Abstract
Despite the current reliance on blood cultures (BCs), the diagnosis of bloodstream infections (BSIs) can be sped up using new technologies performed directly on positive BC bottles. Two methods (the MALDI BioTyper system and FilmArray blood culture identification [BCID] panel) are potentially applicable. In this study, we performed a large-scale clinical evaluation (1,585 microorganisms from 1,394 BSI episodes) on the combined use of the MALDI BioTyper and FilmArray BCID panel compared to a reference (culture-based) method. As a result, the causative organisms of 97.7% (1,362/1,394) of the BSIs were correctly identified by our MALDI BioTyper and FilmArray BCID-based algorithm. Specifically, 65 (5.3%) out of 1,223 monomicrobial BCs that provided incorrect or invalid identifications with the MALDI BioTyper were accurately detected by the FilmArray BCID panel; additionally, 153 (89.5%) out of 171 polymicrobial BCs achieved complete identification with the FilmArray BCID panel. Conversely, full use of the MALDI BioTyper would have resulted in the identification of only 1 causative organism in 97/171 (56.7%) of the polymicrobial cultures. By applying our diagnostic algorithm, the median time to identification was shortened (19.5 h versus 41.7 h with the reference method; P < 0.001), and the minimized use of the FilmArray BCID panel led to a significant cost savings. Twenty-six out of 31 microorganisms that could not be identified were species/genera not designed to be detected with the FilmArray BCID panel, indicating that subculture was not dispensable for a few of our BSI episodes. In summary, the fast and effective testing of BC bottles is realistically adoptable in the clinical microbiology laboratory workflow, although the usefulness of this testing for the management of BSIs remains to be established.
INTRODUCTION
The rapid detection and/or identification (ID) of the microbial pathogens responsible for bloodstream infections (BSIs) is pivotal for reducing infection-related mortality and costs (1), particularly for severely ill patients (2, 3). Although blood culture (BC) continues to be essential for BSI diagnosis, the turnaround time for ID can be accelerated using new technologies that are directly applied to positive BC bottles/broths (4).
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) was recently demonstrated to improve the clinical management of BSIs (5, 6) and allow patients to receive appropriate antimicrobial therapy earlier (i.e., 24 h from the first positive BC) (7). However, despite its well-documented good performance (8–17), this method often fails to identify polymicrobial BCs in their entirety.
Another method is the multiplex PCR-based FilmArray blood culture ID (BCID) panel (bioMérieux, Marcy l'Etoile, France), which is equally as fast as the MALDI-TOF MS (e.g., MALDI BioTyper system [Bruker Daltonics, Bremen, Germany]). This technique was developed for the simultaneous identification of 24 microbial pathogens, including 19 bacterial genera/species and 5 Candida species (and 3 antibiotic resistance determinants). In addition to its high sensitivity and specificity, the FilmArray BCID panel has proven to be accurate for identifying not only monomicrobial but also polymicrobial BCs (18–23). Notably, successful results were obtained with BC broths tested before the bottles became positive or even before the bottles were incubated in the BC instrument as usual (24). To date, the extensive use of the FilmArray BCID panel in routine clinical laboratory practice has been hampered by the high cost of reagents/consumables.
In this study, we developed a BSI diagnostic algorithm that relied on the direct analysis of positive BC bottles/broths using the MALDI BioTyper system supplemented with the FilmArray BCID panel. We evaluated this algorithm in terms of ID accuracy and turnaround time by comparing the results with a reference culture-based procedure.
(This work has been presented in part as a poster at the 24th European Congress of Clinical Microbiology and Infectious Diseases, 10 to 13 May 2014, Barcelona, Spain).
MATERIALS AND METHODS
Setting, design, and samples.
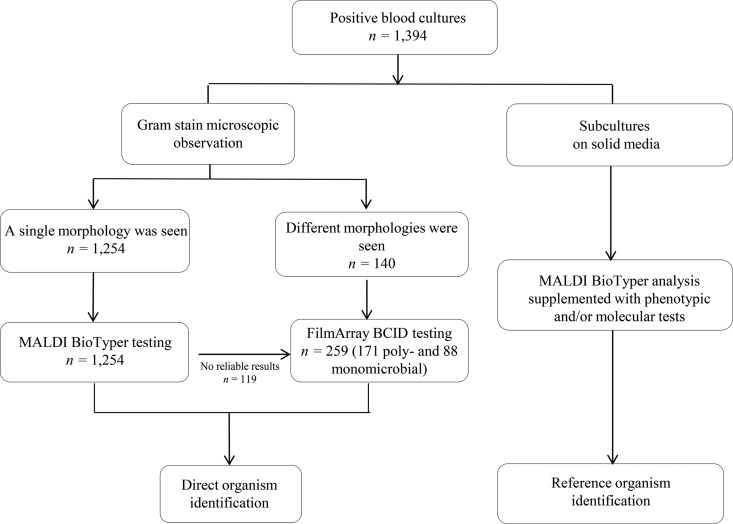
The study was conducted prospectively between December 2013 and April 2015 at the Catholic University of the Sacred Heart Medical Center, which is a large tertiary-care hospital in Rome, Italy. The hospital is entirely served by our clinical microbiology laboratory, which diagnoses an average of 90 BSI episodes per month. Blood samples were inoculated in aerobic and anaerobic Bactec bottles (Becton Dickinson Instrument Systems, Sparks, MD, USA) and incubated at 35°C until they yielded a positive signal or for up to 5 days in a BD Bactec FX automated instrument. Positive BC bottles were submitted for conventional (i.e., Gram stain analysis and culture-based ID) and direct (i.e., MALDI BioTyper system and/or FilmArray BCID panel) tests, according to the BSI diagnostic algorithm described in this study (Fig. 1). Direct MALDI BioTyper testing was performed on BC broths following Gram stain analysis and the report of the results to the treating physician when a single morphology was noted. Conversely, FilmArray BCID testing was performed on BC broths when multiple morphologies were noted and when the MALDI BioTyper analysis failed to provide reliable results (i.e., identifications with scores of <1.8, multiple hits in the top 10 matches list with scores of >1.7 and <1.8 that were suggestive of the presence of >1 microbial species, or the identification of organisms, such as Streptococcus pneumoniae). Only one positive (aerobic) BC bottle per patient episode was used for the direct assays. The direct assays were performed several times daily on weekdays (from 7:30 a.m. to 7:00 p.m., Monday through Saturday). Blood cultures that arrived when the laboratory was closed were stored at room temperature, as previously described (15). All consecutive positive BC bottles representing clinically significant BSI episodes (defined a posteriori as the isolation of bacteria and/or yeasts from the blood in the presence of signs and/or symptoms of infection and/or the isolation of at least one organism that was considered clinically relevant by the treating physician with the support of an infectious disease consultant [25, 26]) were included in the final evaluation of our MALDI BioTyper/FilmArray BCID panel-based diagnostic algorithm. Cultures growing commensal organisms, such as coagulase-negative staphylococcus (CoNS) and viridans streptococcus, were judged to be clinically relevant when these organisms were recovered from >2 BCs in patients with clinical manifestations of infection that were not explained by other causes (15). BSI episodes separated by >1 week were considered to represent different episodes per patient.
FIG 1.
Bloodstream infection diagnostic workflow using direct (MALDI-TOF MS [MALDI BioTyper system] and/or FilmArray BCID panel) or culture-based (reference) microbial identification methods on positive blood culture (BC) broths. According to the developed algorithm, the FilmArray BCID panel assays were performed on BC broths that revealed multiple morphologies by Gram staining and in all cases for which MALDI BioTyper analysis failed to provide reliable results (i.e., identifications with scores of <1.8, multiple hits in the top 10 matches list with scores ranging from >1.7 to <1.8 that were suggestive of the presence of >1 microbial species, and identifications of organisms, such as S. pneumoniae). MALDI-TOF MS, matrix-assisted laser desorption ionization–time of flight mass spectrometry; BCID, blood culture identification.
Culture-based reference ID and susceptibility testing.
Aliquots from each positive BC bottle/broth were subjected to routine Gram staining microscopy and solid-medium subcultures. After isolation from the cultures, bacteria and yeasts were identified by MALDI BioTyper analysis; in cases in which the isolate could not be identified (i.e., ID score was <2.0), conventional phenotypical tests and/or sequencing of the 16S rRNA gene or the rpoB gene (for bacterial isolates) and the internal transcribed spacer 1 (ITS1)-5.8S-ITS2 rRNA gene region (for fungal isolates) were performed as previously described (11, 15, 27). Antimicrobial susceptibility testing of the bacterial isolates was performed as part of the routine BC analyses with the Vitek 2 (bioMérieux) and/or Etest (bioMérieux), and interpreted according to EUCAST guidelines. Additionally, PCR amplification and sequencing analyses were performed to confirm the presence of the blaKPC (for Klebsiella pneumoniae), mecA (for staphylococci), or vanA or vanB (for enterococci) genes obtained with the FilmArray BCID panel assays (see below).
MALDI BioTyper system ID.
MALDI-TOF MS assays were performed starting from 8-ml BC aliquots, as previously described (11, 15). Briefly, pellets containing bacterial or yeast cells were washed and then suspended in distilled water plus absolute ethanol. After centrifugation, each microbial pellet was extracted by adding 70% formic acid plus pure acetonitrile, and 1 μl of the final supernatant was spotted in duplicate onto a polished steel MALDI target plate (Bruker Daltonics). The spots from each sample were overlaid with 1 μl of matrix solution (α-cyano-4-hydroxycinnamic acid in 50% acetonitrile–2.5% trifluoroacetic acid) and allowed to dry. MALDI-TOF mass spectra were acquired using a Microflex LT benchtop mass spectrometer (Bruker Daltonics). The MALDI BioTyper 3.1 software (Bruker Daltonics) was used for spectra analysis and comparison with the reference database (updated with 6,270 species). Each database query returned the top 10 identification matches along with scores ranging from 0.0 to 3.0; the match with highest score was used for species identification. Scores of ≥1.8 were considered valid for species-level ID (14, 28), and samples with a score ranging of >1.7 and <1.8 were repeated once. Scores of <1.7 were considered an unreliable ID, according to the manufacturer's recommendations.
FilmArray BCID panel ID.
The FilmArray BCID panel assays were performed to potentially identify 24 microbial targets (Enterococcus spp., Listeria monocytogenes, Staphylococcus spp., Staphylococcus aureus, Streptococcus spp., Streptococcus agalactiae, S. pneumoniae, Streptococcus pyogenes, Acinetobacter baumannii, Haemophilus influenzae, Neisseria meningitidis, Pseudomonas aeruginosa, Enterobacteriaceae spp., Enterobacter cloacae complex, Escherichia coli, Klebsiella oxytoca, K. pneumoniae, Proteus spp., Serratia spp., Candida albicans, Candida glabrata, Candida krusei, Candida parapsilosis, and Candida tropicalis) and the 3 previously mentioned antibacterial resistance determinants, according to the manufacturer's instructions. Briefly, a 100-μl BC aliquot was diluted in the FilmArray dilution buffer. The diluted sample (300 μl) was injected into the prehydrated FilmArray pouch before loading it into the FilmArray system for the automated analysis steps (i.e., DNA isolation, amplification, and detection/identification). Two internal run controls were included for both the amplification and detection steps. The results were automatically provided by the software based on the post-PCR DNA melting curve data if the quality control reaction mixtures were appropriately detected. When either of the two controls failed, the result was listed as invalid. All samples with invalid or negative results were repeated once.
Data analysis.
Overall, the ID results were assessed at the following levels of comparison: (i) combined use of the MALDI BioTyper system and FilmArray BCID panel versus the reference method, (ii) the MALDI BioTyper system versus the reference method, and (iii) the FilmArray BCID panel versus the reference method. For each comparison, the results were classified as follows: correct, both (test and reference) methods provided the same ID at the species level; incorrect, both methods provided an organism ID that did not match at the species level; and no result, the MALDI BioTyper system and/or FilmArray BCID panel did not provide a reliable or valid ID result. The results of the FilmArray BCID panel were considered correct if the species identified with the reference method was included in the genus/species group that the BCID assay was designed to detect. For each method, concordance with the reference ID results was calculated as the number of correct organism IDs divided by the total number of organisms identified.
For samples in which more than one pathogen was detected, the results were classified as follows: complete identification, the MALDI BioTyper system or FilmArray BCID panel detected all pathogens identified by the reference method; and incomplete identification, at least one of the pathogens identified by the reference method was not detected by the MALDI BioTyper system or FilmArray BCID panel.
For both of the tests and the reference methods, time to final ID (TFI) was defined (measured in hours) as the interval between incubation in the automated instrument and positive signaling of the BC bottle (time to positivity [TTP]) plus the interval between positive BC bottle signaling and organism ID (time to ID [TTI]), according to the equation TFI = TTP + TTI.
The data are presented as numbers (%) or medians (interquartile range [IQR]), as appropriate. To assess the differences between the study methods, the median TFIs were compared using the Wilcoxon matched-pairs signed-rank test. A P value of ≤0.05 was considered statistically significant. The statistical analyses were performed with Stata version 11.1 (StataCorp, College Station, TX, USA).
RESULTS
During the study period, we evaluated 1,394 consecutive BSI episodes, of which 1,223 (87.7%) were monomicrobial infections and 171 (12.3%) were polymicrobial infections. Of the polymicrobial episodes, 154 episodes were caused by two organisms, and 17 episodes were caused by at least three organisms (only 3 grew 4 organisms). Among all of the BSIs, 658 (47.2%) BSIs were due to Gram-negative bacteria only, 511 (36.7%) BSIs were due to Gram-positive bacteria only, 116 (8.3%) BSIs were due to yeasts, 80 (5.7%) BSIs were due to both Gram-negative and Gram-positive bacteria, and 29 (2.1%) BSIs were due to both Gram-negative or Gram-positive bacteria and yeasts. A total of 1,585 organisms were isolated, of which 788 (49.7%, 164 from polymicrobial BCs) organisms were Gram-negative bacteria, 651 (41.1%, 168 from polymicrobial BCs) organisms were Gram-positive bacteria, and 146 (9.2%, 30 from polymicrobial BCs) organisms were yeasts.
Results of combined MALDI BioTyper and FilmArray BCID panel. (i) Overall results.
Of the 1,394 BSI episodes studied, the causative organisms of 97.7% (1,362 BSIs, including 153 polymicrobial episodes) were correctly identified by our MALDI BioTyper and FilmArray BCID-based algorithm (Fig. 1), with accuracy rates ranging from 88.8% for mixed Gram-negative/Gram-positive bacterial episodes to 98.5% for Gram-negative bacterial episodes. Specifically, the MALDI BioTyper system allowed for the identification of the causative organisms of 82.1% of the BSIs (1,144/1,394 of all monomicrobial episodes), and the FilmArray BCID panel allowed for the identification of the causative organisms of another 15.6% of the BSIs (218/1,394, including 65 mono- and 153 polymicrobial episodes). Of the 32 remaining BSIs (2.3%), 17 (all polymicrobial episodes) did not yield a complete ID result, and 15 (14 mono- and 1 polymicrobial episodes) did not yield a correct or valid ID result compared to the reference culture-based method.
Of the total BSI organisms, 97.9% (1,552/1,585) were correctly identified by our MALDI BioTyper and FilmArray BCID panel-based algorithm (Tables 1 and 2), with ID rates of 97.4% (634/651) for Gram-positive bacteria, 98.2% (774/788) for Gram-negative bacteria, and 98.6% (144/146) for yeasts. Of the 33 remaining microorganisms (2.1%, 19 were from polymicrobial cultures), 31 microorganisms failed to be detected, and 2 microorganisms were not correctly identified (1 Enterococcus faecium as Enterococcus faecalis and 1 Staphylococcus capitis as Staphylococcus epidermidis). Twenty-six of the 31 microorganisms were species/genera that the FilmArray BCID panel was not designed to detect (Tables 1 and 2). Four additional organisms were detected by the FilmArray BCID panel but not by the BCs; of them, 2 (1 Enterococcus sp. and 1 C. albicans) were detected in 2 BCs that grew E. coli and S. epidermidis (not shown), and 2 (both CoNS organisms) were detected in 2 BCs with otherwise-detected polymicrobial growth (see Table S1 in the supplemental material).
TABLE 1.
Overall results for direct identification of pathogens from 1,223 monomicrobial BCs using the MALDI-TOF MS (BioTyper) system and FilmArray BCID panel methodsa
| Pathogen identified by the reference methodb | No. of isolates by method type |
Total no. (%) of isolates identifiedd | |||
|---|---|---|---|---|---|
| MALDI BioTyper |
FilmArray BCID panelc |
||||
| Tested | Identified | Tested | Identified | ||
| Gram-negative bacteria | 624 | 605 | 19 | 14 | 619 (99.2) |
| A. baumannii | 21 | 20 | 1 | 1 | 21 (100) |
| Achromobacter xylosoxidans | 2 | 1 | 1 | 0e | 1 |
| Aeromonas caviae | 1 | 0 | 1 | 0 | 0 |
| Aeromonas veronii | 1 | 1 | 0 | ND | 1 |
| Bacteroides fragilis | 7 | 7 | 0 | ND | 7 (100) |
| Bacteroides thetaiotaomicron | 2 | 2 | 0 | ND | 2 |
| Bacteroides uniformis | 1 | 0 | 1 | 0e | 0 |
| B. cepacia | 1 | 1 | 0 | ND | 1 |
| Campylobacter jejuni | 2 | 2 | 0 | ND | 2 |
| Citrobacter freundii | 6 | 6 | 0 | ND | 6 (100) |
| Citrobacter koseri | 4 | 4 | 0 | ND | 4 |
| Enterobacter aerogenes | 9 | 8 | 1 | 1 | 9 (100) |
| E. cloacae | 27 | 26 | 1 | 1 | 27 (100) |
| E. coli | 236 | 233 | 3 | 3 | 236 (100) |
| H. influenzae | 1 | 1 | 0 | ND | 1 |
| Haemophilus parainfluenzae | 1 | 1 | 0 | ND | 1 |
| K. oxytoca | 11 | 11 | 0 | ND | 11 (100) |
| K. pneumoniae | 147 | 143 | 4 | 4 | 147 (100) |
| Morganella morganii | 10 | 10 | 0 | ND | 10 (100) |
| Proteus mirabilis | 21 | 20 | 1 | 1 | 21 (100) |
| Providencia stuartii | 2 | 1 | 1 | 0e | 1 |
| P. aeruginosa | 76 | 74 | 2 | 2 | 76 (100) |
| Ralstonia pickettii | 1 | 1 | 0 | ND | 1 |
| Salmonella spp. | 9 | 9 | 0 | ND | 9 (100) |
| Serratia marcescens | 19 | 18 | 1 | 1 | 19 (100) |
| S. maltophilia | 6 | 5 | 1 | 0e | 5 (83.3) |
| Gram-positive bacteria | 483 | 434 | 49 | 42 | 476 (98.5) |
| Abiotrophia defectiva | 2 | 2 | 0 | ND | 2 |
| Aerococcus urinae | 1 | 1 | 0 | ND | 1 |
| Brevibacterium casei | 2 | 1 | 1 | 0e | 1 |
| Clostridium perfringens | 3 | 3 | 0 | ND | 3 |
| C. septicum | 1 | 0 | 1 | 0e | 0 |
| Corynebacterium striatum | 1 | 0 | 1 | 0e | 0 |
| E. faecalis | 68 | 65 | 3 | 3 | 68 (100) |
| E. faecium | 36 | 34 | 2 | 1 | 35 (97.2) |
| Enterococcus gallinarum | 4 | 3 | 1 | 1 | 4 |
| Leuconostoc mesenteroides | 2 | 2 | 0 | ND | 2 |
| L. monocytogenes | 7 | 7 | 0 | ND | 7 (100) |
| Nocardia farcinica | 1 | 0 | 1 | 0e | 0 |
| Rothia mucilaginosa | 2 | 1 | 1 | 0e | 1 |
| S. aureus | 133 | 129 | 4 | 4 | 133 (100) |
| S. capitis | 15 | 11 | 4 | 3 | 14 (93.3) |
| Staphylococcus caprae | 1 | 1 | 0 | ND | 1 |
| S. epidermidis | 102 | 91 | 11 | 11 | 102 (100) |
| S. haemolyticus | 19 | 16 | 3 | 3 | 19 (100) |
| S. hominis | 24 | 19 | 5 | 5 | 24 (100) |
| Staphylococcus lugdunensis | 1 | 1 | 0 | ND | 1 |
| Staphylococcus warneri | 1 | 1 | 0 | ND | 1 |
| S. agalactiae | 6 | 6 | 0 | ND | 6 (100) |
| S. anginosus | 13 | 10 | 3 | 3 | 13 (100) |
| Streptococcus constellatus | 2 | 2 | 0 | ND | 2 |
| Streptococcus dysgalactiae | 2 | 2 | 0 | ND | 2 |
| Streptococcus gallolyticus | 7 | 7 | 0 | ND | 7 (100) |
| Streptococcus gordonii | 4 | 2 | 2 | 2 | 4 |
| Streptococcus lutetiensis | 1 | 1 | 0 | ND | 1 |
| S. mitis | 7 | 3 | 4 | 4 | 7 (100) |
| S. pneumoniae | 10 | 9 | 1 | 1 | 10 (100) |
| S. pyogenes | 3 | 3 | 0 | ND | 3 |
| Streptococcus sanguinis | 2 | 1 | 1 | 1 | 2 |
| Yeasts | 116 | 105 | 11 | 9 | 114 (98.3) |
| C. albicans | 60 | 56 | 4 | 4 | 60 (100) |
| C. glabrata | 8 | 7 | 1 | 1 | 8 (100) |
| Candida guilliermondii | 1 | 1 | 0 | ND | 1 |
| C. krusei | 3 | 2 | 1 | 1 | 3 |
| Candida lusitaniae | 1 | 1 | 0 | ND | 1 |
| Candida orthopsilosis | 3 | 3 | 0 | ND | 3 |
| C. parapsilosis | 27 | 25 | 2 | 2 | 27 (100) |
| Candida pelliculosa | 1 | 0 | 1 | 0e | 0 |
| C. tropicalis | 11 | 10 | 1 | 1 | 11 (100) |
| R. mucilaginosa | 1 | 0 | 1 | 0e | 0 |
| Total | 1,223 | 1,144 | 79 | 65 | 1,209 (98.8) |
BC, blood culture; MALDI-TOF MS, matrix-assisted laser desorption ionization–time of flight mass spectrometry; BCID, blood culture identification.
Isolates were identified using a reference method, which consisted of Gram stain microscopic examination and subcultures on routine medium, followed by MALDI-TOF MS analysis of bacterial or yeast colonies. Additionally, conventional phenotypical tests and/or sequencing analyses of the 16S rRNA and rpoB genes (for bacterial isolates) and the ITS1-5.8S-ITS2 rRNA gene regions (for yeast isolates) were performed whenever necessary. ND, not done.
FilmArray BCID panel testing of the BCs was performed according to the diagnostic algorithm presented in the study, and also when MALDI BioTyper system testing failed to provide a correct identification (i.e., which was concordant with the reference method identification) or any identification result. Furthermore, for all 9 isolates of S. pneumoniae, direct identification results by the MALDI BioTyper system were confirmed by results obtained with the FilmArray BCID panel. According to the manufacturer's product insert, isolates identified as Enterococcus potentially belonged to Enterococcus species, such as E. casseliflavus, E. durans, E. faecalis, E. faecium, and E. gallinarum; isolates identified as coagulase-negative staphylococci potentially belonged to Staphylococcus species, such as S. capitis, S. caprae, S. cohnii, S. epidermidis, S. haemolyticus, S. hominis, S. kloosii, S. lugdunensis, S. microti, S. nepalensis, S. saccharolyticus, S. saprophyticus, S. simiae, S. warneri, and S. xylosus; and isolates identified as Streptococcus potentially belonged to Streptococcus species, such as S. anginosus, S. equinus, S. gallolyticus, S. gordonii, S. mitis, S. mutans, S. oralis, S. parasanguinis, S. salivarius, S. sanguinis, and S. uberis.
Percentages for the species with <5 isolates were not calculated.
Not identifiable by the method.
TABLE 2.
Overall results for direct identification of pathogens from 171 polymicrobial BCs using the FilmArray BCID panel methoda
| Pathogen identified by the reference method (no. of isolates)b | No. (%) of isolates identified with FilmArray BCID panelc |
|---|---|
| Gram-negative bacteria (164) | 155 (94.5) |
| A. baumannii (12) | 12 (100) |
| Campylobacter rectus (1) | 0d |
| C. koseri (1) | 1 |
| C. freundii (2) | 1 |
| E. aerogenes (1) | 1 |
| E. cloacae (6) | 6 (100) |
| E. coli (38) | 38 (100) |
| K. oxytoca (2) | 2 |
| K. pneumoniae (36) | 36 (100) |
| M. morganii (3) | 0d |
| P. mirabilis (20) | 20 (100) |
| P. stuartii (1) | 0d |
| P. aeruginosa (33) | 33 (100) |
| S. marcescens (5) | 5 (100) |
| S. maltophilia (3) | 0d |
| Gram-positive bacteria (168) | 158 (94.0) |
| Bacillus cereus (2) | 0d |
| C. perfringens (2) | 0d |
| Clostridium tertium (1) | 0d |
| E. faecalis (43) | 42 (97.7) |
| E. faecium (11) | 11 (100) |
| E. gallinarum (3) | 3 |
| L. monocytogenes (1) | 1 |
| Parvimonas micra (1) | 0d |
| S. aureus (17) | 17 (100) |
| S. capitis (3) | 1 |
| S. epidermidis (47) | 47 (100) |
| S. haemolyticus (18) | 18 (100) |
| S. hominis (10) | 9 (90.0) |
| S. agalactiae (1) | 1 |
| S. anginosus (1) | 1 |
| S. gallolyticus (1) | 1 |
| S. mitis (3) | 3 |
| S. pneumoniae (2) | 2 |
| S. sanguinis (1) | 1 |
| Yeasts (30) | 30 (100) |
| C. albicans (19) | 19 (100) |
| C. glabrata (2) | 2 |
| C. krusei (1) | 1 |
| C. parapsilosis (6) | 6 (100) |
| C. tropicalis (2) | 2 |
| Total (362) | 343 (94.7) |
BC, blood culture; BCID, blood culture identification.
Isolates were identified using a reference method, which consisted of Gram stain microscopic examination and subcultures on routine medium, followed by MALDI-TOF MS analysis of bacterial or yeast colonies. Additionally, conventional phenotypical tests and/or sequencing analyses of the 16S rRNA and rpoB genes (for bacterial isolates) and the ITS1-5.8S-ITS2 rRNA gene regions (for yeast isolates) were performed whenever necessary.
FilmArray BCID panel testing of the polymicrobial BCs was performed according to the diagnostic algorithm presented in the study. According to the manufacturer's product insert, isolates identified as Enteric potentially belonged to enteric species, such as Cedecea davisae, Citrobacter spp., Cronobacter sakazakii, Enterobacter spp., Kluyvera ascorbata, Leclercia adecarboxylata, Raoultella spp., Salmonella spp., Shigella spp., and Yokenella regensburgei; isolates identified as Enterococcus potentially belonged to Enterococcus species, such as E. casseliflavus, E. durans, E. faecalis, E. faecium, and E. gallinarum; isolates identified as coagulase-negative staphylococci potentially belonged to Staphylococcus species, such as S. capitis, S. caprae, S. cohnii, S. epidermidis, S. haemolyticus, S. hominis, S. kloosii, S. lugdunensis, S. microti, S. nepalensis, S. saccharolyticus, S. saprophyticus, S. simiae, S. warneri, and S. xylosus; isolates identified as Streptococcus potentially belonged to Streptococcus species, such as S. anginosus, S. equinus, S. gallolyticus, S. gordonii, S. mitis, S. mutans, S. oralis, S. parasanguinis, S. salivarius, S. sanguinis, and S. uberis. Percentages for the species with <5 isolates were not calculated.
Not identifiable by the method.
(ii) Monomicrobial cultures.
The MALDI BioTyper system identified 1,144 (93.5%) out of 1,223 isolates recovered from monomicrobial cultures, including 605 (96.9%) out of 624 Gram-negative bacteria, 105 (90.5%) out of 116 yeasts, and 434 (89.9%) out of 483 Gram-positive bacteria (Table 1). Additionally, the FilmArray BCID panel identified 65 (5.3%) isolates that were initially tested but not identified with the MALDI BioTyper system. As shown, all 10 isolates of S. pneumoniae (9 of which were identified with MALDI BioTyper) were tested and identified as such by the FilmArray BCID panel.
(iii) Polymicrobial cultures.
The FilmArray BCID panel identified 343 (94.7%) of the 362 isolates recovered from polymicrobial cultures, including 155 (94.5%) out of 164 Gram-negative bacteria, 158 (94.0%) out of 168 Gram-positive bacteria, and 30 (100%) out of 30 yeasts (Table 2). In 31 of the 171 polymicrobial episodes, BCs were not directly processed with the FilmArray BCID panel, because different Gram stain morphologies were not observed (Fig. 1). However, the MALDI BioTyper results indicated no reliable ID (15 episodes) or suggested the presence of more than one species (16 episodes).
As detailed in Table S1 in the supplemental material, the FilmArray BCID panel provided incomplete ID results for all of the polymicrobial assortments of BCs tested, including Gram-negative bacteria alone (n = 5; 34 BCs tested), Gram-positive bacteria alone (n = 3; 28 BCs tested), Gram-negative and Gram-positive bacteria (n = 8; 80 BCs tested), or bacteria (Gram-negative/Gram-positive) and yeasts (n = 1; 29 BCs tested). In the last group, an incomplete ID was reported for only 1 BC that included Staphylococcus hominis (mecA positive), C. albicans, and Stenotrophomonas maltophilia, with the last organism giving an expected negative result (the species was not detectable by the FilmArray BCID panel).
The 362 polymicrobial BC broths were also tested with the MALDI BioTyper (see Table S1 in the supplemental material). As expected, the method correctly identified 84 (51.2%) out of 164 Gram-negative bacteria and 21 (12.5%) out of 168 Gram-positive bacteria. Importantly, the MALDI BioTyper missed the identification of all 30 Candida species; no ID was produced in 19 out of 29 mixed bacterial/yeast BSIs, and an incomplete ID result was obtained for the remaining 10 BSIs. As previously mentioned, all but 1 of the 29 BSIs gave a complete ID result with the FilmArray BCID panel method.
(iv) Time savings.
The median TTP for all 1,394 BCs included in this study was 12.2 h (IQR, 8.2 to 17.5 h), with 10.4 h (IQR, 7 to 15.1 h) for BCs containing Gram-negative bacteria, 15.2 h (IQR, 10.3 to 18.5 h) for BCs containing Gram-positive bacteria, and 16.4 h (IQR, 10.3 to 28.0 h) for BCs containing yeasts. For polymicrobial BCs, the median TTP was 10.5 h (IQR, 6 to 16.0 h). For each BSI episode, the median TFI for the direct method (MALDI BioTyper system and/or FilmArray BCID panel) was 19.5 h (IQR, 14.3 to 26.5 h), which was in contrast to the TFI of 41.7 h (IQR, 35.5 to 53.0 h) for the culture-based reference method (P < 0.001). Depending on whether Gram-negative or Gram-positive bacteria (and/or yeasts) were present, TFIs were 17.2 to 21.5 h and 40.6 to 49.5 h, respectively.
Detection of antibiotic resistance markers by FilmArray BCID.
Using the FilmArray BCID panel, the oxacillin resistance determinant mecA was correctly identified for 103 out of 121 Staphylococcus spp., including 8 methicillin-resistant S. aureus organisms, 5 methicillin-susceptible S. aureus organisms, 64 oxacillin-resistant CoNS organisms, and 26 oxacillin-susceptible CoNS organisms. In 8 BC broths with S. aureus plus CoNS, the FilmArray panel detected mecA without being able to distinguish which of the organisms was carrying the resistance gene. Subsequent molecular analysis revealed that the S. aureus isolate was mecA positive and the CoNS was mecA negative in 2 cases, the S. aureus isolate was mecA positive and the CoNS was mecA positive in 2 cases, and the S. aureus isolate was mecA negative and the CoNS was mecA positive in 4 cases. False-positive mecA detection was observed in 2 polymicrobial BCs that grew only Gram-negative bacteria and 2 BCs that grew CoNS organisms that tested mecA negative during the confirmatory molecular analysis. Additionally, the FilmArray BCID panel correctly reported 2 positive and 60 negative vanA or vanB results for the enterococci and 19 positive blaKPC results for the K. pneumoniae organisms.
DISCUSSION
To the best of our knowledge, this study represents the first large-scale clinical evaluation of the use of the MALDI BioTyper system coupled to the FilmArray BCID panel for the direct detection/identification of the causative organism of BSIs from positive BCs. Through integrated testing with both methods, our diagnostic algorithm allowed the successful identification of ∼98% of the BSI-causing organisms studied and more importantly potentiated the utility of each method individually. This approach resulted in 65/1,223 (5.3%) monomicrobial cultures whose organisms could not be correctly detected or that failed to be identified with the MALDI BioTyper system but were accurately identified with the FilmArray BCID panel; a total of 9 of the 65 microorganisms were Candida yeasts. Furthermore, 153/171 (89.5%) polymicrobial cultures contained organisms that were identified in their completeness (although at least one of the microorganisms present in these cultures was identified by the FilmArray BCID panel).
Conversely, the full use of the MALDI BioTyper system (i.e., use of the method not on the basis of the initial [Gram-stain driven] discrimination between BCs with mono- or polymicrobial growth) would have resulted in the identification of only 1 causative organism in 97/171 (56.7%) of the polymicrobial cultures. Exceptionally, the second causative organism would have been identified in 3/171 (1.7%) of the polymicrobial cultures (1 E. coli + 1 K. pneumoniae, 1 E. coli + 1 S. epidermidis, and 1 K. pneumoniae + 1 S. epidermidis); in 1 additional polymicrobial culture (1 E. coli + 1 E. faecalis + 1 E. faecium), only E. faecium would have been identified out of the two enterococcal species present in the BC broth (see Table S1 in the supplemental material).
In theory, MALDI-TOF MS is capable of identifying a wide range of pathogens in broths from BC bottles. However, the method requires manual processing, such as centrifugation or filtration concentration, prior to analysis, and the MALDI-TOF MS procedures for BC are still being standardized (13, 29, 30). Low microbial loads, especially low fungal loads (<104 CFU/ml), can be a relevant cause of ineffective direct ID using the MALDI BioTyper system (11, 28). Taking advantage of our consolidated experience with this method (11, 15), we used a starting volume of 8 ml instead of the 1.5 ml used elsewhere (28) for each of the positive BC broths subjected to multiple preanalytical treatments (including the centrifugation and washing steps). Therefore, we noted that the direct MALDI BioTyper method had a high performance (≥90%) in monomicrobial BCs that were positive for Gram-positive bacteria or yeasts. Both of these organisms may present more difficulties for a species-level ID than with Gram-negative bacteria (∼97% identified in this study), because the formic acid and the MALDI matrix solution cannot efficiently break down their thick or robust cell walls. Specifically, the MALDI BioTyper generated a correct species ID for 73.3% of S. capitis, 79.2% of S. hominis, 84.2% of Staphylococcus haemolyticus, 89.2% of S. epidermidis (among staphylococci), and 76.9% of Streptococcus anginosus (among streptococci) organisms; in contrast, 96.9% of S. aureus, 94.4% to 95.6% of E. faecium or E. faecalis, and 9 of the 10 S. pneumoniae organisms isolated from monomicrobial BC broths were correctly identified. Despite known limitations with this streptococcal species due to its relatedness to Streptococcus mitis group members, MALDI BioTyper showed high accuracy for the identification of S. pneumoniae organisms, which was confirmed by the FilmArray BCID results obtained with the same isolates.
The FilmArray BCID panel is one of the newly developed rapid diagnostic methods for BSIs. Recent studies reported the performance of this assay with BCs collected primarily from adult patients (18–22, 24). Two of these studies compared the FilmArray BCID panel with MALDI-TOF MS (22, 24); most recently, Bhatti et al. (20) evaluated the FilmArray BCID panel in comparison with the (other FDA-cleared) Verigene BC system (Nanosphere, Northbrook, IL, USA) (20). In that study, both platforms correctly identified Gram-positive and Gram-negative bacteria in 92% of the monomicrobial cultures tested; this ID rate was almost identical to the rate obtained by Blaschke et al. (18) and Altun et al. (19) in clinical performance evaluations of the FilmArray BCID panel. Zheng et al. (21) evaluated the FilmArray BCID panel by specifically testing BCs collected from pediatric patients. In the study by Rand et al. (22), the slight superiority of the FilmArray BCID panel over the MALDI-TOF MS system (Vitek MS; bioMérieux) was demonstrated for 151 monomicrobial BC bottles, although the authors excluded 18 monomicrobial organisms that were not identified, as expected from the manufacturer's database. Interestingly, the FilmArray BCID panel identified 20/21 (95.2%) organisms, in contrast to the 1 organism identified with the Vitek MS system from 7/10 (70.0%) polymicrobial cultures analyzed in that study (22).
One strength of our study is that the cultures with polymicrobial growth accounted for a relatively large percentage of BCs (171/1,394 BCs [12.3%]). Testing these cultures (which were ∼7- to 17-fold higher in number than those tested elsewhere [19, 22]) allowed us not only to confirm and strengthen the usefulness of the FilmArray BCID panel for the diagnosis of BSI but also to establish the validity of a clinical laboratory diagnostic strategy that applied the FilmArray BCID assay to selected clinical specimens/situations. The method has several advantages; for instance, it contains antibiotic resistance determinants that make the FilmArray BCID panel particularly useful in cases of BSI due to organisms, such as S. aureus and Enterococcus spp., for which the rapid detection of the mecA and vanA or vanB genes might impact therapeutic decisions. Nonetheless, based on our diagnostic algorithm, we did not triage all of the BCs with monomicrobial growth for S. aureus or Enterococcus identified by the MALDI BioTyper for antimicrobial resistance testing. However, clinical microbiology laboratories, such as ours, are equipped with molecular assay alternatives to the FilmArray BCID panel that allow rapid (and more inexpensive) testing at least for methicillin resistance in S. aureus organisms. After the initial investment, excessive costs (pricing for the FilmArray is in the range of €150 to 159/test) might arise through integral (not circumstantiated) use of the FilmArray BCID panel. The estimated cost savings with our diagnostic strategy, which minimizes the use of FilmArray BCID panel (218 BCs tested versus 1,394 testable BCs) is €176,480. In contrast, full use of the MALDI BioTyper (1,394 BCs tested) would have resulted in an overall reagent/consumable expenditure of €4,042.6 (€2.9/sample). However, this difference is not so remarkable if we consider the technologist time/labor involved with the MALDI BioTyper processing, which is considerably higher than that of the FilmArray BCID panel, because this assay requires only 3 min of hands-on processing and approximately 1 h of instrument time for an individual test.
Despite differing hands-on time requirements, as expected, both methods (alone or in combination) led to a significantly shortened TFI by 22.2 h compared with routine (conventional) methods. Due to the design of our study, we did not include an analysis of patient outcomes. However, it is plausible that a rapid and precise ID to the species level and a rapid differentiation between more virulent organisms (S. aureus versus L. monocytogenes) or between organisms with predictable antimicrobial resistance (Enterobacter spp. versus K. pneumoniae) may have helped our physicians in the clinical and/or therapeutic management of BSI patients. Additional studies will be necessary to clearly establish the clinical impact of our diagnostic strategy, particularly in light of the evidence that performing rapid ID for patients with positive BCs does not affect antimicrobial therapies without resistance genotyping and/or clinical intervention (31). However, the implementation of such an algorithm would imply that some positive BCs are unnecessarily subjected to the MALDI BioTyper/FilmArray BCID panel testing in daily clinical laboratory practice because they would be judged to be clinically significant only a posteriori.
One limitation of our study is the lack of or insufficient testing of certain pathogens. For example, both methods can detect N. meningitidis, but no culture grew this microorganism; other pathogens, such as H. influenzae, Burkholderia cepacia, Clostridium septicum, and Rhodotorula mucilaginosa, were tested only once. The list of microorganisms included in the FilmArray BCID panel may encompass almost 95% of the organisms commonly found in positive BCs; however, 26/31 (83.9%) ID failures in our study were due to organisms that were undetectable by the method. Future improvements might allow the spectrum of microbial targets detected by the FilmArray BCID panel to be enlarged. However, at the time of this writing, subculture was indispensable for a small number of BSI episodes, and the growth of colonies was needed to perform susceptibility testing for all BSI episodes. Another drawback of the FilmArray BCID panel is the rate of false-positive results (especially false-positive Enterococcus species results) that occurs despite a product recently optimized by the manufacturer to improve sample specificity. This optimization was designed to mitigate the cross-reactivity of the Enterococcus assay with Staphylococcus organisms (heavy growth of CoNS was noted to cause this effect [31]) and was shown to have a positive impact on the FilmArray BCID panel performance (32).
In conclusion, we demonstrated the reliability of a rapid, accurate, and cost-effective approach for the direct ID of the microorganisms most commonly isolated from BCs. We used two innovative methods (the FilmArray BCID panel and MALDI BioTyper system) with differing but excellent performances to achieve fast and efficient BC testing for the clinical microbiology laboratory. Whether this approach can be an important and useful tool for the management of BSI remains to be established in the future.
Supplementary Material
ACKNOWLEDGMENTS
We thank Leopoldo Dimiziani for providing technical support with the Microflex LT MALDI-TOF during this study, and the American Journal Experts staff for their assistance in editing the manuscript.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02590-15.
REFERENCES
- 1.Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Peterson LE, Musser JM. 2014. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect 69:216–225. doi: 10.1016/j.jinf.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Delle Rose D, Sordillo P, Gini S, Cerva C, Boros S, Rezza G, Meledandri M, Gallo MT, Prignano G, Caccese R, D'Ambrosio M, Citterio G, Rocco M, Leonardis F, Natoli S, Fontana C, Favaro M, Celeste MG, Franci T, Testore GP, Andreoni M, Sarmati L. 2015. Microbiologic characteristics and predictors of mortality in bloodstream infections in intensive care unit patients: a 1-year, large, prospective surveillance study in 5 Italian hospitals. Am J Infect Control 43:1178–1183. [DOI] [PubMed] [Google Scholar]
- 3.Oriol I, Sabé N, Melilli E, Lladó L, González-Costello J, Soldevila L, Carratalà J. 2015. Factors influencing mortality in solid organ transplant recipients with bloodstream infection. Clin Microbiol Infect 21:1104.e9–1104.e14. [DOI] [PubMed] [Google Scholar]
- 4.Opota O, Croxatto A, Prod'hom G, Greub G. 2015. Blood culture-based diagnosis of bacteraemia: state of the art. Clin Microbiol Infect 21:313–322. doi: 10.1016/j.cmi.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Tan KE, Ellis BC, Lee R, Stamper PD, Zhang SX, Carroll KC. 2012. Prospective evaluation of a matrix-assisted laser desorption ionization–time of flight mass spectrometry system in a hospital clinical microbiology laboratory for identification of bacteria and yeasts: a bench-by-bench study for assessing the impact on time to identification and cost-effectiveness. J Clin Microbiol 50:3301–3308. doi: 10.1128/JCM.01405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clerc O, Prod'hom G, Vogne C, Bizzini A, Calandra T, Greub G. 2013. Impact of matrix-assisted laser desorption ionization time-of-flight mass spectrometry on the clinical management of patients with Gram-negative bacteremia: a prospective observational study. Clin Infect Dis 56:1101–1107. doi: 10.1093/cid/cis1204. [DOI] [PubMed] [Google Scholar]
- 7.Vlek AL, Bonten MJ, Boel CH. 2012. Direct matrix-assisted laser desorption ionization time-of-flight mass spectrometry improves appropriateness of antibiotic treatment of bacteremia. PLoS One 7:e32589. doi: 10.1371/journal.pone.0032589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Scola B, Raoult D. 2009. Direct identification of bacteria in positive blood culture bottles by matrix-assisted laser desorption ionisation time-of-flight mass spectrometry. PLoS One 4:e8041. doi: 10.1371/journal.pone.0008041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevenson LG, Drake SK, Murray PR. 2010. Rapid identification of bacteria in positive blood culture broths by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 48:444–447. doi: 10.1128/JCM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christner M, Rohde H, Wolters M, Sobottka I, Wegscheider K, Aepfelbacher M. 2010. Rapid identification of bacteria from positive blood culture bottles by use of matrix-assisted laser desorption–ionization time of flight mass spectrometry fingerprinting. J Clin Microbiol 48:1584–1591. doi: 10.1128/JCM.01831-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spanu T, Posteraro B, Fiori B, D'Inzeo T, Campoli S, Ruggeri A, Tumbarello M, Canu G, Trecarichi EM, Parisi G, Tronci M, Sanguinetti M, Fadda G. 2012. Direct MALDI-TOF mass spectrometry assay of blood culture broths for rapid identification of Candida species causing bloodstream infections: an observational study in two large microbiology laboratories. J Clin Microbiol 50:176–179. doi: 10.1128/JCM.05742-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagacé-Wiens PR, Adam HJ, Karlowsky JA, Nichol KA, Pang PF, Guenther J, Webb AA, Miller C, Alfa MJ. 2012. Identification of blood culture isolates directly from positive blood cultures by use of matrix-assisted laser desorption ionization–time of flight mass spectrometry and a commercial extraction system: analysis of performance, cost, and turnaround time. J Clin Microbiol 50:3324–3328. doi: 10.1128/JCM.01479-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JH, Ho PL, Kwan GS, She KK, Siu GK, Cheng VC, Yuen KY, Yam WC. 2013. Direct bacterial identification in positive blood cultures by use of two commercial matrix-assisted laser desorption ionization–time of flight mass spectrometry systems. J Clin Microbiol 51:1733–1739. doi: 10.1128/JCM.03259-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez RM, Bauerle ER, Fang FC, Butler-Wu SM. 2014. Evaluation of three rapid diagnostic methods for direct identification of microorganisms in positive blood cultures. J Clin Microbiol 52:2521–2529. doi: 10.1128/JCM.00529-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiori B, D'Inzeo T, Di Florio V, De Maio F, De Angelis G, Giaquinto A, Campana L, Tanzarella E, Tumbarello M, Antonelli M, Sanguinetti M, Spanu T. 2014. Performance of two resin-containing blood culture media in detection of bloodstream infections and in direct matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) broth assays for isolate identification: clinical comparison of the BacT/Alert Plus and Bactec Plus systems. J Clin Microbiol 52:3558–3567. doi: 10.1128/JCM.01171-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Sánchez B, Sánchez-Carrillo C, Ruiz A, Marín M, Cercenado E, Rodríguez-Créixems M, Bouza E. 2014. Direct identification of pathogens from positive blood cultures using matrix-assisted laser desorption-ionization time-of-flight mass spectrometry. Clin Microbiol Infect 20:O421-–O427. doi: 10.1111/1469-0691.12455. [DOI] [PubMed] [Google Scholar]
- 17.Bidart M, Bonnet I, Hennebique A, Kherraf ZE, Pelloux H, Berger F, Cornet M, Bailly S, Maubon D. 2015. An in-house assay is superior to Sepsityper for direct matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry identification of yeast species in blood cultures. J Clin Microbiol 53:1761–1764. doi: 10.1128/JCM.03600-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaschke AJ, Heyrend C, Byington CL, Fisher MA, Barker E, Garrone NF, Thatcher SA, Pavia AT, Barney T, Alger GD, Daly JA, Ririe KM, Ota I, Poritz MA. 2012. Rapid identification of pathogens from positive blood cultures by multiplex polymerase chain reaction using the FilmArray system. Diagn Microbiol Infect Dis 74:349–355. doi: 10.1016/j.diagmicrobio.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altun O, Almuhayawi M, Ullberg M, Ozenci V. 2013. Clinical evaluation of the FilmArray blood culture identification panel in identification of bacteria and yeasts from positive blood culture bottles. J Clin Microbiol 51:4130–4136. doi: 10.1128/JCM.01835-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatti MM, Boonlayangoor S, Beavis KG, Tesic V. 2014. Evaluation of FilmArray and Verigene systems for rapid identification of positive blood cultures. J Clin Microbiol 52:3433–3436. doi: 10.1128/JCM.01417-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng X, Polanco W, Carter D, Shulman S. 2014. Rapid identification of pathogens from pediatric blood cultures by use of the FilmArray blood culture identification panel. J Clin Microbiol 52:4368–4371. doi: 10.1128/JCM.02133-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rand KH, Delano JP. 2014. Direct identification of bacteria in positive blood cultures: comparison of two rapid methods, FilmArray and mass spectrometry. Diagn Microbiol Infect Dis 79:293–297. doi: 10.1016/j.diagmicrobio.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Southern TR, VanSchooneveld TC, Bannister DL, Brown TL, Crismon AS, Buss SN, Iwen PC, Fey PD. 2015. Implementation and performance of the BioFire FilmArray blood culture identification panel with antimicrobial treatment recommendations for bloodstream infections at a midwestern academic tertiary hospital. Diagn Microbiol Infect Dis 81:96–101. doi: 10.1016/j.diagmicrobio.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Almuhayawi M, Altun O, Strålin K, Ozenci V. 2014. Identification of microorganisms by FilmArray and matrix-assisted laser desorption ionization–time of flight mass spectrometry prior to positivity in the blood culture system. J Clin Microbiol 52:3230–3236. doi: 10.1128/JCM.01084-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elzi L, Babouee B, Vögeli N, Laffer R, Dangel M, Frei R, Battegay M, Widmer AF. 2012. How to discriminate contamination from bloodstream infection due to coagulase-negative staphylococci: a prospective study with 654 patients. Clin Microbiol Infect 18:E355–E361. doi: 10.1111/j.1469-0691.2012.03964.x. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Jiménez MC, Muñoz P, Valerio M, Alonso R, Martos C, Guinea J, Bouza E. 2015. Candida biomarkers in patients with candidaemia and bacteraemia. J Antimicrob Chemother 70:2354–2361. doi: 10.1093/jac/dkv090. [DOI] [PubMed] [Google Scholar]
- 27.De Carolis E, Vella A, Vaccaro L, Torelli R, Posteraro P, Ricciardi W, Sanguinetti M, Posteraro B. 2014. Development and validation of an in-house database for matrix-assisted laser desorption ionization–time of flight mass spectrometry-based yeast identification using a fast protein extraction procedure. J Clin Microbiol 52:1453–1458. doi: 10.1128/JCM.03355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paolucci M, Foschi C, Tamburini MV, Ambretti S, Lazzarotto T, Landini MP. 2014. Comparison between MALDI-TOF MS and FilmArray blood culture identification panel for rapid identification of yeast from positive blood culture. J Microbiol Methods 104:92–93. doi: 10.1016/j.mimet.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Yan Y, He Y, Maier T, Quinn C, Shi G, Li H, Stratton CW, Kostrzewa M, Tang YW. 2011. Improved identification of yeast species directly from positive blood culture media by combining Sepsityper specimen processing and Microflex analysis with the matrix-assisted laser desorption ionization Biotyper system. J Clin Microbiol 49:2528–2532. doi: 10.1128/JCM.00339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fothergill A, Kasinathan V, Hyman J, Walsh J, Drake T, Wang YF. 2013. Rapid identification of bacteria and yeasts from positive-blood-culture bottles by using a lysis-filtration method and matrix-assisted laser desorption ionization–time of flight mass spectrum analysis with the SARAMIS database. J Clin Microbiol 51:805–809. doi: 10.1128/JCM.02326-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerjee R, Teng CB, Cunningham SA, Ihde SM, Steckelberg JM, Moriarty JP, Shah ND, Mandrekar JN, Patel R. 2015. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 61:1071–1080. doi: 10.1093/cid/civ447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altun O, Özenci V. 2015. FilmArray: correction of previously false-positive results by improved software. J Clin Microbiol 53:750. doi: 10.1128/JCM.02508-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.