Abstract
Blood citrulline and intestinal fatty acid binding protein were determined as biomarkers for intestinal mucositis. Biomarker levels were correlated with corresponding serum 1,3-beta-d-glucan levels in 56 samples obtained from 33 cases with underlying hematological malignancies receiving induction chemotherapy. No correlation between biomarkers of intestinal mucositis and BDG levels was observed. (This study has been registered at ClinicalTrials.gov under registration no. NCT01576653.)
TEXT
A polysaccharide cell wall component of most fungal species and a useful serum biomarker for early diagnosis of invasive fungal infections (IFIs) is (1→3)-β-d-glucan (BDG) (1, 2). False-positive results have been linked to use of cellulose-containing hemodialysis membranes, blood derivatives, broad-spectrum antibiotics, and severe mucositis (3). Intestinal mucositis is a frequently observed adverse event of induction chemotherapy in patients with underlying hematological malignancies (4). Severe mucositis is characterized by a loss of integrity of the intestinal mucosal barrier, increasing the likelihood of translocation of bacterial and/or fungal commensals of the gastrointestinal tract (5). Whether intestinal mucositis is a cause of false-positive BDG serum levels has been insufficiently evaluated to date mainly because of difficulties in diagnosing and defining mucositis in hematological malignancy patients in routine clinical procedures. Biopsy of the gastrointestinal tract is the diagnostic gold standard but is often hindered by the unfavorable risk-benefit ratio in those with severe underlying conditions and increased risk of bleeding.
Thus, several clinical scores have been implemented for diagnosis and grading of mucositis (6, 7), but these are prone to subjective interpretation. Therefore, noninvasive diagnostic biomarkers for mucositis have been increasingly investigated. Serum citrulline, a nonprotein amino acid produced by intact enterocytes (8, 9), and intestinal fatty acid binding protein (IFABP), a low-molecular-mass cytosolic protein found in tissues involved in the uptake, intracellular metabolism, and transport of long-chain fatty acids (10, 11), are two of the most promising biomarkers for detection of loss of enterocytes and, therefore, mucosal barrier injury.
(Data of the manuscript have been previously presented, in part, at the Interscience Conference of Antimicrobial Agents and Chemotherapy [ICAAC], San Diego, CA, 2015 [12], and at Trends in Medical Mycology [TIMM], Lisbon, Portugal, 2015 [13]).
The objective of this study was to investigate whether serum BDG levels are falsely elevated in hematological malignancy patients with intestinal mucositis. A total of 56 same-day plasma and serum samples were prospectively obtained from 33 consecutive cases (i.e., 33 admissions corresponding to 24 patients; maximum, 2 samples per case) with underlying hematological malignancies receiving induction chemotherapy for acute leukemia. None of our patients fulfilled criteria for IFI according to 2008 revised definitions for IFIs from the European Organization for Research and Treatment of Cancer Invasive Fungal Infections Cooperative Group/National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) (14, 15). Samples were collected between July 2012 and May 2013 at the University Hospital of Graz, Austria, and a maximum of two samples per case were included (in those with two samples, samples were always obtained >4 days apart). BDG levels were determined prospectively, while citrulline and IFABP levels were determined retrospectively in serum/plasma samples after immediate sample storage at −70°C at the Medical University of Graz, Graz, Austria. BDG levels were determined using the automated protocol Fungitell assay (Associates of Cape Cod, Inc., East Falmouth, MA, USA), as described previously (16). IFABP was determined using the Quantikine Human FABP2/I-FABP immunoassay (R&D Systems Europe Ltd., Oxford, United Kingdom) according to the manufacturer's instruction. Citrulline was determined from plasma samples at the University Hospital Leuven, Leuven, Belgium, using liquid chromatography-tandem mass spectrometry as described elsewhere (17). Mucositis was classified according to citrulline levels: >30 μmol/liter (no evidence of mucositis), 20 to 30 μmol/liter (partial villus atrophy), 10 to 20 μmol/liter (destructive mucosal lesions), and <10 μmol/liter (diffuse destructive mucositis) (18, 19). IFABP was used as a second marker for mucositis to better support the mucositis classifications based on citrulline levels. In contrast to the citrulline results, IFABP levels are highly elevated in cases of intestinal mucosal barrier damage, with 100-fold increases in patients with intestinal ischemia compared to healthy controls (10).
For statistical analyses, a cutoff value of 10 μmol/liter citrulline was used as an indicator of extensive mucositis. The study adhered to the Declaration of Helsinki (1996) and good clinical practice. The study protocol was approved by the local ethics committee, Medical University Graz, Graz, Austria (EC no. 23-343), and was registered at ClinicalTrials.gov (ClinicalTrials registration no. NCT01576653). Informed consent was obtained from all participating patients. For statistical analysis, SPSS Version 23 was used (SPSS Inc., Chicago, IL, USA). For continuous variables, medians plus interquartile ranges (IQR) or means plus 95% confidence intervals (95% CI) are displayed as appropriate. Correlation analysis was performed by using Spearman ρ analyses due to the nonnormality of our data, and data are presented in scatterplots. Retrospective power analysis revealed that a sample size of 48 mucositis samples (8 samples were derived from patients without mucositis) provides at least 80% power (with alpha = 0.05) to detect a correlation of r = 0.44 (i.e., r2 = 0.19) or higher for correlations of three variables. The chi-square test was used for categorical data. A P value of <0.05 was considered statistically significant.
Demographic data, underlying diseases, and biomarker levels for patients, cases, and samples are displayed in Table 1. All patients were receiving mold-active antifungal prophylaxis with posaconazole oral suspension, as recommended in clinical guidelines. Additionally, all included patients were able to tolerate solid food. All but two patients were on systemic antibiotic treatment/prophylaxis at the time of sample collection. The most common antibiotics prescribed at the time of sampling were levofloxacin (23 samples, 42.6%), cefepime (21 samples, 38.9%), linezolid (7 samples, 13%), and meropenem (6 samples, 11.1%). Additionally, all patient received prophylaxis for oral mucositis containing chlorhexidine mouth wash and nystatin oral solution. The overall specificity of serum BDG testing in our cohort was 0.95 (95% CI, 0.85 to 0.98).
TABLE 1.
Demographic data, underlying diseases, and 1,3-beta-d-glucan, citrulline, and intestinal fatty acid binding protein levels in the study populationa
| Parameter | Value(s) |
||
|---|---|---|---|
| Patients | Cases | Samples | |
| n | 24 | 33 | 56 |
| Sex: females/males | 10 (41.7)/14 (58.3) | 15 (45.5)/18 (54.5) | 28 (50)/28 (50) |
| Age, yrs (range) | 54 (20–67) | 57 (20–67) | 59 (20–67) |
| Underlying disease | |||
| Acute myeloid leukemia | 18 (75) | 26 (78.8) | 46 (82.1) |
| Acute lymphoblastic leukemia | 3 (12.5) | 4 (12.1) | 5 (8.9) |
| Myelodysplastic syndrome | 2 (8.3) | 2 (6.1) | 3 (5.4) |
| Aplastic anemia | 1 (4.2) | 1 (3) | 2 (3.6) |
| Citrulline categories (μmol/liter) | |||
| Median citrulline level (IQR) | 14.3 (8.6–33.6) | 14.1 (8.8–18.2) | 13.8 (8.7–18.2) |
| <10 (diffuse destructive mucositis) | 8 (33.3) | 11 (33.3) | 17 (30.4) |
| 10–20 (destructive mucosal lesions) | 12 (50) | 18 (54.5) | 31 (55.4) |
| 20–30 (partial villus atrophy) | 1 (4.2) | 1 (3) | 4 (7.1) |
| ≥30 (no evidence of mucositis) | 3 (12.5) | 3 (9.1) | 4 (7.1) |
| BDG categories (pg/ml) | |||
| Median BDG level (IQR) | 15.4 (7.4–39.9) | 15.4 (5–35.8) | 15.4 (6.8–30.9) |
| ≥80 pg/ml | 2 (8.3) | 2 (6.1) | 3 (5.4) |
| <80 pg/ml | 22 (91.7) | 31 (93.9) | 53 (94.6) |
| Median IFABP level in pg/ml (IQR) | 524.6 (309.8–804.3) | 483.5 (332.3–849.7) | 514.9 (337.9–792.6) |
| Severe diarrhea | 3 (12.5) | 3 (9.1) | 4 (7.1) |
| Citrulline < 10 μmol/liter (diffuse destructive mucositis) | 1 (33.3) | 1 (33.3) | 2 (50) |
| Citrulline 10–20 μmol/liter (destructive mucosal lesions) | 2 (66.6) | 2 (66.6) | 2 (50) |
Data represent absolute numbers plus percentages, unless otherwise noted. Data in the “Patients” column represent the first sample per patient within the study period; data in the “Cases” column represent 33 admissions corresponding to 24 patients; data in the “Samples” column represent all samples obtained from the included patients within the study period. Abbreviations: BDG, 1,3-beta-d-glucan; IQR, 25%-to-75% interquartile range; IFABP, intestinal fatty acid binding protein.
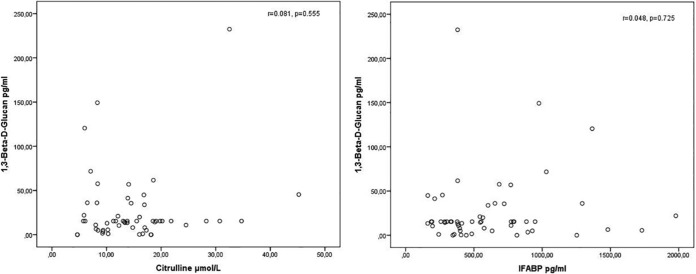
Positive BDG serum levels (>80 pg/ml) were observed in three serum samples: 2 (11.8%) of 17 samples with citrulline levels of <10 μmol/liter had false-positive BDG levels (120.5 pg/ml and 149.3 pg/ml, respectively; corresponding IFABP levels, 1,366.3 pg/ml and 976.1 pg/ml, respectively), and 1 (2.6%) of 39 samples with citrulline levels of ≥10 μmol/liter had a false-positive BDG level (232.4 pg/ml; corresponding IFABP level, 380.1 pg/ml; P = 0.160). Median BDG levels in samples with citrulline levels of <10 μmol/liter and ≥10 μmol/liter were 15.4 pg/ml in both groups. Serum IFABP levels showed a strong negative correlation with plasma citrulline levels (Spearman ρ r = −0.601; P = <0.001). Additionally, IFABP values were significantly higher in the mucositis group (i.e., n = 17 with <10 μmol/liter citrulline) than in the group without severe mucositis according to the citrulline levels (814.8 pg/ml versus 380.9 pg/ml; P = 0.004), supporting a mucositis diagnosis based on citrulline levels. Correlations between BDG and citrulline or IFABP levels are displayed in Fig. 1.
FIG 1.
Correlation of 1,3-beta-d-glucan (BDG) with citrulline and intestinal fatty acid binding protein (IFABP) in same-day plasma and serum samples. Correlation analyses were performed by Spearman ρ analysis and yielded no significant correlations between BDG and citrulline (r = 0.081) and BDG and IFABP (r = 0.048).
Our main objective in this study was to determine whether underlying intestinal mucositis in hematological malignancy patients is a potential cause of falsely elevated serum BDG levels. As surrogate markers for mucositis, we used citrulline and IFABP. The two markers showed excellent correlation with each other, supporting the diagnosis of intestinal mucositis; however, no correlation between either citrulline or IFABP levels and serum BDG levels was observed. Additionally, only 2 of 17 samples from patients with biomarker levels suggestive of diffuse intestinal mucosal lesions yielded positive serum BDG levels.
As fungi, especially Candida spp., are commensals in the gastrointestinal tract, mucosal barrier damage is considered a risk factor for developing IFIs (20, 21). Antifungal prophylaxis is therefore a recommended practice in patients with hematological malignancies receiving induction chemotherapy or hematopoietic stem cell transplantation (22). In our setting, oral posaconazole suspension was used as prophylactic agent in all patients. Antifungal prophylaxis is likely to result in a decreased fungal burden in the gastrointestinal tract and therefore in a reduced amount of fungal components in serum in cases of mucosal barrier damage. This may be particularly true for the posaconazole suspension that was used in all our patients. Due to the liquid formulation, the posaconazole suspension is more fungicidal in the upper gastrointestinal tract (i.e., oral, esophagus) than the newer tablet formulation. As fungal colonization is mainly found in the upper gastrointestinal tract, the posaconazole suspension may cause a more substantial reduction of fungal colonization than the tablet or the intravenous form. While the fact that antifungal prophylaxis was used in all participants may be considered a limitation from a scientific standpoint (i.e., we could not evaluate whether mucositis is associated with false-positive BDG levels in the absence of antifungal prophylaxis), it may be considered a strength from a clinical standpoint, as this reflects clinical practice in most specialized centers.
The specificity of serum BDG testing in our study was 95%, which is comparable to that measured in other studies in patients with hematological malignancy (23). Our results therefore clearly indicate that mucositis may not be a major cause of false-positive BDG levels in patients with induction chemotherapy who are receiving antifungal prophylaxis. These findings have important clinical implications, as false-negative BDG test results in the presence of antifungal therapy or prophylaxis have been shown to occur rarely. This is supported by a study conducted by Koo et al., who were able to demonstrate that systemic antifungal therapy did not alter the performance of serum BDG determination methods (24).
Our study was limited by indirect assessment of intestinal mucositis by citrulline and IFABP determinations. No endoscopies to verify intestinal mucosal barrier damage were performed. Additionally, citrulline and IFABP are both produced in the lower gastrointestinal tract and may not be very reliable biomarkers in patients with exclusively oral mucositis. Oral mucositis may have therefore been underestimated in our study. Small sample sizes represented another important limitation.
In conclusion, we did not observe elevated serum BDG levels in hematological malignancy patients with underlying intestinal mucositis who received antifungal prophylaxis, indicating that serum BDG may be a specific biomarker in this setting.
ACKNOWLEDGMENTS
R. B. Raggam received travel grants from Pfizer and Merck. A. Wölfler received research grants and speaker honoraria from Merck. M. Hoenigl received research grants from Merck and Pfizer; served on the speakers' bureaus of Pfizer, Gilead, Astellas, Basilea, and Merck, and received travel grants from Astellas, Merck, Gilead, and Pfizer. I. Spriet received research grants from Merck and Pfizer and speaker honoraria and travel grants from Pfizer, Merck, and Gilead. The rest of us declare that we have no conflicts of interest.
Funding Statement
The funders had no role in the study design, data collection, analysis, interpretation, or decision to publish, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
REFERENCES
- 1.Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME. 2011. Beta-d-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis 52:750–770. doi: 10.1093/cid/ciq206. [DOI] [PubMed] [Google Scholar]
- 2.Prattes J, Hoenigl M, Rabensteiner J, Raggam RB, Prueller F, Zollner-Schwetz I, Valentin T, Hönigl K, Fruhwald S, Krause R. 2014. Serum 1,3-beta-d-glucan for antifungal treatment stratification at the intensive care unit and the influence of surgery. Mycoses 57:679–686. doi: 10.1111/myc.12221. [DOI] [PubMed] [Google Scholar]
- 3.Ellis M, Al-Ramadi B, Finkelman M, Hedstrom U, Kristensen J, Ali-Zadeh H, Klingspor L. 2008. Assessment of the clinical utility of serial beta-d-glucan concentrations in patients with persistent neutropenic fever. J Med Microbiol 57:287–295. doi: 10.1099/jmm.0.47479-0. [DOI] [PubMed] [Google Scholar]
- 4.Benson AB III, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA Jr, McCallum R, Mitchell EP, O'Dorisio TM, Vokes EE, Wadler S. 2004. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol 22:2918–2926. doi: 10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]
- 5.Bougnoux ME, Diogo D, Francois N, Sendid B, Veirmeire S, Colombel JF, Bouchier C, Van Kruiningen H, d'Enfert C, Poulain D. 2006. Multilocus sequence typing reveals intrafamilial transmission and microevolutions of Candida albicans isolates from the human digestive tract. J Clin Microbiol 44:1810–1820. doi: 10.1128/JCM.44.5.1810-1820.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly JP, Muus P, Schattenberg A, De Witte T, Horrevorts A, DePauw BE. 1992. A scheme for daily monitoring of oral mucositis in allogeneic BMT recipients. Bone Marrow Transplant 9:409–413. [PubMed] [Google Scholar]
- 7.Potting CM, Blijlevens NA, Donnelly JP, Feuth T, Van Achterberg T. 2006. A scoring system for the assessment of oral mucositis in daily nursing practice. Eur J Cancer Care (Engl) 15:228–234. doi: 10.1111/j.1365-2354.2005.00644.x. [DOI] [PubMed] [Google Scholar]
- 8.Lutgens LC, Deutz N, Granzier-Peeters M, Beets-Tan R, De Ruysscher D, Gueulette J, Cleutjens J, Berger M, Wouters B, von Meyenfeldt M, Lambin P. 2004. Plasma citrulline concentration: a surrogate end point for radiation-induced mucosal atrophy of the small bowel. A feasibility study in 23 patients. Int J Radiat Oncol Biol Phys 60:275–285. doi: 10.1016/j.ijrobp.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 9.Barzał JA, Szczylik C, Rzepecki P, Jaworska M, Anuszewska E. 2014. Plasma citrulline level as a biomarker for cancer therapy-induced small bowel mucosal damage. Acta Biochim Pol 61:615–631. [PubMed] [Google Scholar]
- 10.Lieberman JM, Sacchettini J, Marks C, Marks WH. 1997. Human intestinal fatty acid binding protein: report of an assay with studies in normal volunteers and intestinal ischemia. Surgery 121:335–342. doi: 10.1016/S0039-6060(97)90363-9. [DOI] [PubMed] [Google Scholar]
- 11.Paulussen RJ, Veerkamp JH. 1990. Intracellular fatty-acid-binding proteins. Characteristics and function. Subcell Biochem 16:175–226. doi: 10.1007/978-1-4899-1621-1_7. [DOI] [PubMed] [Google Scholar]
- 12.Prattes J, Raggam RB, Vanstraelen K, Rabensteiner J, Hoegenauer C, Krause R, Prueller F, Woelfler A, Spriet I, Hoenigl M. 2015. Abstr 48th Intersci Conf Antimicrob Agents Chemother (ICAAC), San Diego, CA, USA, guided poster walk, presentation 1477. [Google Scholar]
- 13.Prattes J, Raggam RB, Vanstraelen K, Rabensteiner J, Hoegenauer C, Krause R, Prueller F, Woelfler A, Spriet I, Hoenigl M. 2015. Abstr Trends in Medical Mycology (TIMM), Lisbon, Portugal, presentation P452. [Google Scholar]
- 14.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, Consensus Group . 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoenigl M, Strenger V, Buzina W, Valentin T, Koidl C, Wolfler A, Seeber K, Valentin A, Strohmeier AT, Zollner-Schwetz I, Raggam RB, Urban C, Lass-Florl C, Linkesch W, Krause R. 2012. European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) host factors and invasive fungal infections in patients with haematological malignancies. J Antimicrob Chemother 67:2029–2033. doi: 10.1093/jac/dks155. [DOI] [PubMed] [Google Scholar]
- 16.Prüller F, Wagner J, Raggam RB, Hoenigl M, Kessler HH, Truschnig-Wilders M, Krause R. 2014. Automation of serum (1–>3)-beta-d-glucan testing allows reliable and rapid discrimination of patients with and without candidemia. Med Mycol 52:455–461. doi: 10.1093/mmy/myu023. [DOI] [PubMed] [Google Scholar]
- 17.Demacker PN, Beijers AM, van Daal H, Donnelly JP, Blijlevens NM, van den Ouweland JM. 2009. Plasma citrulline measurement using UPLC tandem mass-spectrometry to determine small intestinal enterocyte pathology. J Chromatogr B Analyt Technol Biomed Life Sci 877:387–392. doi: 10.1016/j.jchromb.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 18.Crenn P, Messing B, Cynober L. 2008. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr 27:328–339. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Crenn P, Vahedi K, Lavergne-Slove A, Cynober L, Matuchansky C, Messing B. 2003. Plasma citrulline: a marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology 124:1210–1219. doi: 10.1016/S0016-5085(03)00170-7. [DOI] [PubMed] [Google Scholar]
- 20.Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R. 1994. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg 220:751–758. doi: 10.1097/00000658-199412000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nucci M, Anaissie E. 2001. Revisiting the source of candidemia: skin or gut? Clin Infect Dis 33:1959–1967. doi: 10.1086/323759. [DOI] [PubMed] [Google Scholar]
- 22.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 23.Marchetti O, Lamoth F, Mikulska M, Viscoli C, Verweij P, Bretagne S, European Conference on Infections in Leukemia (ECIL) Laboratory Working Groups . 2012. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant 47:846–854. doi: 10.1038/bmt.2011.178. [DOI] [PubMed] [Google Scholar]
- 24.Koo S, Bryar JM, Page JH, Baden LR, Marty FM. 2009. Diagnostic performance of the (1–>3)-beta-d-glucan assay for invasive fungal disease. Clin Infect Dis 49:1650–1659. doi: 10.1086/647942. [DOI] [PubMed] [Google Scholar]