Abstract
Accurate detection of carbapenemase-producing Gram-negative bacilli is of utmost importance for the control of nosocomial spread and the initiation of appropriate antimicrobial therapy. The modified Hodge test (MHT), a carbapenem inactivation assay, has shown poor sensitivity in detecting the worldwide spread of New Delhi metallo-β-lactamase (NDM). Recent studies demonstrated that NDM is a lipoprotein anchored to the outer membrane in Gram-negative bacteria, unlike all other known carbapenemases. Here we report that membrane anchoring of β-lactamases precludes detection of carbapenemase activity by the MHT. We also show that this limitation can be overcome by the addition of Triton X-100 during the test, which allows detection of NDM. We propose an improved version of the assay, called the Triton Hodge test (THT), which allows detection of membrane-bound carbapenemases with the addition of this nonionic surfactant. This test was challenged with a panel of 185 clinical isolates (145 carrying known carbapenemase-encoding genes and 40 carbapenemase nonproducers). The THT displayed test sensitivity of >90% against NDM-producing clinical isolates, while improving performance against other carbapenemases. Ertapenem provided the highest sensitivity (97 to 100%, depending on the type of carbapenemase), followed by meropenem (92.5 to 100%). Test specificity was not affected by the addition of Triton (87.5% and 92.5% with ertapenem and meropenem, respectively). This simple inexpensive test confers a large improvement to the sensitivity of the MHT for the detection of NDM and other carbapenemases.
INTRODUCTION
Detection of carbapenemase producers in clinical laboratories is of major importance to define appropriate empirical antimicrobial therapy and to implement infection control measures. Acquired carbapenemases belong to three of the four known classes of β-lactamases, namely, Ambler class A (KPC, SME, NMC-A, IMI-1, and some allelic variants of GES), Ambler class B or metallo-β-lactamases (MBLs) (e.g., VIM, IMP, NDM, and SPM), and Ambler class D or oxacillinases (OXAs) (e.g., OXA-48 and OXA-181) (1).
The modified Hodge test (MHT) is a phenotypic screening test to identify carbapenemase producers, being recommended by the Clinical and Laboratory Standards Institute (CLSI) for Enterobacteriaceae with elevated carbapenem MICs or reduced disk diffusion inhibition zones (2). This test is based on the inactivation of a carbapenem by carbapenemase-producing strains, which enables a susceptible indicator strain to extend growth toward a disk containing this antibiotic, along the streak of inoculum of the tested strain. The MHT has shown excellent sensitivity in the detection of class A and class D carbapenemase producers (3–6). Unfortunately, the MHT performs poorly in the detection of NDM-producing isolates, with sensitivity below 50% (3–7). Because NDMs are Zn(II)-dependent enzymes, it has been suggested that the deficits of this cation in commercial media could be responsible for these false-negative results (4). Indeed, Zn(II) availability has been shown to be crucial for bacterial fitness when resistance to antibiotics depends on class B enzymes (8). However, supplementation of culture media with up to 100 μg/ml zinc sulfate failed to reverse these false-negative results (4, 9), suggesting the presence of other mechanisms responsible for this deficient performance.
Recent experiments have shown that NDM-1 is a lipoprotein anchored to the outer membrane in Gram-negative bacteria, unlike all other known carbapenemases, which have been characterized as soluble periplasmic enzymes (10, 11). This cellular localization is consistent with the presence of a canonical lipidation sequence (LSGC), called the lipobox, proximal to the signal peptide of NDM-1 (and all NDM variants) (10). In this work, we show that false-negative results with the MHT can be attributed to membrane anchoring of NDM. We propose a simple improvement of the MHT, called the Triton Hodge test (THT), which allows detection of these membrane-bound carbapenemases by addition of a nonionic surfactant during the test.
MATERIALS AND METHODS
Bacterial isolates. (i) Isogenic Escherichia coli DH5α strains.
Isogenic E. coli DH5α strains harboring native and chimeric variants of the NDM-1 and VIM-2 genes with different cellular localizations were used to explore the effects of membrane anchoring of MBLs on the performance of the MHT. E. coli DH5α was used for expression of plasmid pMBLe containing blaNDM-1 or blaVIM-2, which retain the native peptide leader of each β-lactamase (see below). MBL mutants NDM-1 C26A (NDM-1 in which the lipobox was disrupted by replacing Cys with Ala at the indicated position), V-NDM-1 (NDM-1 in which the first 47 amino acids were replaced by the first 42 residues of VIM-2), and N-VIM-2 (VIM-2 in which the first 42 amino acids were replaced by the first 47 residues of NDM-1) were constructed and subcloned into plasmid pMBLe (see below). These plasmids allow expression of membrane-bound variants, i.e., native NDM-1 and N-VIM-2, and soluble periplasmic variants, i.e., native VIM-2, NDM-1 C26A, and V-NDM-1 (11).
Briefly, full-length blaNDM-1 and blaVIM-2 (including their native peptide leaders) were amplified using the following primers and subcloned into the NdeI and HindIII sites of pMBLe: blaNDM-1, NDM1NdeIFw (5′-TATACATATGGAATTGCCCAATATTATGCACC-3′) and NDM1HindIIIRv (5′-GACGTAAGCTTCTAGCGCAGCTTGTCGGC-3′); blaVIM-2, VIM2NdeIFw (5′-GACATCATATGTTCAAACTTTTGAGTAAGTTATTGGTC-3′) and VIM2HindIIIRv (5′-GACGTAAGCTTCTACTCAACGACTGAGCGATTTGTG-3′). All PCRs were carried out using Platinum Pfx DNA polymerase (Invitrogen) with the following thermal cycle: 3 min at 95°C, 30 cycles of 15 s at 95°C, 30 s at 55°C, and 1 min at 68°C, and 10 min at 68°C. The NDM-1 C26A mutant gene was generated from pMBLe-blaNDM-1 by site-directed mutagenesis, as described previously (12), using the primers NDM-1-C26AFw (5′-CATTGATGCTGAGCGGGGCGATGCCCGGTGAAATC-3′) and NDM-1-C26ARv (5′-GATTTCACCGGGCATCGCCCCGCTCAGCATCAATG-3′). V-NDM-1 and N-VIM-2 were constructed by overlap-extension PCR using the overlapping primers VIM2-B (5′-ATTCGGTGCGAGCTGGCGGAAAACCAGATCCCCGACCGGAATTTCGC-3′), NDM1-C (5′-GATCTGGTTTTCCGCCCAGCTCGCACCG-3′), NDM1-D (5′-ACCATCGGCAATCTGGTAAAGCCGGACCTCGCCAAACCGTTGGTCGCC-3′), and VIM2-E (5′-GAGGTCCGGCTTTACCAGATTGCCG-3′), with external primers VIM2NdeIFw, NDM1NdeIFw, VIM2StHindIIIRv, and NDM1StHindIIIRv. All constructs were verified by DNA sequencing (University of Maine). The plasmid pMBLe was subsequently introduced into E. coli DH5α as described previously (13).
(ii) Panel of clinical isolates.
A total of 185 clinical isolates were included (145 isolates carrying known carbapenemase-encoding genes and 40 isolates without carbapenemase production). The carbapenemases represented were the class A carbapenemases KPC-2, KPC-3, GES-3, GES-5, NMC-A, and SME-1b (n = 25), the class B carbapenemases NDM-1, IMP-1, IMP-8, IMP-13, IMP-16, IMP-18, SPM-1, VIM-1, VIM-2, and VIM-11 (n = 100), and the class D carbapenemases OXA-48, OXA-163, OXA-181, OXA-247, and OXA-438 (n = 20). Strains were isolated from clinical specimens, as follows: urine, 35%; blood, 35%; respiratory tract, 15%; other sites (e.g., bone, abdomen, and cerebrospinal fluid), 15%. Only a single isolate per patient was included in the panel. The isolates, belonging to the collection of the National and Regional Reference Laboratory (INEI) for the Latin American region, represent submissions from very diverse locations (21 countries and 1,226 laboratories surveyed in 2010 to 2014) and thus are expected to display minimum clonal and enzyme bias. When pulsed-field gel electrophoresis (PFGE) results were available (14), strains included were nonclonal. Panel isolates were previously identified using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Microflex; Bruker, Germany). Only strains that met the score cutoff values recommended by the manufacturer for species-level identification (scores of ≥2.000) and that showed ≥10% score differences between the first two best matches in the database (15) were included in the study. Proteus isolates were excluded due to their frequent swarming during the assays, which prevented interpretation of the phenotypic confirmatory tests.
Antimicrobial susceptibility testing.
The MICs of imipenem, meropenem, and ertapenem were determined by the broth microdilution method (with a panel prepared in-house), according to CLSI guidelines (2).
Characterization of mechanisms of resistance.
PCR analysis followed by DNA sequencing of the amplicons was considered the standard method for characterization of β-lactamases. Strains were analyzed for blaNDM, blaVIM, blaIMP, blaSPM, blaKPC, blaOXA-48-like, blaSME, blaIMI/NMC-A, blaGES, blaCTX-M, blaPER, and blaCMY as described previously (16, 17). Outer membrane porin profiles of non-carbapenem-susceptible, carbapenemase nonproducers were determined by SDS-PAGE (18, 19). Overexpression of chromosomal AmpC was evaluated by spectrophotometric analyses, as described previously (20).
Cell fractionation and NDM-1 detection.
Lysogeny broth medium (25 ml) was inoculated with E. coli DH5α pMBLe NDM-1, Providencia rettgeri 15758, Serratia marcescens 17468, or Enterobacter cloacae 17464, and cells were grown at 37°C, with shaking, to an optical density at 600 nm (OD600) of 1 (in the case of E. coli DH5α, expression of NDM-1 was induced, at an OD600 of 0.4, by the addition of 50 μM isopropyl-β-d-thiogalactopyranoside [IPTG]). Cells were pelleted, resuspended in 10 mM HEPES, 200 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF) (pH 7.4), and disrupted by sonication. Cell debris was then removed by centrifugation at 14,000 × g for 20 min at 4°C, and total protein concentrations were determined using the Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Scientific). Equal amounts of cleared homogenates (5 mg total protein) were subjected to ultracentrifugation at 150,000 × g for 1 h at 4°C. Membrane (pellet) and soluble (supernatant) fractions were separated and concentrated 5-fold for electrophoresis. NDM-1 protein was detected in bacterial fractions by SDS-PAGE followed by Western blotting with polyclonal antibodies against NDM-1 (kindly provided by Robert Bonomo, Case Western Reserve University, Cleveland, OH) and immunoglobulin G-alkaline phosphatase conjugates.
Phenotypic confirmatory assays. (i) MHT.
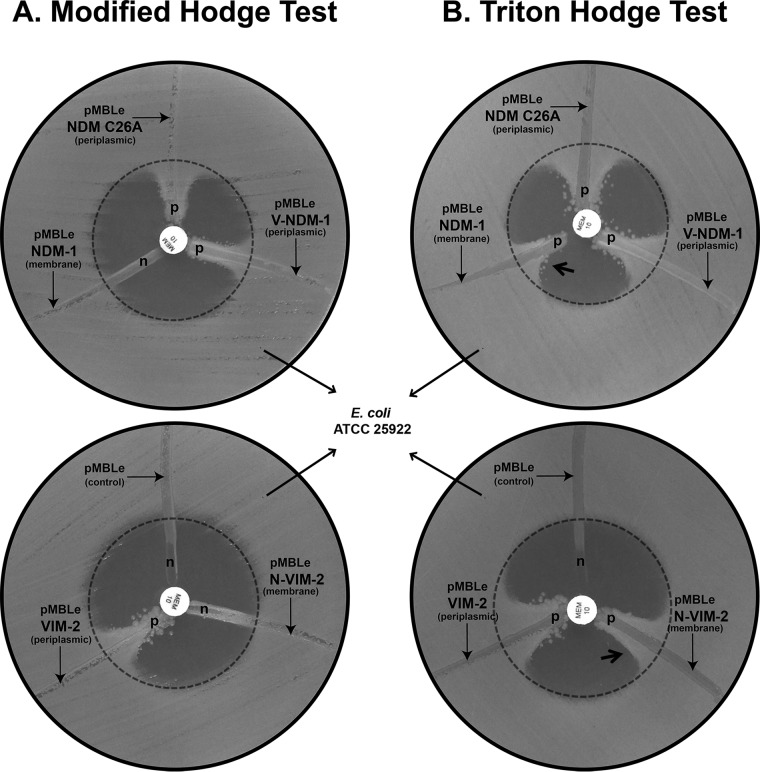
The MHT was performed as described previously (2). Briefly, a 1:10 dilution of an inoculum of the indicator organism E. coli ATCC 25922, adjusted to a 0.5 McFarland turbidity standard, was used to inoculate the surfaces of plates containing Mueller-Hinton agar (MHA) (Becton Dickinson, BBL), by swabbing. After the plates were allowed to stand at room temperature for 10 min, disks (BBL) containing meropenem (10 μg) or ertapenem (10 μg) were placed on the agar plates. Subsequently, three to five colonies of the test organisms (from an agar plate grown overnight) were inoculated onto the plate in a straight line out from the edge of the disk, using a 10-μl loop. Plates were examined after overnight incubation at 35°C. For carbapenem hydrolysis screening, growth of the indicator strain toward the carbapenem disk was interpreted as a positive or weakly positive result, depending on the magnitude of the enhanced growth (measured with a ruler, as described previously [21]). Isolates that allowed growth of the indicator strain up to 3 mm were recorded as weakly positive, while those with growth of >3 mm were labeled positive. The choice of this cutoff value was based on the fact that, in our experience, most discrepancies in result interpretation occur in cases in which growth of the indicator strain is less than 3 mm. The absence of growth of the indicator strain toward the carbapenem disks was interpreted as a negative result. For test isolates that produced substances that inhibited the growth of the indicator strain (a clear area was seen around the streak), the MHT result was recorded as uninterpretable. Two laboratory staff members read all test results independently (discrepancies were resolved by a third observer). Figure 1 illustrates the components of the MHT.
FIG 1.
Results of the modified Hodge test (MHT) (A) and the Triton Hodge test (THT) (B) for Escherichia coli DH5α strains harboring wild-type and mutant variants of NDM-1 and VIM-2 genes. Meropenem (MEM) (10 μg) was used as the substrate. The cellular localization (membrane or periplasmic) of the MBLs is depicted in parentheses. p, meropenem was hydrolyzed by the streaked cells; n, meropenem was not hydrolyzed by the streaked cells. The arrow pointing towards indentation indicates strains with a negative MHT that became positive for the THT. For more technical details, refer to reference 2, where the MHT components and test interpretation guidelines are included.
(ii) Triton Hodge test.
For solubilization of membrane proteins, the MHT was performed on a MHA plate (Becton Dickinson, BBL) flooded with 50 μl of pure Triton X-100 reagent (0.2% [vol/vol] in the MHA plate). Briefly, the detergent was dripped in the center of the plate and quickly distributed by streaking a swab over the entire sterile agar surface 4 to 6 times, until the detergent was completely absorbed. Delays of more than 10 min in streaking the Triton X-100 might alter the agar surface around the Triton X-100 drop. Flooded plates were stored at 4°C until use. Before inoculation with the indicator organism, excess surface moisture was removed by evaporation at 35°C. E. coli DH5α laboratory strains were included as controls in each THT assay performed. In addition to meropenem and ertapenem, we used imipenem, a substrate not included in CLSI recommendations for the MHT (2), in the standardization of the THT, with the final aim of looking for the optimal test conditions. However, imipenem showed more false-positive results than other carbapenems in preliminary assays and therefore was excluded from further analysis. The THT was also challenged with non-Enterobacteriaceae isolates, which are not included in the CLSI recommendations for the MHT (2). Test result interpretation was performed as defined for the MHT (see above).
MHA used in phenotypic confirmatory assays.
A method comparison between MHA batches with different Zn(II) concentrations was performed with isolates expressing different MBLs. We included a batch of MHA from Laboratorios Britania (Argentina) with a Zn(II) concentration of 14.6 ± 0.5 ppm (wt/wt) in the dehydrated medium or 0.54 to 0.57 μg/ml in the hydrated medium (as declared in the certificate of analysis and determined by atomic absorption-acetylene flame by a university reference laboratory at the Physics School, Faculty of Pharmacy and Biochemistry, University of Buenos Aires) and the reference BD/BBL batch, recommended as reference MHA for MBL detection (20), with a Zn(II) concentration of 23.5 ± 0.5 ppm (wt/wt) in the dehydrated medium or 0.87 to 0.91 μg/ml in the hydrated medium, according to data provided by the university reference laboratory.
Triton plate stability assay.
Triton-flooded agar plates were stored in sealed packages at 4°C and examined every 2 weeks with one NDM-1-producing P. rettgeri isolate, one OXA-48-producing E. coli isolate, one KPC-2-producing Klebsiella pneumoniae isolate, and one carbapenemase nonproducer (a CTX-M-15-producing K. pneumoniae clinical isolate).
RESULTS
Membrane anchoring of MBLs gives rise to false-negative MHT results.
NDM-1 is bound to the outer membrane in its native form. In order to explore the impact of MBL membrane anchoring on the performance of the MHT, we tested E. coli DH5α strains with soluble and membrane-anchored variants of NDM-1 and VIM-2. We challenged the MHT with an E. coli DH5α strain expressing membrane-bound NDM-1 and two E. coli DH5α strains expressing soluble (periplasmic) variants of NDM-1, namely, NDM-1 C26A, containing a mutation in the lipidation site that precludes membrane anchoring, and V-NDM-1, a chimera of NDM-1 and the N-terminal peptide leader of VIM-2, which also gives rise to a soluble enzyme. As shown in Fig. 1A, the MHT results were negative for membrane-bound NDM-1 but positive for both E. coli strains expressing the soluble NDM-1 variants. To validate these findings, we tested an E. coli DH5α strain expressing VIM-2 in its native soluble form, compared to an isogenic strain expressing N-VIM-2. N-VIM-2 is a membrane-anchored variant of VIM-2 resulting from replacement of the native signal peptide of VIM-2 by that of NDM-1, including the lipidation site. The strain expressing soluble VIM-2 gave a clear positive MHT result. Conversely, the strain expressing the chimeric membrane-anchored N-VIM-2 presented a negative MHT result (Fig. 1A). These experiments clearly show that membrane anchoring of β-lactamases to the bacterial membrane gives rise to false-negative MHT results.
Detaching membrane-bound MBLs with a nonionic surfactant improves MHT performance.
We aimed to solubilize NDM-1 by adding a nonionic detergent. We tested E. coli DH5α strains expressing soluble and membrane-anchored variants of NDM-1 and VIM-2 on MHA plates that had been treated previously with Triton X-100. Carbapenemase-like patterns were clearly observed for E. coli DH5α strains expressing the membrane-bound NDM-1 and N-VIM-2 chimera upon the addition of Triton (Fig. 1B), in contrast to the negative results observed in the absence of detergent (Fig. 1A). As expected, isogenic E. coli DH5α strains expressing the soluble enzymes VIM-2, NDM-1 C26A variant, and V-NDM-1 also tested positive with the MHT in the presence of detergent (Fig. 1B). These results suggest that the addition of a nonionic detergent to the test plate can reverse the false-negative MHT results observed for membrane-bound β-lactamases, probably through release of the lipid anchor. We propose naming this modification the Triton Hodge test (THT).
Cellular localization of NDM-1 in clinical isolates.
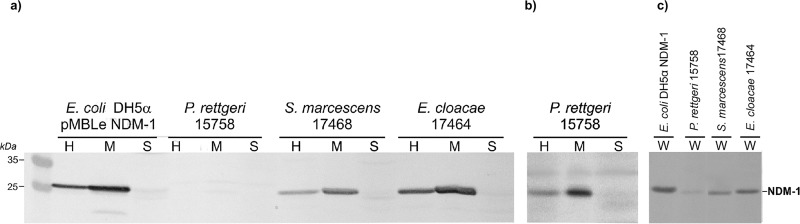
We evaluated the cellular localization of NDM-1 in different species of clinical isolates, to validate the generality of this approach. NDM-1 was detected in the membrane fraction of Providencia rettgeri, Serratia marcescens, and Enterobacter cloacae clinical isolates (Fig. 2), as observed for the model E. coli DH5α strain. In contrast, no traces of NDM-1 could be detected in the soluble periplasmic fraction of any of these strains. P. rettgeri M15758 showed visibly smaller amounts of NDM-1, compared to the other two tested strains, as evidenced in both whole cells and membrane fractions. Among these strains, only E. cloacae M17464 tested positive (indentation of <3 mm) for carbapenemase activity by the MHT (Table 1). These results encouraged us to evaluate the use of Triton to improve NDM detection in clinical strains.
FIG 2.
Membrane localization of NDM-1 in laboratory strain E. coli DH5α pMBLe NDM-1 and clinical strains P. rettgeri 15758, S. marcescens 17468, and E. cloacae 17464. Western blot detection of NDM-1 in the membrane (M) and soluble (S) fractions derived from bacterial homogenates (H), using standard (a) or extended (from 5 to 20 min) (b) colorimetric reaction of the secondary antibody, and in whole cells (W) (c) is shown.
TABLE 1.
Detection of clinical isolates producing NDM-1 carbapenemase using the modified Hodge test and the Triton Hodge test
| Species | Isolate | Acquired β-lactamase(s) | MIC (μg/ml)a |
Assay resultb |
|||||
|---|---|---|---|---|---|---|---|---|---|
| MHT |
THT |
||||||||
| IMP | MEM | ERT | MEM | ERT | MEM | ERT | |||
| Acinetobacter baumannii (n = 3) | 17042 | NDM-1 | ≥16 | ≥16 | ND | − | − | + | + |
| 17232 | NDM-1 | ≥16 | ≥16 | ND | − | + (weak)c | + | + | |
| 17575 | NDM-1 | ≥16 | ≥16 | ND | + (weak) | + | + | + | |
| Acinetobacter pittii (n = 2) | 15274 | NDM-1 | ≥16 | ≥16 | ≥2 | − | − | + | + |
| 15373 | NDM-1 | ≥16 | ≥16 | ≥2 | − | − | + | + | |
| Citrobacter amalonaticus (n = 1) | 19108 | NDM-1 | ≥16 | 8 | ≥2 | − | − | + | + |
| Citrobacter braakii (n = 1) | 19329 | NDM-1 | ≥16 | ≥16 | ≥2 | − | − | + | + |
| Citrobacter freundii (n = 3) | 15375 | NDM-1 + CTX-M-15 | 8 | 2 | ≥2 | − | + (weak) | + | + |
| 17571 | NDM-1 + CTX-M-15 | 2 | 8 | ≥2 | + (weak) | + | + | + | |
| 17572 | NDM-1 | 8 | ≥16 | ≥2 | + (weak) | + | + | + | |
| Enterobacter aerogenes (n = 1) | 17568 | NDM-1 | 8 | 8 | ≥2 | − | + | + | + |
| E. cloacae (n = 3) | 17464 | NDM-1 | ≥16 | ≥16 | ≥2 | + (weak) | + (weak) | + | + |
| 17581 | NDM-1 + PER-2 | ≥16 | ≥16 | ≥2 | − | − | + | + | |
| 19074 | NDM-1 + CTX-M-2 | ≥16 | 8 | ≥2 | − | − | + | + | |
| E. coli (n = 6) | 15792 | NDM-1 + CTX-M-15 | 2 | 4 | ≥2 | + | + | + | + |
| 17386 | NDM-1 | ≥16 | ≥16 | ≥2 | − | − | + | + | |
| 17574 | NDM-1 + CMY-2 | 2 | ≥16 | ≥2 | + (weak) | + (weak) | + | + | |
| 17758 | NDM-1 | ≥16 | ≥16 | ≥2 | − | − | + | + | |
| 19269 | NDM-1 | 4 | ≥16 | ≥2 | − | − | + | + | |
| 19426 | NDM-1 | 8 | 8 | ≥2 | − | − | + | + | |
| K. pneumoniae (n = 6) | 13717 | NDM-1 + CTX-M-15 | 4 | 4 | ≥2 | − | − | + | + |
| 17047 | NDM-1 + CTX-M-2 | ≥16 | ≥16 | ≥2 | − | + (weak) | + | + | |
| 17277 | NDM-1 + ESBLd | ≥16 | ≥16 | ≥2 | − | + (weak) | + (weak) | + | |
| 17579 | NDM-1 + PER-2 | ≥16 | ≥16 | ≥2 | + (weak) | + (weak) | + | + | |
| 17619 | NDM-1 + CTX-M-15 | 4 | ≥16 | ≥2 | − | − | + | + | |
| 17624 | NDM-1 + CTX-M-15 | 4 | ≥16 | ≥2 | − | − | + | + | |
| Morganella morganii (n = 2) | 17569 | NDM-1 | 4 | 8 | 1 | − | − | + | + |
| 17570 | NDM-1 | 8 | 8 | 1 | − | − | + | + | |
| P. rettgeri (n = 7) | 15758 | NDM-1 | 8 | 8 | ≥2 | − | − | − | + (weak) |
| 15973 | NDM-1 + PER-2 | ≥16 | 16 | ≥2 | − | − | + | + | |
| 17154 | NDM-1 | ≥16 | 8 | ≥2 | − | − | + | + | |
| 17156 | NDM-1 | ≥16 | ≥16 | ≥2 | − | − | + | + | |
| 17159 | NDM-1 | ≥16 | 8 | ≥2 | − | − | + | + | |
| 17560 | NDM-1 | ≥16 | ≥16 | ≥2 | − | − | + | + | |
| 17561 | NDM-1 + PER-2 | ≥16 | ≥16 | 1 | − | − | + | + | |
| Providencia stuartii (n = 4) | 17600 | NDM-1 | 8 | 4 | 0.5 | − | − | − | + (weak) |
| 17617 | NDM-1 | ≥16 | 2 | 1 | − | − | + | + | |
| 17638 | NDM-1 | 8 | 2 | ≤0.5 | + (weak) | + (weak) | + | + | |
| 17687 | NDM-1 + PER-2 | ≥16 | ≥16 | ≥2 | − | − | − | + (weak) | |
| S. marcescens (n = 1) | 17468 | NDM-1 | 8 | 8 | ≥2 | − | − | + | + |
IMP, imipenem; MEM, meropenem; ERT, ertapenem; ND, not determined; ESBL, extended-spectrum β-lactamase.
The overall results were as follows: MHT, meropenem, 8 positive isolates/40 total isolates (20%); ertapenem, 13 positive isolates/40 total isolates (32.5%); THT, meropenem, 37 positive isolates/40 total isolates (92.5%); ertapenem, 40 positive isolates/40 total isolates (100%).
An area of enhanced growth of the indicator strain of ≤3 mm was categorized as a weak positive result for the indicated assay.
A strain with synergism between aztreonam and amoxicillin-clavulanate disks (phenotypic test indicating ESBL production) but negative results of PCRs targeting the usual ESBL genes.
Comparative performance of the MHT and THT for carbapenemase detection among clinical isolates.
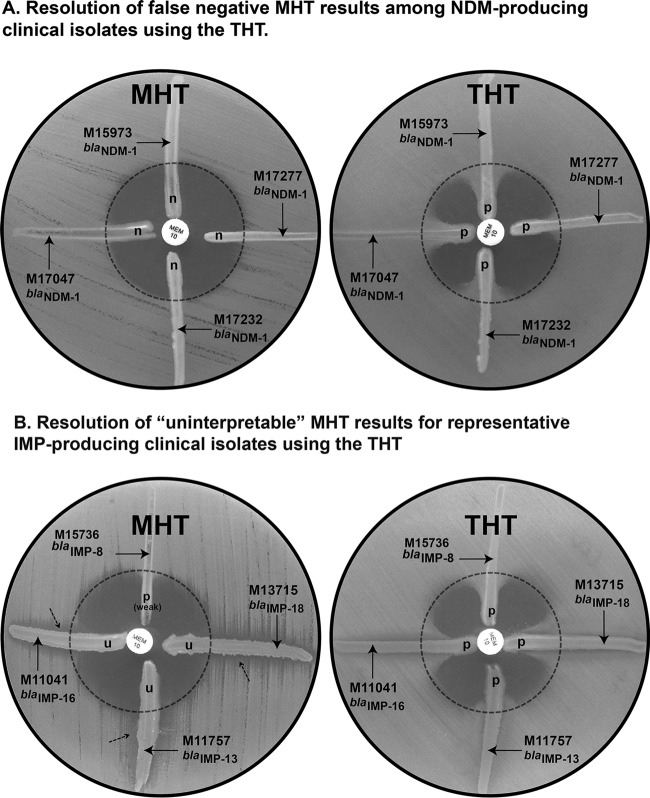
We challenged the MHT and the THT with a panel of clinical isolates with distinct profiles of susceptibility to carbapenems. Figure 3A shows the performance of the THT and the MHT for representative NDM producers. The MHT performed poorly in the detection of NDM-producing isolates (20% and 32.5% sensitivity for meropenem and ertapenem, respectively) (Table 1). Most NDM producers with positive MHT results showed weak enhanced growth (indentation of <3 mm) of the indicator strain (Table 1). In contrast, the sensitivities of the THT were 100% with ertapenem and 92.5% with meropenem for NDM-1-producing strains (false-negative results corresponded to Providencia isolates) (Table 1). Surprisingly, the THT also performed better than the MHT for organisms producing other types of MBLs, especially among Pseudomonas isolates (Table 2 and Fig. 3B). Only one IMP-13-producing Pseudomonas aeruginosa isolate showed an uninterpretable result in the Triton test. The THT showed performance comparable to that of the MHT against class A and class D enzymes (Table 2). Overall, the sensitivities of the THT for carbapenemase detection were 97% (141 of 145 positive results) and 99% (143/145 positive results) for meropenem and ertapenem, respectively, compared to 67% (97/145 positive results) and 72% (105/145 positive results) for the MHT.
FIG 3.
Comparative results of the modified Hodge test (MHT) and the Triton Hodge test (THT) for representative carbapenemase-producing clinical isolates. Results were obtained using meropenem (MEM) (10 μg) as the substrate. Strains included P. rettgeri M15973, K. pneumoniae M17047, Acinetobacter baumannii M17232, and K. pneumoniae M17277 (A) and E. cloacae M15736, P. aeruginosa M11041, P. aeruginosa M11757, and P. aeruginosa M13715 (B). p, meropenem was hydrolyzed by the streaked cells; n, meropenem was not hydrolyzed by the streaked cells; u, uninterpretable result (dashed arrow, inhibition of growth of the indicator strain E. coli ATCC 25922).
TABLE 2.
Results of the modified Hodge test and the Triton Hodge test and carbapenem susceptibility of selected carbapenemase-producing and nonproducing clinical isolates
| Group and β-lactamase | Bacterial species | % of resistance (% of nonsusceptibility)a to: |
No. (%) positive |
|||||
|---|---|---|---|---|---|---|---|---|
| MHT |
THT |
|||||||
| IMP | MEM | ERT | MEM | ERT | MEM | ERT | ||
| MBLs (n = 60) | ||||||||
| Enterobacteriaceae (n = 33) | ||||||||
| IMP-8 (n = 15) | C. freundii (n = 1), E. coli (n = 2), Enterobacter asburiae (n = 1), E. cloacae (n = 7), Klebsiella oxytoca (n = 1), K. pneumoniae (n = 2), S. marcescens (n = 1) | 27 (75) | 27 (75) | 33 (87.5) | 15 (100) | 15 (100) | 15 (100) | 15 (100) |
| VIM-1 (n = 1) | E. coli (n = 1) | 100 | 100 | 100 | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| VIM-2 (n = 16) | E. cloacae (n = 6), K. pneumoniae (n = 2), P. rettgeri (n = 4), P. stuartii (n = 4) | 68.8 (81.3) | 81.3 (81.3) | 81.3 (100) | 16 (100) | 16 (100) | 16 (100) | 16 (100) |
| VIM-11 (n = 1) | E. cloacae (n = 1) | 0 (0) | 0 (0) | 100 | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| All Enterobacteriaceae | 33 (100) | 33 (100) | 33 (100) | 33 (100) | ||||
| Non-Enterobacteriaceae (n = 27) | ||||||||
| IMP-1 (n = 4) | Acinetobacter junii (n = 2), Acinetobacter ursingii (n = 2) | 100 | 100 | ND | 4 (100) | 4 (100) | 4 (100) | 4 (100) |
| IMP-13 (n = 4) | P. aeruginosa (n = 4) | 50 (50) | 50 (50) | ND | 2 (50) (uninterp, 2) | 2 (50) (uninterp, 2) | 3 (75) (uninterp, 1) | 3 (75) (uninterp, 1) |
| IMP-16 (n = 4) | P. aeruginosa (n = 4) | 100 | 100 | ND | 2 (50) (uninterp, 2) | 2 (50) (uninterp, 2) | 4 (100) | 4 (100) |
| IMP-18 (n = 1) | P. aeruginosa (n = 1) | 100 | 100 | ND | 0 (0) (uninterp, 1) | 0 (0) (uninterp, 1) | 1 (100) | 0 (0) (uninterp, 1) |
| VIM-2 (n = 11) | P. aeruginosa (n = 5), Pseudomonas chlororaphis (n = 1), Pseudomonas fulva (n = 1), Pseudomonas monteili (n = 1), Pseudomonas oleovorans (n = 1), Pseudomonas putida (n = 2) | 100 | 100 | ND | 2 (18) (uninterp, 5)b | 5 (45) (uninterp, 5)b | 11 (100) | 11 (100) |
| VIM-11 (n = 1) | P. aeruginosa (n = 1) | 100 | 100 | ND | 0 (0) (uninterp, 1) | 0 (0) (uninterp, 1) | 1 (100) | 1 (100) |
| SPM-1 (n = 2) | P. aeruginosa (n = 2) | 100 | 100 | ND | 2 (100) | 2 (100) | 2 (100) | 2 (100) |
| All non-Enterobacteriaceae | 12 (44) | 15 (56) | 26 (96) | 25 (93) | ||||
| All MBLs (n = 60) | 45 (75) | 48 (80) | 59 (98) | 58 (97) | ||||
| Class A carbapenemases (n = 25) | ||||||||
| Enterobacteriaceae (n = 19) | ||||||||
| KPC-2 (n = 14) | Citrobacter braakii (n = 1), C. freundii (n = 1), E. coli (n = 3), E. cloacae (n = 2), Leclercia adecarboxylata (n = 1), K. oxytoca (n = 1), K. pneumoniae (n = 5) | 50 (85.7) | 50 (71.4) | 64.2 (85.7) | 14 (100) | 14 (100) | 14 (100) | 14 (100) |
| KPC-3 (n = 1) | K. pneumoniae (n = 1) | 100 | 100 | 100 | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| GES-3 (n = 1) | K. pneumoniae (n = 1) | 100 | 100 | 100 | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| NMC-A (n = 1) | E. cloacae (n = 1) | 100 | 100 | 100 | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| SME-1b (n = 2) | S. marcescens (n = 2) | 100 | 50 (50) | 0 (0) | 1 (50) (uninterp, 1) | 1 (50) (uninterp, 1) | 2 (100) | 2 (100) |
| All Enterobacteriaceae | 18 (95) | 18 (95) | 19 (100) | 19 (100) | ||||
| Non-Enterobacteriaceae (n = 6) | ||||||||
| KPC-2 (n = 5) | P. aeruginosa (n = 5) | 100 | 100 | ND | 5 (100) | 5 (100) | 5 (100) | 5 (100) |
| GES-5 (n = 1) | P. aeruginosa (n = 1) | 100 | 100 | ND | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| All non-Enterobacteriaceae | 6 (100) | 6 (100) | 6 (100) | 6 (100) | ||||
| All class A carbapenemases (n = 25) | 24 (96) | 24 (96) | 25 (100) | 25 (100) | ||||
| Class D carbapenemases (n = 20) | ||||||||
| OXA-48 (n = 5) | E. coli (n = 3), K. oxytoca (n = 1), K. pneumoniae (n = 1) | 20 (80) | 40 (100) | 80 (100) | 5 (100) | 5 (100) | 5 (100) | 5 (100) |
| OXA-163 (n = 11) | C. freundii (n = 1), E. cloacae (n = 3), E. coli (n = 2), K. pneumoniae (n = 3), P. stuartii (n = 2) | 18.1 (27.3) | 27.3 (27.3) | 100 | 11 (100) | 11 (100) | 11 (100) | 11 (100) |
| OXA-181 (n = 1) | K. pneumoniae (n = 1) | 100 | 100 | 100 | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| OXA-247 (n = 2) | E. coli (n = 1), K. pneumoniae (n = 1) | 50 (100) | 50 (100) | 50 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) |
| OXA-438 (n = 1) | E. coli (n = 1) | 100 | 100 | 100 | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| All class D carbapenemases (n = 20) | 20 (100) | 20 (100) | 20 (100) | 20 (100) | ||||
| Carbapenemase nonproducers (n = 40) | ||||||||
| ESBLs + porin loss (n = 20)c | E. aerogenes (n = 1), E. cloacae (n = 2), K. pneumoniae (n = 16), S. marcescens (n = 1) | 10 (50) | 85 (90) | 95 (100) | 1 (5) | 2 (10) | 2 (10) | 3 (15) |
| Overexpression of chromosomal AmpC + porin loss (n = 18) | E. aerogenes (n = 2), E. cloacae (n = 14), E. coli (n = 2) | 61.1 (100) | 44.4 (88.9) | 100 | 1 (6) | 1 (6) | 1 (6) | 2 (11) |
| CMY-2 (n = 2) | E. coli (n = 1), K. pneumoniae (n = 1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| All nonproducers (n = 40) | 2 (5) | 3 (7.5) | 3 (7.5) | 5 (12.5) | ||||
Intermediate plus resistant isolates for the indicated carbapenem (when applied). IMP, imipenem; MEM, meropenem; ERT, ertapenem; ND, not determined; uninterp, uninterpretable test due to inhibition of growth of the indicator strain along the tested isolate.
Four isolates of P. aeruginosa and one isolate of P. putida producing VIM-2 with uninterpretable results.
The ESBLs included were CTX-M-2 (n = 16) and CTX-M-15 (n = 4).
The MHT has been largely associated with false-positive results among ESBL/AmpC producers (20, 22). Thus, we challenged the THT with a panel of carbapenem-resistant, non-carbapenemase-producing clinical isolates. The THT displayed a false-positive rate similar to that of the MHT (Table 2). The greatest specificity was observed with meropenem, a stable substrate for AmpC/CTX-M enzymes (false-positive results were seen for one K. pneumoniae CTX-M-2 producer, one E. cloacae AmpC hyperproducer that already had a positive MHT result, and one CTX-M-2-producing K. pneumoniae isolate). False-positive results with ertapenem included the former strains and two additional E. cloacae isolates that produced either CTX-M or AmpC.
Triton-flooded plates stored up to 12 weeks showed similar results with the control strains. The studies were not continued beyond that time because MH storage for longer periods is not recommended, according to the manufacturer's guidelines.
Effects of Zn(II) contents of growth media on test performance.
We compared the test performances using reference media and an alternative commercial MHA brand with different zinc contents. The proportion of NDM-1 producers that tested positive in this alternative medium, containing 0.54 to 0.57 μg/ml Zn(II), paralleled the results obtained with reference MHA with 0.87 to 0.91 μg/ml Zn(II). The MHT displayed 20% and 32.5% positive results with meropenem and ertapenem, respectively, while values for the THT were 92.5% (meropenem) and 100% (ertapenem). The areas of enhanced growth of the indicator strain were similar regardless of the MHA batch. Clinical isolates producing other types of MBLs showed equivalent results in both MHAs (data not shown).
DISCUSSION
Effective screening of carbapenemase producers in clinical microbiology laboratories requires the development of sensitive and inexpensive methods. The widely used MHT fails in the detection of NDM-1 producers (3–7, 9). Here we show that these false-negative results are due to the fact that NDM-1 is a membrane-bound lipoprotein and that, in contrast to previous suggestions (23, 24), the Zn(II) levels in commercial media do not sensibly affect the detection of NDM producers.
NDM-1 is a membrane-anchored lipoprotein associated with the outer membrane through a lipid moiety covalently bound to a Cys residue (10, 11). This feature is common to all NDM variants, making them different from the rest of the periplasmic, soluble metal-dependent carbapenemases, such as VIM-2. Here we also show that NDM-1 is bound to the membrane in clinical strains, being absent in the soluble fraction. Engineered soluble (i.e., not membrane-bound) variants of NDM-1 can be detected by the MHT, as opposed to membrane-anchored variants. These results suggest that the membrane-bound nature of NDM precludes carbapenemase detection by the MHT, by preventing the release of the enzyme into the extracellular medium. Indeed, all clinical strains producing NDM-1 with negative MHT results became positive upon addition of the nonionic surfactant Triton X-100, which is able to solubilize membrane lipoproteins (25–28). These results are consistent with other reports suggesting that Triton X-100 is able to release NDM-1 from membranes of different bacteria while preserving β-lactam activity (L. González, G. Bahr, T. Nakashige, E. Nolan, R. Bonomo, and A. Vila, submitted for publication).
We propose the addition of a nonionic surfactant as a simple and inexpensive strategy to improve the performance of the MHT for the detection of NDM producers. This modification, named the Triton Hodge test (THT), also improves the detection of organisms producing other soluble class B enzymes, while not affecting the detection of class A and D producers. This is possibly due to enhanced periplasmic release of the soluble β-lactamases. The enhanced detection was also observed in bacteria not currently included in CLSI recommendations for the MHT, such as the non-Enterobacteriaceae, in which the number of uninterpretable results, largely associated with this group, was significantly reduced. Thus, the THT represents an attractive alternative to other methods (29). On the other hand, the occurrence of false detection of carbapenemase production using this approach among isolates with reduced carbapenem susceptibility due to dual mechanisms (ESBLs/AmpC plus decreased porins) was similar to that observed for the MHT. Therefore, for areas with high prevalences of these types of strains, the positive predictive value of THT would be low, paralleling that of the MHT (20, 22).
Ertapenem is the best substrate to screen carbapenemase producers among Enterobacteriaceae. Indeed, it was the only compound enabling carbapenemase detection with Providencia isolates (which show lower endogenous expression of NDM-1 and lower MIC values). The use of this carbapenem, however, might increase false-positive results from carbapenemase nonproducers, as we observed in tests with Klebsiella and Enterobacter isolates. The use of meropenem as a second substrate, if possible, is an alternative approach to reduce the number of isolates that require confirmation by other methods. Among non-Enterobacteriaceae, the two carbapenems yielded almost identical results, except for an IMP-18-producing strain that was detected only with meropenem.
Based on our results, we provide additional recommendations to clinical microbiology laboratories, aiming to improve routine detection of NDM and other carbapenemase producers with the THT. (i) The long-term stability of Triton-flooded plates (up to 12 weeks) enables early preparation and fractionation in aliquots as an efficient alternative to daily on-site plate preparation. (ii) The THT can be performed with MHA from commercial sources proposed as reference for MBL detection (23), such as Becton Dickinson BBL medium, as well as media from other commercial manufacturers with adequate Zn(II) levels to ensure MBL activity (≥0.54 μg/ml in the hydrated medium).
The present study demonstrates that addition of the nonionic surfactant Triton X-100 to the MHT represents a simple and inexpensive variant of this popular test that allows NDM-1 detection and, at the same time, provides better sensitivity for isolates producing other carbapenemases. These features make it a good candidate as a diagnostic tool for routine laboratories.
ACKNOWLEDGMENTS
We thank Stella Maris Cristaldo for technical assistance, Jorge Meda and Luciana Icardi (Laboratorios Britania, Argentina) for kindly providing agar media for this study and the results of cation measurements for the culture media, and Roberto Melano for providing OXA-48 and OXA-181 reference strains.
No conflicts of interests are declared.
Funding Statement
Work at ANLIS was supported by the regular federal budget of the Ministry of Health of Argentina.
REFERENCES
- 1.Bush K, Jacoby G. 2010. Updated functional classification of β-lactamases. Antimicrob Agents Chemother 54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 3.Doyle D, Peirano G, Lascols C, Lloyd T, Church DL, Pitout JD. 2012. Laboratory detection of Enterobacteriaceae that produce carbapenemases. J Clin Microbiol 50:3877–3880. doi: 10.1128/JCM.02117-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girlich D, Poirel L, Nordmann P. 2012. Value of the modified Hodge test for detection of emerging carbapenemases in Enterobacteriaceae. J Clin Microbiol 50:477–479. doi: 10.1128/JCM.05247-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castanheira M, Deshpande LM, Mathai D, Bell JM, Jones RN, Mendes RE. 2011. Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: report from the SENTRY Antimicrobial Surveillance Program, 2006–2007. Antimicrob Agents Chemother 55:1274–1278. doi: 10.1128/AAC.01497-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito R, Koyano S, Dorin M, Higurashi Y, Misawa Y, Nagano N, Kaneko T, Moriya K. 2015. Evaluation of a simple phenotypic method for the detection of carbapenemase-producing Enterobacteriaceae. J Microbiol Methods 108:45–48. doi: 10.1016/j.mimet.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Bonnin RA, Naas T, Poirel L, Nordmann P. 2012. Phenotypic, biochemical, and molecular techniques for detection of metallo-β-lactamase NDM in Acinetobacter baumannii. J Clin Microbiol 50:1419–1421. doi: 10.1128/JCM.06276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meini M, Tomatis P, Weinreich D, Vila AJ. 2015. Quantitative description of a protein fitness landscape based on molecular features. Mol Biol Evol 32:1774–1787. doi: 10.1093/molbev/msv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HK, Park JS, Sung H, Kim MN. 2015. Further modification of the modified Hodge test for detecting metallo-β-lactamase-producing carbapenem-resistant Enterobacteriaceae. Ann Lab Med 35:298–305. doi: 10.3343/alm.2015.35.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King D, Strynadka N. 2011. Crystal structure of New Delhi metallo-β-lactamase reveals molecular basis for antibiotic resistance. Protein Sci 20:1484–1491. doi: 10.1002/pro.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez L, Bahr G, Bonomo RA, Vila A. 2014. Membrane anchoring of carbapenemase NDM-1 favors protein stability and resistance transfer. Abstr 54th Intersci Conf Antimicrob Agents Chemother, abstr C-162c. [Google Scholar]
- 12.Moran-Barrio J, Lisa M, Vila A. 2012. In vivo impact of Met221 substitution in GOB metallo-β-lactamase. Antimicrob Agents Chemother 56:1769–1773. doi: 10.1128/AAC.05418-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi K, Kumar A, Schweizer H. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J Microbiol Methods 64:391–397. doi: 10.1016/j.mimet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almuzara M, Barberis C, Traglia G, Famiglietti A, Ramirez MS, Vay C. 2015. Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry for species identification of nonfermenting Gram-negative bacilli. J Microbiol Methods 112:24–27. doi: 10.1016/j.mimet.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Pasteran F, Veliz O, Ceriana P, Lucero C, Rapoport M, Albornoz E, Gomez S, Corso A. 2015. Evaluation of the Blue-Carba test for rapid detection of carbapenemases in Gram-negative bacilli. J Clin Microbiol 53:1996–1998. doi: 10.1128/JCM.03026-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melano R, Corso A, Petroni A, Centron D, Orman B, Pereyra A, Moreno N, Galas M. 2003. Multiple antibiotic-resistance mechanisms including a novel combination of extended-spectrum β-lactamases in a Klebsiella pneumoniae clinical strain isolated in Argentina. J Antimicrob Chemother 52:36–42. doi: 10.1093/jac/dkg281. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Martínez L, Hernández-Allés S, Abertí S, Tomás J, Benedi V, Jacoby G. 1996. In vivo selection of porin-deficient mutants of Klebsiella pneumoniae with increased resistance to cefoxitin and expanded-spectrum cephalosporins. Antimicrob Agents Chemother 40:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamzehpour M, Pecher J, Plesiat P, Kohler T. 1995. OprK and OprM define two genetically distinct multidrug efflux systems in Pseudomonas aeruginosa. Antimicrob Agents Chemother 39:2392–2396. doi: 10.1128/AAC.39.11.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasteran F, Mendez T, Rapoport M, Guerriero L, Corso A. 2010. Controlling false-positive results obtained with the Hodge and Masuda assays for detection of class A carbapenemase in species of Enterobacteriaceae by incorporating boronic acid. J Clin Microbiol 48:1323–1332. doi: 10.1128/JCM.01771-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribeiro VB, Linhares AR, Zavascki AP, Barth AL. 2014. Performance of quantification of modified Hodge test: an evaluation with Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae isolates. Biomed Res Int doi: 10.1155/2014/139305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvalhaes CG, Picão RC, Nicoletti AG, Xavier DE, Gales AC. 2010. Cloverleaf test (modified Hodge test) for detecting carbapenemase production in Klebsiella pneumoniae: be aware of false positive results. J Antimicrob Chemother 65:249–251. doi: 10.1093/jac/dkp431. [DOI] [PubMed] [Google Scholar]
- 23.Dortet L, Bre'chard L, Poirel L, Nordmann P. 2014. Impact of the isolation medium for detection of carbapenemase-producing Enterobacteriaceae using an updated version of the Carba NP. J Med Microbiol 63:772–776. doi: 10.1099/jmm.0.071340-0. [DOI] [PubMed] [Google Scholar]
- 24.Cooper GL, Louie A, Baltch AL, Chu RC, Smith RP, Ritz WJ, Michelsen P. 1993. Influence of zinc on Pseudomonas aeruginosa susceptibilities to imipenem. J Clin Microbiol 31:2366–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnaitman CA. 1971. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol 108:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shang ES, Summers TA, Haake DA. 1996. Molecular cloning and sequence analysis of the gene encoding LipL41, a surface-exposed lipoprotein of pathogenic Leptospira species. Infect Immun 64:2322–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crago AM, Koronakis V. 1998. Salmonella InvG forms a ring-like multimer that requires the InvH lipoprotein for outer membrane localization. Mol Microbiol 30:47–56. doi: 10.1046/j.1365-2958.1998.01036.x. [DOI] [PubMed] [Google Scholar]
- 28.Jones N. 1999. Surfactants in membrane solubilisation. Int J Pharm 177:137–159. doi: 10.1016/S0378-5173(98)00345-7. [DOI] [PubMed] [Google Scholar]
- 29.Pasteran F, Veliz O, Rapoport M, Guerriero L, Corso A. 2011. Sensitive and specific modified Hodge test for KPC and metallo-β-lactamase detection in Pseudomonas aeruginosa by use of a novel indicator strain, Klebsiella pneumoniae ATCC 700603. J Clin Microbiol 49:4301–4303. doi: 10.1128/JCM.05602-11. [DOI] [PMC free article] [PubMed] [Google Scholar]