter vesicles) on the basis of their different metabolic profiles and different responses to nonsurgical treatments (20). CE3a cysts are true transitional cysts because they may be active or inactive, while CE3b cysts are active. This classification was developed on the basis of analysis of cystic echinococcosis cysts in the liver, but, in a number of cases, it can also be applied in the management and follow-up of cysts located elsewhere. One of the advantages of the classification is that, at least for hepatic CE, it facilitates a more rational treatment approach based on cyst stage and size (20).
FIG 2.
World Health Organization Informal Working Group on Echinococcosis classification of ultrasound images for cystic echinococcosis cysts (31). CE, cystic echinococcosis; CL, cystic lesion. “A” indicates CE3a, and “B” indicates CE3b (see the text for details). (Reprinted from reference 31 with permission of the publisher.)
Cysts that are not accessible to ultrasound can be studied using other imaging modalities such as computed tomography (CT) or magnetic resonance imaging (MRI). MRI is better than CT at detecting the structural, stage-defining features of cysts seen on ultrasound. There is a very good level of agreement between ultrasound imaging and MRI for liver cystic echinococcosis stages CE1 to CE4. MRI has the shortcoming of being unable to identify certain details of the cyst wall such as calcification. If an ultrasound cannot be performed, a MRI with T2-weighted sequences (in particular, True Fisp and HASTE [half-Fourier acquisition single-shot turbo spin echo] sequences) should be obtained as these sequence best detect liquid content in the cyst matrix (21).
MRI imaging with MR cholangiopancreatography (MCRP) also has a role in the preoperative evaluation of complications such as cysto-biliary fistulas. It performs as well as endoscopic retrograde cholangiopancreatography (ERCP) in the recognition of biliary obstruction and is not invasive. Cystobiliary fistulas become apparent only after rupture of the endocyst (mostly stages CE2 and CE3b), and the sensitivity and specificity of MRCP to detect a cysto-biliary fistula in these stages are 75% and 95%, respectively (22).
Clinical laboratory analysis, including chemistry and hematology testing, is nonspecific in patients with cystic echinococcosis. For those with biliary obstruction, elevated levels of bilirubin, transaminases, and gamma-glutamyl transferase may be observed. In the setting of cyst leakage into the biliary tree or cyst rupture, significant elevation in gamma-glutamyl transferase and alkaline phosphatase may also be observed, together with eosinophilia, which is usually absent in intact cysts.
Immunodiagnostics play an ancillary role in diagnosis due to limitations in sensitivity and specificity. However, serology may be useful to support or confirm a diagnosis of cystic echinococcosis. World Health Organization/World Organisation for Animal Health recommendations include sequential testing based on a screening and confirmatory test model (4). Primary screening methodologies include enzyme-linked immunosorbent assays (ELISA), indirect hemagglutination antibody tests (IHAT), latex agglutination (LAT), immunofluorescence antibody tests (IFAT), and immunoelectrophoresis (IEP), with ELISA being the most common. These methods have various levels of sensitivity and somewhat poor specificity, especially in patients with other helminthic infections. For many assays, the antigen material used for testing is a crude or purified preparation from animal liver hydatid cysts and is likely a significant source of variability in test performance (6, 23).
Many factors, including technical issues such as the quality of the antigen preparation mentioned above, as well as host factors, such as the immune status of the subject, can influence assay sensitivity. The sensitivity of the test is also dependent on the integrity of the cyst wall and the development stage of the cyst. Early cysts, such as CE1 cysts, usually have their antigens sequestered from the host's immune system and might present with a negative serologic test result, and patients with inactive CE4 and CE5 cysts are also often seronegative. Cystic lesions located in sanctuary sites, such as the eye or brain, are also usually missed by serologic evaluation. It is therefore important to remember that a negative serologic test result does not exclude the presence of cystic echinococcosis. Serology is positive in around 80% to 94% of hepatic echinococcosis cases and 65% of pulmonary cases (23).
Significant cross-reactivity is seen with other parasitic conditions, including alveolar echinococcosis, cysticercosis, fascioliasis, and filariasis, as well as with other nonparasitic conditions, including malignancy. Patients with reactive primary screening serology should have a confirmatory test. Recommended methods for secondary testing include immunoblot assays to test for reactivity with E. granulosus antigen subunits, identification of specific IgG subclasses (i.e., IgG1 and/or IgG4), and arc 5 precipitation testing. Although specificity is much improved with these assays compared to primary test methods, cross-reactivity with alveolar echinococcosis and cysticercosis is not completely eliminated (6). The use of recombinant antigens for serodiagnosis has also been studied and was shown to improve sensitivity over the use of native antigens or their purified subunits, but further research is needed to improve the clinical usefulness of serodiagnosis (24).
New testing modalities that could be used in small laboratories to facilitate epidemiologic studies in countries where the disease is endemic are being developed. A loop-mediated isothermal amplification (LAMP) assay is such an example. The assay is able to accurately detect 5 different species of Echinococcus (E. granulosus sensu stricto, E. equinus, E. ortleppi, E. canadensis, and E. felidis) (25).
In patients requiring diagnostic aspiration (rarely indicated), the cyst fluid can be examined microscopically to confirm the detection of Echinococcus spp. by observing protoscolices and/or free hooklets. A wet unstained mount procedure is simple to perform and is often adequate for diagnosis. Several staining methods, including those employing Ziehl-Neelsen stain, Wheatley trichrome or Ryan trichrome blue stain, Baxby stain, and modified Baxby stain, can improve the visualization of hooklets (whether isolated or associated with protoscolices) (26). Cyst material can also be identified on histologic tissue sections (see Fig. 1B to F). The acellular laminated layer and thin cellular, germinal layer with brood capsules and protoscolices can often be visualized in mature echinococcal cysts.
TREATMENT AND PREVENTION
The management of cystic echinococcosis is complex and is beyond the scope of this review. The WHO-IWGE published an “Expert Consensus for the Diagnosis and Treatment of Cystic and Alveolar Echinococcosis in Humans” in 2010 (20). Given the lack of trials comparing the different treatment options, the recommendations are based on the opinions of experts in the field. Even among those respected authorities, there is great variation in the management of the disease worldwide (27).
Surgery has been considered the gold standard, but alternatives exist for selected patients and are now considered the first management options. In general, there are four different management modalities: percutaneous therapy, surgery, chemotherapy, and observation without intervention (watch and wait). The expertise of the personnel and the availability of resources, the stage, size, and location of the cyst, and the presence of symptoms/complications are the main elements taken into consideration in choosing among these options. There is no test of cure, and long-term follow-up with imaging is required to evaluate the efficacy of treatment, as serology results may remain positive for years even after successful treatment. When possible, patients should be evaluated in a reference center.
Although benzimidazoles have been the cornerstone of medical therapy since the late 1980s, many issues (e.g., duration of therapy) remain unresolved (28). Many other compounds have been tested experimentally without success, and, despite the effect of the synergy between benzimidazoles and other agents such as metformin seen in vitro, there is still a great need to invest in the development of new chemotherapeutic agents (28, 29).
The prevention of the disease depends on the interruption of the life cycle of E. granulosus. For example, regular screening and treatment of infected dogs have been successful in eradicating the disease in areas of endemicity such as New Zealand (10). Restricting the feeding of home-slaughtered livestock to dogs and vaccinating the intermediate host (e.g., sheep) are other available control measures.
CONCLUSION
Cystic echinococcosis is a complex disease. It continues to be a major public health problem in many countries despite being, in principle, preventable, treatable, and eradicable. There are many unanswered questions and unresolved problems. For example, the evidence available to guide treatment is poor and well-designed trials that could guide therapy are overdue. In the interim, adoption of the stage-specific approach advocated by the WHO-IWGE, at least for liver CE, would greatly reduce mismanagement (27). There is also a clear need for research into development of diagnostics and prevention/control programs that takes into account the social, political, and economical situation of the affected communities (30).
Biography

Nelson Iván Agudelo Higuita is an Assistant Professor of Medicine at the University of Oklahoma Health Sciences Center. He also serves as the Associate Program Director for the Internal Medicine Residency Program. He earned his doctoral degree from the Autonomous University of Honduras. He then completed a residency in Internal Medicine and a fellowship in Infectious Diseases, both at the University of Oklahoma Health Sciences Center. He has special interest in tropical diseases and other geographic and travel-related infections.
Funding Statement
This work was partly funded by the European Union through grant no. 602051 (project title: HERACLES—Human cystic Echinococcosis ReseArch in CentraL and Eastern Societies), FP7-HEALTH-2013-INNOVATION-1 (to E. Brunetti).
REFERENCES
- 1.Nakao M, Lavikainen A, Yanagida T, Ito A. 2013. Phylogenetic systematics of the genus Echinococcus (Cestoda: Taeniidae). Int J Parasitol 43:1017–1029. doi: 10.1016/j.ijpara.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Xiao N, Qiu J, Nakao M, Li T, Yang W, Chen X, Schantz PM, Craig PS, Ito A. 2005. Echinococcus shiquicus n. sp., a taeniid cestode from Tibetan fox and plateau pika in China. Int J Parasitol 35:693–701. doi: 10.1016/j.ijpara.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Hüttner M, Nakao M, Wassermann T, Siefert L, Boomker JD, Dinkel A, Sako Y, Mackenstedt U, Romig T, Ito A. 2008. Genetic characterization and phylogenetic position of Echinococcus felidis (Cestoda: Taeniidae) from the African lion. Int J Parasitol 38:861–868. doi: 10.1016/j.ijpara.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Lymbery AJ, Jenkins EJ, Schurer JM, Thompson RC. 2015. Echinococcus canadensis, E borealis, and E intermedius. What's in a name? Trends Parasitol 31:23–29. doi: 10.1016/j.pt.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Boufana B, Qiu J, Chen X, Budke CM, Campos-Ponce M, Craig PS. 2013. First report of Echinococcus shiquicus in dogs from eastern Qinghai-Tibet plateau region, China. Acta Trop 127:21–24. doi: 10.1016/j.actatropica.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Eckert J, Gemmell MA, Meslin F-X, Pawłowski ZS (ed). 2001. WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health (Office International des Epizooties), Paris, France, and World Health Organization, Geneva, Switzerland. [Google Scholar]
- 7.Díaz A, Casaravilla C, Allen JE, Sim RB, Ferreira AM. 2011. Understanding the laminated layer of larval Echinococcus II: immunology. Trends Parasitol 27:264–273. doi: 10.1016/j.pt.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Schneider R, Gollackner B, Schindl M, Tucek G, Auer H. 2010. Echinococcus canadensis G7 (pig strain): an underestimated cause of cystic echinococcosis in Austria. Am J Trop Med Hyg 82:871–874. doi: 10.4269/ajtmh.2010.09-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budke CM, Deplazes P, Torgerson PR. 2006. Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis 12:296–303. doi: 10.3201/eid1202.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig PS, McManus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, Gavidia CM, Gilman RH, Gonzalez AE, Lorca M, Naquira C, Nieto A, Schantz PM. 2007. Prevention and control of cystic echinococcosis. Lancet Infect Dis 7:385–394. doi: 10.1016/S1473-3099(07)70134-2. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez Rojas CA, Romig T, Lightowlers MW. 2014. Echinococcus granulosus sensu lato genotypes infecting humans–review of current knowledge. Int J Parasitol 44:9–18. doi: 10.1016/j.ijpara.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Craig PS, Li T, Qiu J, Zhen R, Wang Q, Giraudoux P, Ito A, Heath D, Warnock B, Schantz P, Yang W. 2008. Echinococcosis and Tibetan communities. Emerg Infect Dis 14:1674–1675. doi: 10.3201/eid1410.071636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moro P, Schantz PM. 2006. Cystic echinococcosis in the Americas. Parasitol Int 55(Suppl):S181–S186. doi: 10.1016/j.parint.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 14.Sadjjadi SM, Mikaeili F, Karamian M, Maraghi S, Sadjjadi FS, Shariat-Torbaghan S, Kia EB. 2013. Evidence that the Echinococcus granulosus G6 genotype has an affinity for the brain in humans. Int J Parasitol 43:875–877. doi: 10.1016/j.ijpara.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Ammann RW, Eckert J. 1996. Cestodes echinococcus. Gastroenterol Clin North Am 25:655–689. doi: 10.1016/S0889-8553(05)70268-5. [DOI] [PubMed] [Google Scholar]
- 16.Demircan O, Baymus M, Seydaoglu G, Akinoglu A, Sakman G. 2006. Occult cystobiliary communication presenting as postoperative biliary leakage after hydatid liver surgery: are there significant preoperative clinical predictors? Can J Surg 49:177–184. [PMC free article] [PubMed] [Google Scholar]
- 17.Yalin R, Aktan AO, Yegen C, Dosluoglu HH. 1992. Significance of intracystic pressure in abdominal hydatid disease. Br J Surg 79:1182–1183. doi: 10.1002/bjs.1800791127. [DOI] [PubMed] [Google Scholar]
- 18.Saylam B, Coskun F, Demiriz B, Vural V, Comcali B, Tez M. 2013. A new and simple score for predicting cystobiliary fistula in patients with hepatic hydatid cysts. Surgery 153:699–704. doi: 10.1016/j.surg.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Anonymous. 2003. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop 85:253–261. doi: 10.1016/S0001-706X(02)00223-1. [DOI] [PubMed] [Google Scholar]
- 20.Brunetti E, Kern P, Vuitton DA. 2010. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 114:1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Stojkovic M, Rosenberger K, Kauczor HU, Junghanss T, Hosch W. 2012. Diagnosing and staging of cystic echinococcosis: how do CT and MRI perform in comparison to ultrasound? PLoS Negl Trop Dis 6:e1880. doi: 10.1371/journal.pntd.0001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosch W, Stojkovic M, Janisch T, Heye T, Werner J, Friess H, Kauffmann GW, Junghanss T. 2008. MR imaging for diagnosing cysto-biliary fistulas in cystic echinococcosis. Eur J Radiol 66:262–267. doi: 10.1016/j.ejrad.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Biava MF, Dao A, Fortier B. 2001. Laboratory diagnosis of cystic hydatic disease. World J Surg 25:10–14. doi: 10.1007/s002680020002. [DOI] [PubMed] [Google Scholar]
- 24.Hernández-González A, Santivanez S, Garcia HH, Rodriguez S, Munoz S, Ramos G, Orduna A, Siles-Lucas M. 2012. Improved serodiagnosis of cystic echinococcosis using the new recombinant 2B2t antigen. PLoS Negl Trop Dis 6:e1714. doi: 10.1371/journal.pntd.0001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wassermann M, Mackenstedt U, Romig T. 2014. A loop-mediated isothermal amplification (LAMP) method for the identification of species within the Echinococcus granulosus complex. Vet Parasitol 200:97–103. doi: 10.1016/j.vetpar.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Clavel A, Varea M, Doiz O, Lopez L, Quilez J, Castillo FJ, Rubio C, Gomez-Lus R. 1999. Visualization of hydatid elements: comparison of several techniques. J Clin Microbiol 37:1561–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nabarro LE, Amin Z, Chiodini PL. 2015. Current management of cystic echinococcosis: a survey of specialist practice. Clin Infect Dis 60:721–728. doi: 10.1093/cid/ciu931. [DOI] [PubMed] [Google Scholar]
- 28.Vuitton DA. 2009. Benzimidazoles for the treatment of cystic and alveolar echinococcosis: what is the consensus? Expert Rev Anti Infect Ther 7:145–149. doi: 10.1586/14787210.7.2.145. [DOI] [PubMed] [Google Scholar]
- 29.Loos JA, Cumino AC. 2015. In vitro anti-echinococcal and metabolic effects of metformin involve activation of AMP-activated protein kinase in larval stages of Echinococcus granulosus. PLoS One 10:e0126009. doi: 10.1371/journal.pone.0126009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunetti E, Garcia HH, Junghanss T. 2011. Cystic echinococcosis: chronic, complex, and still neglected. PLoS Negl Trop Dis 5:e1146. doi: 10.1371/journal.pntd.0001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. 2001. PAIR: puncture, aspiration, injection, re-aspiration—an option for the treatment of cystic echinococcosis. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/67207/1/WHO_CDS_CSR_APH_2001.6.pdf. [Google Scholar]