Abstract
Candida bloodstream infections (BSI) are associated with significant morbidity, mortality, and increased health care costs. Early treatment is essential, because delayed therapy detrimentally impacts clinical outcomes. The FDA recently approved the first culture-independent direct molecular detection method for Candida BSIs (T2Candida). The speed and sensitivity of this assay give it the potential to improve patient care, but the reagents and instrumentation are expensive. We used an analytic decision tree model to compare the cost-effectiveness of T2Candida-directed antifungal therapy (T2DT) to that of either empirical therapy (ET) or blood culture-directed therapy (BCDT). The costs included those of T2Candida testing, antifungal treatment, and hospital length of stay. The effectiveness measure was survival status at hospital discharge. T2DT was less costly and more effective than BCDT but was less costly and less effective than ET with an echinocandin (incremental cost-effectiveness ratio, $111,084 per additional survivor). One-way sensitivity analyses demonstrated that the cost-effectiveness of T2DT was highly dependent on Candida BSI prevalence and the cost of antifungal therapy and T2Candida test reagents. The use of T2DT reduced the number of unnecessarily treated patients by 98% relative to that with ET. Reduced drug exposure might lessen the possibility of drug-related adverse events and may also prevent the development of antifungal resistance or emergence of drug-resistant Candida species. The greatest benefit of T2Candida appears to be the ability to confidently withhold or stop empirical antifungal therapy in low-to-moderate-risk patients who are unlikely to benefit from treatment.
INTRODUCTION
Candida spp. are the fourth leading cause of bloodstream infections (BSI) in hospitalized patients and the third most common cause of BSI in the intensive care unit (ICU) (1). These infections are not only prevalent but are also associated with significant morbidity, mortality, and increased heath care costs. Attributable mortality rates range from 5 to 71%, depending on the patient population (2). Early treatment of infected patients is critical, because delays in effective therapy significantly impact outcomes (3–5), but difficulties in making a microbiological diagnosis often contribute to delayed treatment.
Blood culture is the current gold standard for diagnosing Candida BSI; however, culture is relatively insensitive and requires several days to complete. As a result of the limitations of culture, patients with risk factors for invasive candidiasis (IC) are often given immediate empirical antifungal therapy (6). Although empirical therapy reduces delays, it also subjects some patients to unnecessary treatment, which in turn increases expenses, may cause adverse side effects, and potentially contributes to the emergence of antifungal resistance (7–10). More rapid and sensitive diagnostic methods are needed for the early and accurate diagnosis of Candida BSI.
The U.S. Food and Drug Administration (FDA) recently approved the first in vitro diagnostic assay for direct detection of Candida, the T2Candida (T2 Biosystems, Inc.) (11). This rapid test detects the five most common Candida spp. directly from whole-blood specimens with high sensitivity and specificity compared to blood culture (11). The assay has the potential to speed diagnosis, reduce treatment delays, and minimize unnecessary antifungal drug use. The purpose of this study was to investigate the factors contributing to the cost-effectiveness of T2Candida testing compared to current practice, which includes empirical or blood culture-directed treatment.
MATERIALS AND METHODS
An analytic decision tree model was constructed to compare the cost-effectiveness of T2Candida-directed antifungal therapy to that of either empirical therapy or blood culture-directed therapy for the treatment of candidemia.
Perspective and time horizon.
A hospital perspective was adopted for the model and included costs and outcomes accrued over a treatment episode. Each treatment episode began when a blood culture was ordered for a suspected infection (t = 0) and ended upon the cessation of treatment related to the suspected infection.
Management strategies.
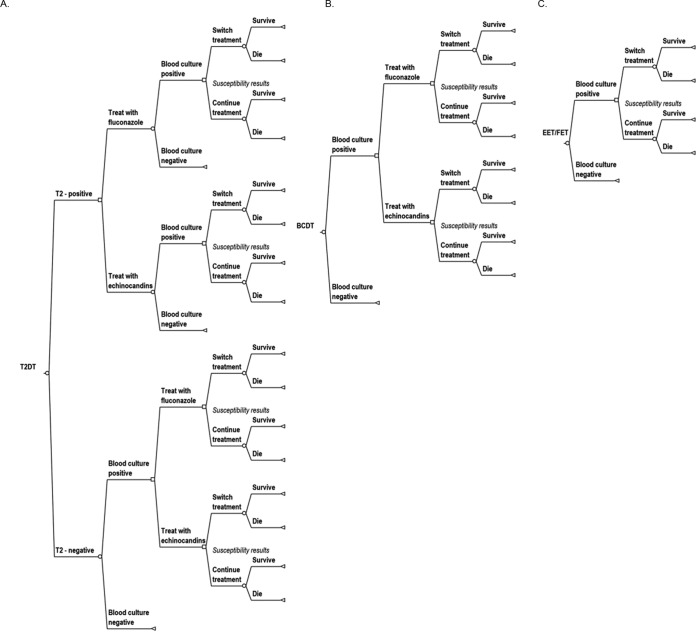
Four different antifungal therapeutic interventions were compared: (i) T2Candida-directed therapy (T2DT), (ii) echinocandin empirical therapy (EET), (iii) fluconazole empirical therapy (FET), and (iv) blood culture-directed therapy (BCDT) (Fig. 1). Antifungal therapy was initiated at the beginning of the episode (t = 0) for EET, FET, and T2DT. We assumed that the 3- to 5-h turnaround time required for T2DT testing did not significantly impact patient outcomes. For BCDT, therapy was initiated only after Candida was identified in a positive blood culture. The antifungal drug class selected for T2DT and BCDT was determined by species-level identification of the causative organism. An echinocandin was selected initially for Candida glabrata or C. krusei, and fluconazole was used when C. albicans, C. tropicalis, or C. parapsilosis was detected. The initial therapy was then modified if phenotypic susceptibility results indicated insufficient or excessive treatment. Therapy was defined as appropriate if the patient's isolate tested susceptible to the antifungal drug used and inappropriate if the isolate was resistant. We assumed all isolates were susceptible to echinocandins.
FIG 1.
Treatment arm decision trees. In all arms, when a species-level identification is made, using either T2Candida or blood culture rapid species identification methods, antifungal treatment was based on the organism. C. albicans, C. tropicalis, and C. parapsilosis were treated with fluconazole, while C. krusei and C. glabrata were treated with echinocandins. For the T2DT and EET/FET arms, treatment was stopped if the blood culture was negative. In all arms, treatment can be altered based on susceptibility results. Infections treated with an echinocandin were switched to fluconazole if the organism isolated was fluconazole susceptible. Infections treated with fluconazole were escalated to an echinocandin if the isolated organism was fluconazole resistant. (A) T2Candida-directed therapy (T2DT) decision tree. All T2Candida tests were followed by a confirmatory blood culture. (B) Blood culture-directed therapy (BCDT) decision tree. (C) Empirical therapy decision tree: echinocandin empirical therapy (EET) or fluconazole empirical therapy (FET).
Analysis parameters.
The input probabilities (Table 1) and cost parameters (Table 2) for the baseline model are described below.
TABLE 1.
Model probabilities
| Probabilitya | Mean value (reference[s]) (%) | 95% CI (%) | β distribution |
|---|---|---|---|
| In ICU | 42 (14–17) | 37–47 | f(156.92, 216.7) |
| Prevalence of Candida BSI | 3 (13) | 1.5–5 | f(10.75, 347.51) |
| Mortality without empirical therapy | |||
| Non-ICU | 23 (5, 28, 31) | 17–30 | f(37.73, 124.89) |
| ICU | 43 (5, 28, 31) | 32–55 | f(30.95, 40.53) |
| Reduction in raw mortality due to empirical therapy (ICU and non-ICU) | 48 (3) | 33–63 | f(19.67, 21.31) |
| Distribution of Candida spp. (35) | |||
| C. albicans | 43.7 | ||
| C. tropicalis | 8.7 | ||
| C. parapsilosis | 15.5 | ||
| C. glabrata | 26.3 | ||
| C. krusei | 3.3 | ||
| Other Candida species | 2.5 | ||
| Resistance to fluconazole | |||
| C. albicans | 0.5 (26, 27) | 0.1–1.1 | f(3, 662) |
| C. tropicalis | 3.7 | 1.2–7.5 | f(5, 129) |
| C. parapsilosis | 2.6 | 1.8–6.7 | f(9, 222) |
| C. glabrata | 10.7 | 7.9–13.9 | f(43, 359) |
| C. krusei | 100 | ||
| T2Candida performance (11) | |||
| C. albicans/C. tropicalis sensitivity | 92.3 | 86.5–96.5 | f(96, 8) |
| C. albicans/C. tropicalis specificity | 98.9 | 98.4–99.4 | f(1,679, 18) |
| C. parapsilosis sensitivity | 94.2 | 86.5–98.8 | f(49, 3) |
| C. parapsilosis specificity | 99.3 | 98.8–99.6 | f(1,739, 13) |
| C. glabrata/C. krusei sensitivity | 88.1 | 81.2–93.6 | f(89, 12) |
| C. glabrata/C. krusei specificity | 99.3 | 99.8–100 | f(1,699, 1) |
All probabilities were defined by β distributions, with the exception of the distribution of Candida species, which were defined by a Dirichlet distribution.
TABLE 2.
Time and cost parametersa
| Parameter | Mean value (reference[s]) | 95% CI | γ distribution |
|---|---|---|---|
| LOS (days) | |||
| Avg | 21 (18–20) | 16.9–29 | 16.5 + f(6.33, 0.632) |
| Reduction in LOS due to early treatment | 5.5 (4, 18) | 0.3– 13.7 | f(1.494, 2.945) |
| Duration of treatment (days) | |||
| Infected patients | 16.5 (6, 21, 22) | 15–18.7 | 14 + f(6.925, 0.361) |
| Noninfected subjects | 5 | ||
| Reduction in duration of therapy due to early treatment | 1 (40) | 0.3–2.2 | f(2.838, 1.938) |
| Time to blood culture identification of species (days) | |||
| C. albicans | 1.8 (41–44) | 0.8–2.7 | f(11.111, 0.144) |
| C. tropicalis | 1.2 (41–44) | 0.2–2.6 | f(2.367, 0.423) |
| C. parapsilosis | 2.9 (41–44) | 0.5–3.0 | f(5.165, 0.29) |
| C. glabrata | 4.1 (41–44) | 1.0–5.8 | f(5.297, 0.547) |
| C. krusei | 1.2(41–44) | 0.03–3.2 | f(1.095, 0.822) |
| Time to negative culture result (days) | 5 | ||
| Time to susceptibility results after ID (days) | 1.9 | 1.1–2.9 | f(17.827, 0.107) |
| Costs ($)b | |||
| Non-ICU (per day) | 1,500 (28) | 857–2,319 | f(16, 93.75) |
| ICU (per day) | 3,300 (18, 28) | 1,886–5,103 | f(16, 206.25) |
| Fluconazole (per day) | 5.4 (29) | 3.10–8.40 | f(16, 0.34) |
| Echinocandins (per day) | 76 | 43–118 | f(16, 4.75) |
| T2Candida test (per test) | 265 | 151–410 | f(16, 16) |
| Rapid species identification (per test) | 129 |
All time and cost variables were defined by gamma distributions.
A standard deviation of 25% was assumed for all costs.
Patient population.
This cost-effectiveness model was applied to a cohort of nonneutropenic patients at risk for IC. This population, referred to as the test population, was defined as inpatients with signs and symptoms sufficient to conduct a blood culture that had at least one risk factor for IC (12). Within this test population, we assumed the prevalence of IC was 3% for the baseline model (13). The distribution of Candida spp. causing these infections is given in Table 1. Based on published literature, we predicted that 42% of patients would be managed in the ICU, and 58% of patients would be on the general ward (14–17).
LOS.
We assumed the baseline length of stay (LOS) for BCDT to be 21 days for both survivors and nonsurvivors (18–20). We also assumed that the early initiation of appropriate antifungal therapy (through EET, FET, or T2DT) reduced the LOS by 5.5 days (4, 18). Thus, patients who received early initiation of appropriate therapy had an LOS of 15.5 days, and patients who received inappropriate treatment initially or had delayed treatment had an LOS of 21 days.
Treatment duration.
The infected patients received an average duration of 16.5 days of treatment (2.5 days for mycological success [21, 22], followed by 14 days of additional treatment following the first negative blood culture result [6]). We assumed that the duration of antifungal therapy did not exceed the LOS; therefore, early appropriate treatment decreased therapy by 1 day (i.e., total duration, 15.5 days). Uninfected patients, as defined by a negative blood culture in either of the empirical therapy arms or those in the T2DT arm with a false-positive T2Candida test result, would be treated for 5 days before the blood culture was either finalized as negative or a pathogen other than Candida was identified.
Performance characteristics of laboratory diagnostic methods.
Blood culture was used as the diagnostic gold standard. We assumed that a rapid identification method, such as the FilmArray blood culture identification panel (BioFire Diagnostics, UT) or the Yeast Traffic Light PNA-FISH method (AdvanDX, MA), was used to identify Candida spp. detected in positive blood cultures, providing species identification in 1 to 2 h (23–25). If a T2Candida test had already been performed, this rapid identification method would not be necessary, and the associated laboratory costs were reduced accordingly (see “Cost Considerations,” below). An additional 1.9 days (our unpublished data) was included for the phenotypic susceptibility results, and antifungal susceptibility patterns were based on published literature (Table 1) (26, 27). The performance of the T2Candida assay compared to that of blood culture was based on the results from a recently published accuracy study (Table 1) (11).
Cost considerations.
Costs attributable to Candida infection included those of laboratory testing, antifungal therapy, and inpatient hospital costs (Table 2). Because the test population consisted of patients who were already hospitalized and at risk for IC, only the additional hospital costs (i.e., those associated with increased LOS) that were incurred due to the infection were included in the analysis. Daily costs of hospitalization for ICU and non-ICU wards were derived from two recent cost evaluations of Candida treatments (18, 28). The total costs of hospitalization were calculated by multiplying the LOS by the average daily costs.
The laboratory costs included reagent costs for either T2Candida testing or the rapid molecular tests used to identify the organism directly from positive blood culture broths. We assumed that if the initial T2Candida test failed, it would be repeated at no charge without a clinically significant delay in result turnaround time.
The costs of antifungal medications were derived from the federal supply schedule, which provides the prices at which pharmaceuticals are purchased by federal agencies (29). As recommended, these costs were inflated by 21% to better reflect the average cost paid by hospitals (30). We based our analysis on either the cost of micafungin, which represented the echinocandin class of antifungals, or intravenous fluconazole, depending on the treatment used. The total costs of antifungal medications were calculated by multiplying the duration of treatment by the daily drug costs.
Effectiveness measurements.
Effectiveness was measured by the survival of infected patients. We assumed the baseline crude mortality rate to be 43% in the ICU and 23% in the general ward when treatment was delayed until blood culture results were available (BCDT) or if the initial therapy was determined to be inappropriate based on phenotypic susceptibility testing (5, 28, 31). Earlier initiation of appropriate treatment in the EET, FET, or T2DT arms reduced mortality to 48% of the baseline rates (3), resulting in a mortality rate of 20.6% in the ICU and 11% in the general ward. The survival of noninfected patients was not influenced by early antifungal treatment. Therefore, their survival would have no effect on the incremental difference in effectiveness between treatment strategies and was excluded from the analysis. The incremental cost-effectiveness ratio (ICER) was expressed as the total costs per surviving infected patient per episode of care.
Sensitivity analysis.
One-way sensitivity analyses were conducted to assess the impact of different model assumptions on the cost-effectiveness results. The parameters (model inputs) in Fig. 2 were varied one at a time, over a range of plausible values, while all other inputs were fixed at their baseline level. Probabilistic sensitivity analyses were also performed to assess statistical uncertainty. Various combinations of model inputs were resampled 1,000 times in order to determine the uncertainty in the output. The range of parameter values for combination testing was drawn from the distributions defined in Tables 1 and 2. β distributions were used for probabilities, and γ distributions were used for costs, as recommended by guidelines (32). For costs, γ distributions were used to approximate a normal distribution to within a standard deviation of 25% of the baseline estimate. γ distributions were also used to model LOS and treatment duration to reflect the skewed distribution observed in these parameters.
FIG 2.
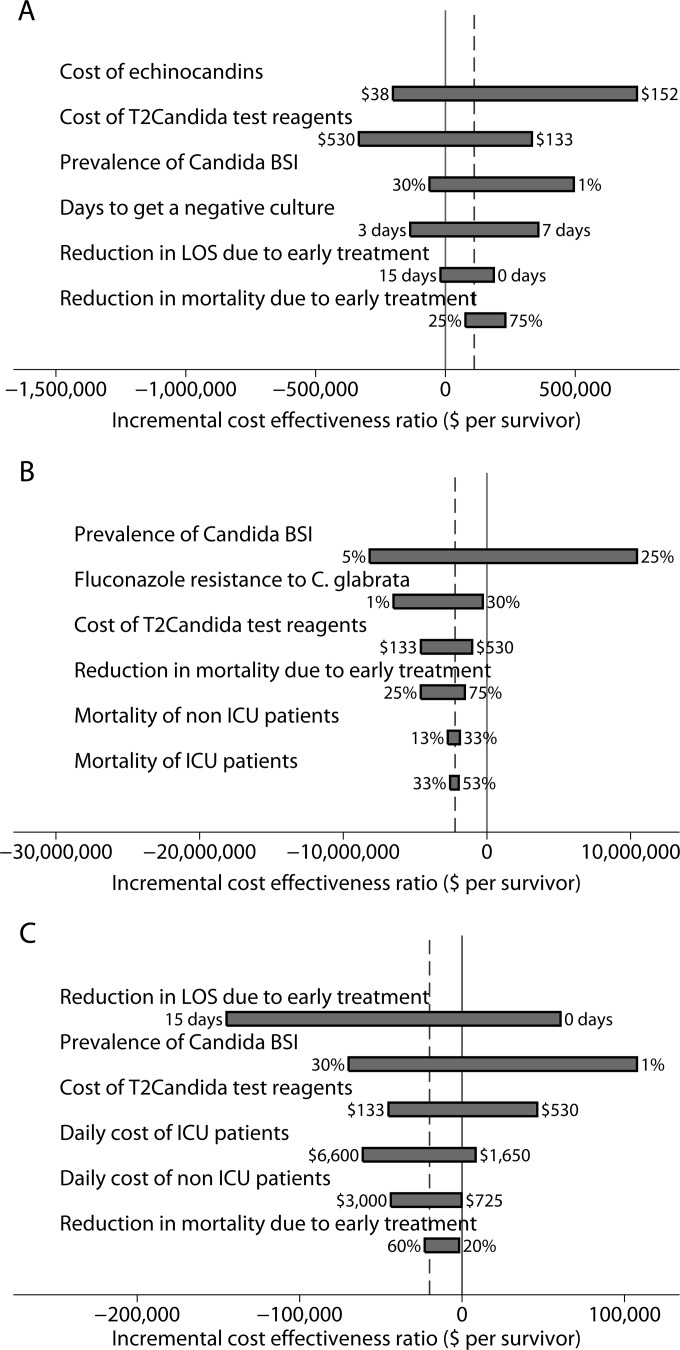
One-way sensitivity analysis to assess impact of individual parameters on cost-effectiveness of treatment arms. In each panel, the dotted line represents the baseline model parameters. (A) One-way analysis of EET versus T2DT. ICERs below zero (solid line) represent parameter values for which EET dominated T2DT. EET dominated T2DT when the cost of echinocandins was <$62, the cost of T2Candida test reagents was >$332, the prevalence of Candida BSI was >7.2%, the number of days to get an alternate diagnosis (uninfected patients) was <4 days, or the reduction in the LOS due to early treatment was >13 days. (B) One-way analysis of FET versus T2DT. ICERs above zero represent parameter values for which FET no longer dominated T2DT. FET no longer dominated T2DT when C. glabrata fluconazole resistance was >20%. (C) One-way analysis of T2DT versus BCDT. ICERs above zero represent parameter values for which T2DT no longer dominated BCDT. T2DT no longer dominated BCDT when the prevalence of Candida BSI was <2.4%, the number of reduced hospital days due to early treatment was <4.4 days, the cost of T2Candida test reagents was >$330, or the average daily costs of patients diagnosed in the ICU or the non-ICU ward was <$2,243 or $734, respectively.
Additional details of the analyses and a glossary of terms can be found in the Appendix.
RESULTS
In a test population of 10,000 at-risk patients, approximately 300 patients had a Candida bloodstream infection detected by blood culture. The T2Candida test would have detected 83% of these infections and would have falsely identified 180 infections. Empirical therapy with either an echinocandin or fluconazole led to significantly more patients being treated unnecessarily than did the T2DT approach (9,700/10,000 [97%] versus180/10,000 [1.8%] of patients treated unnecessarily). The total numbers of antifungal drug doses for empirical versus T2DT and BCDT were 53,151, 5,659, and 5,521 doses, respectively. Overall, the hospital LOS was similar for the EET (4,651 days), FET (4,788 days), and T2DT (4,851 days) arms but was prolonged when blood culture was used to inform treatment decisions (6,301 days).
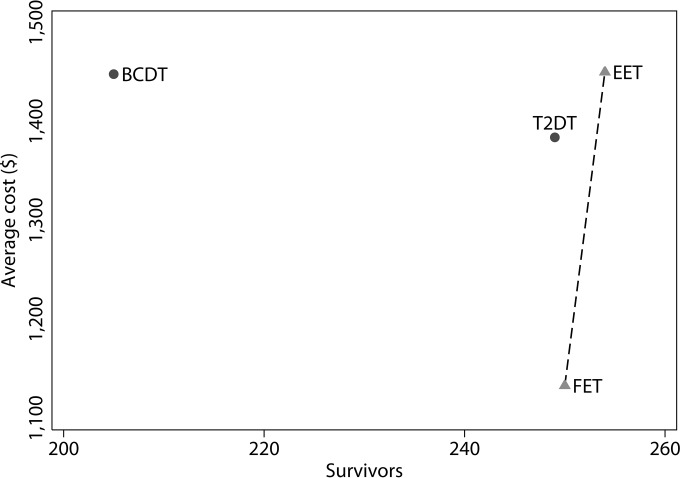
The mortality rates and average costs per patient tested are summarized in Table 3. In the baseline analysis model, EET was the most effective treatment strategy, while FET was the least costly. T2DT was more effective than BCDT but was also slightly less effective than FET and EET. Out of the approximately 300 infected patients, BCDT resulted in the fewest number of survivors (n = 205; 95% confidence interval [CI], 201 to 209 survivors), while T2DT (n = 249; 95% CI, 244 to 254 survivors), FET (n = 250; 95% CI, 245 to 255 survivors), and EET (n = 254; 95% CI, 249 to 259 survivors) saved a similar number of patients. The incremental cost-effectiveness ratio was $644,440 per each additional survivor between FET and EET and $111,084 per each additional survivor between T2DT and EET; in other words, EET had the lowest mortality rate but would cost an additional $644,440 per survivor compared to FET or $111,084 per survivor compared to T2DT (Fig. 3).
TABLE 3.
Cost-effectiveness results
| Treatment | Mortality (%) | No. of survivors at discharge (95% CI) | Avg cost per patient tested (95% CI) ($) |
|---|---|---|---|
| BCDT | 31.6 | 205 (201–209) | 1,448 (1,405–1,475) |
| T2DT | 17.2 | 249 (244–254) | 1,384 (1,352–1,407) |
| FET | 16.6 | 250 (245–255) | 1,138 (1,106–1,170) |
| EET | 15.2 | 254 (249–259) | 1,450 (1,412–1,475) |
FIG 3.
Cost-effectiveness frontier. A treatment strategy is dominated by another strategy if it is both more costly and less effective. Both BCDT and T2DT are dominated by FET, because FET is both more effective and less costly. The cost-effectiveness frontier includes treatment strategies that are not dominated by others. The frontier depicts the optimal strategies at various levels of cost and effectiveness. The slope of the lines connecting the treatment strategies (EET versus T2DT not shown) represents the incremental cost-effectiveness ratio (ICER), which provides the additional cost for each additional unit of effectiveness. The cost-effectiveness frontier is represented by the dotted line connecting FET and EET. The ICER between these two treatment strategies is $644,440 per survivor.
Sensitivity analysis. (i) EET versus T2DT.
The results of the sensitivity analysis between EET and T2DT were most sensitive to variations in the cost of echinocandins, the cost of T2Candida test reagents, the prevalence of Candida BSI, the number of days to get an alternative diagnosis, the reduction in mortality due to early appropriate treatment, and the daily cost of ICU admission (Fig. 2). Five of these factors had sufficient impact to change the conclusions when varied over their plausible range. EET was less costly than T2DT and therefore dominated when (i) the cost of echinocandins was <$62, (ii) the cost of T2Candida test reagents was >$332, (iii) the prevalence of Candida BSI was >7.2%, (iv) the number of days to reach an alternative diagnosis (uninfected patients) was <4 days, or (v) the reduction in LOS due to early appropriate treatment was >13 days (Fig. 2). Our conclusions were relatively insensitive to all other factors. Probabilistic sensitivity analysis results indicated that EET was always more effective than T2DT but was more costly 71.3% of the time (see Fig. S1 in the supplemental material).
(ii) FET versus T2DT.
The results of the sensitivity analysis between FET and T2DT were most sensitive to variations in the prevalence of Candida BSI, the cost of T2Candida test reagents, the reduction in mortality due to early appropriate treatment, predicted fluconazole resistance rates, and the mortality rates in the non-ICU and ICU wards (Fig. 2). Only one of these factors had sufficient impact to change the conclusions when varied over their plausible range; FET was less effective than T2DT, and therefore no longer dominated, when the prevalence of C. glabrata resistance to fluconazole was >20% (Fig. 2). Probabilistic sensitivity analysis results indicated that FET was always less costly than T2DT and was more effective 89.1% of the time (see Fig. S1 in the supplemental material).
(iii) T2DT versus BCDT.
The results of the sensitivity analysis between T2DT and BCDT were most sensitive to the reduction in LOS due to early appropriate treatment, the prevalence of Candida infections, the cost of T2Candida test reagents, the daily costs of ICU patients, the daily costs of non-ICU patients, and the reduction in mortality due to early appropriate treatment (Fig. 2). Only four of these factors had sufficient impact to change the conclusions when varied over their plausible range. T2DT no longer dominated BCDT when one of the following conditions was met: (i) the prevalence of Candida BSI in the test population was <2.4%, (ii) the number of reduced hospital days due to early appropriate treatment was <4.4 days, (iii) the cost of T2Candida test reagents was >$330, or (iv) the average daily cost of patients diagnosed in the ICU or the non-ICU ward was <$2,243 or $734, respectively. The cost-effectiveness of T2DT relative to BCDT was relatively insensitive to all other factors. Probabilistic sensitivity analysis results indicated that compared to BCDT, T2DT was always more effective and less costly 51% of the time (see Fig. S1 in the supplemental material).
DISCUSSION
The FDA recently approved the first culture-independent direct molecular diagnostic method for Candida BSIs. The speed and sensitivity of the T2Candida assay have the potential to improve patient care, but the reagents and instrumentation are expensive. We conducted a cost-effectiveness analysis to compare T2DT with EET, FET, and BCDT. Although T2DT was less costly and more effective than BCDT, it was also more costly than FET and less effective than empirical treatment (either EET or FET). Assuming a hospital with 500 beds, 5,100 annual at-risk admissions, and an IC prevalence of 3%, we estimate that the annual cost to the institution would be $7,058,400 for T2DT, $7,384,800 for BCDT, $5,803,800 for FET, and $7,395,000 for EET. Compared to BCDT, the use of T2DT reduced Candida BSI mortality from 31.6% to 17.2%, which was only slightly higher than that of FET (16.6%) or EET (15.2%). Therefore, although T2DT is less costly and more effective than BCDT, it remains unclear whether T2DT is a cost-effective alternative to empirical therapy.
Perhaps the greatest potential benefit of T2DT is in the support of antifungal stewardship initiatives. T2DT reduced the number of unnecessarily treated patients by 98% relative to empirical treatments. Not only might reduced drug exposure lessen the possibility of drug-related adverse events, it might also prevent the development of antifungal resistance (33). The association of azole or echinocandin use with resistance or a shift toward more-resistant Candida spp. has yet to be definitely demonstrated. Nonetheless, significant reductions in unnecessary antifungal therapy may justify the implementation of a slightly less-cost-effective treatment approach.
Our findings differ from a recently published analysis of the cost-effectiveness of T2DT, which found T2Candida testing to be both more effective and less costly than alternative treatment strategies (34). The discrepancy between studies is likely explained by variations in a few key assumptions used to form the baseline model, including the choice of comparators and the timing of the initiation of antifungal therapy. Bilir et al. (34) compared T2DT to a mixed-treatment strategy, in which 40% of patients received EET, and the remaining 60% received BCDT. Our analysis was based on pure strategies in which all patients in a cohort received identical treatment. We conducted an analysis of mixed strategies and found that T2DT dominated the EET-BCDT mixed strategy when the proportion of patients receiving BCDT was >12% (see Fig. S2 in the supplemental material). We also found that a mixed FET-EET strategy dominates T2DT whenever the proportion of patients treated with echinocandin is <21% (see Fig. S2 in the supplemental material). Our analysis also differed because we assumed that empirical therapy and T2DT both began immediately following specimen collection for blood culture, rather than the 24-h delay in empirical therapy compared to T2DT (34). We reasoned that because of our test population definition, if clinical suspicion for IC was sufficient to trigger T2Candida testing, it would also be sufficient to prompt EET or FET. The impact of this decision is significant, as studies show that even short delays in the initiation of antifungal therapy increase mortality and LOS parameters, thus favoring T2DT over empirical therapy.
While we attempted to model our analysis based on published literature and clinical practice, there were several model assumptions that were favorable to the cost-effectiveness of T2Candida. First, we did not account for the initial capital required to purchase the T2Dx instrument, which is required to run the T2Candida assay. We also assumed that all T2Candida assay failures would be rectified by repetition at no cost to the hospital. In the clinical trial, approximately 12% of T2Candida tests failed to produce a result, with only 36.7% of these resolved upon initial retest (11). Failure rates may vary based on institution and might improve with experience; thus, we elected not to include this variable in our analysis.
In addition to factors that may have favored T2DT, there were other limitations to our analysis. For example, the baseline model is limited by the uncertainty surrounding many of the input variables. To address this concern, we conducted extensive sensitivity analyses to assess the robustness of our conclusions and found that the results were relatively insensitive to variation in most of the input parameters. Disease prevalence and the mortality attributable to Candida were the two most important factors affecting model predictions.
In clinical practice, the actual prevalence of IC is likely to vary based on institution and patient population (35). Interestingly, FET was less costly and more effective than T2DT when the prevalence was varied over a range from 1% to 30%, but T2DT was less costly than EET when the prevalence remained at <7.2% and more costly than BCDT when the prevalence was <2.4%. This suggests that the optimal use of T2DT may be in a moderate-risk setting where the IC prevalence is around 5% and empirical or prophylactic antifungal therapy is prescribed routinely.
We also evaluated the extent to which our results were sensitive to the mortality rate associated with Candida BSI. Although many studies have shown that early appropriate treatment reduces mortality (3, 14, 36), others have suggested that this might not be the case in the ICU (37, 38). The sensitivity analysis revealed that although the absolute costs were sensitive to mortality rates, the relative cost ranking among treatment strategies was insensitive even if we assumed that early appropriate treatment had no effect on mortality. Although it is possible that our assumptions regarding mortality may induce calibration errors (i.e., the absolute magnitude of the ICER may be incorrect), we believe that conclusions regarding the relative cost-effectiveness of the strategies are robust to our assumptions regarding mortality.
Blood culture has limited sensitivity for diagnosing invasive candidiasis, failing to detect as many as 50% of infections (39). Therefore, it is possible that patients with positive T2Candida results but negative blood cultures actually have infections. The impact that this scenario might have on the cost-effectiveness of different treatment approaches is difficult to estimate without data from prospective studies that thoroughly evaluate the clinical significance of T2Candida-positive/blood-culture-negative cases. As a preliminary investigation, an analysis in which a negative blood culture did not rule out the possibility of IC was conducted. At-risk patients who received EET, FET, or T2DT remained on that therapy for a full course of treatment despite a negative blood culture. Positive blood culture results, with subsequent phenotypic susceptibility testing, were still used to determine the appropriateness of the early antifungal therapy, and escalation/deescalation of therapy was prescribed accordingly. Based on these assumptions, the cost per patient for EET, FET, and T2DT would increase to $2,136, $1,185, and $1,391, respectively. The change in cost was minimal for T2DT and FET, but the cost of EET increased by $686 per patient. The level of effectiveness remained unchanged from the baseline model, because all infected patients eventually received the appropriate treatment based on positive blood culture results. The number of patients receiving potentially unnecessary antifungals using this approach, however, increased significantly (data not shown). In contrast to the baseline model, this analysis tests an extreme assumption that blood cultures lack appreciable sensitivity for diagnosing IC and that the T2Candida assay performs with 100% specificity. In clinical practice, however, no test is completely accurate, and the interpretation of a negative blood culture or positive T2Candida result must be done in the context of host risk factors for IC.
Compared to empirical therapy approaches, T2Candida diagnostic testing is a more costly and slightly less effective approach for the management of suspected candidemia. The absolute differences in cost and effectiveness, however, were small. The cost difference between these strategies was approximately $300, which is minimal compared to the overall cost of a hospitalized patient at risk for IC. Additionally, the differences in the mortality rates of the T2DT, EET, and FET strategies were <2%. Model-derived conclusions can be altered based on varying key input parameters over a range that would be observed at different institutions. Ideally, prospective randomized studies are required to evaluate the impact of T2DT on patient outcomes and hospital-associated costs as institutions begin to implement this groundbreaking technology.
APPENDIX
Glossary of terms. (i) Dominance.
An alternative is said to dominate other alternatives if it is less costly and more effective.
(ii) Efficient frontier.
The efficient frontier consists of the set of nondominated alternatives. In a cost-effectiveness analysis, this set represents the best attainable trade-off between cost and effectiveness. The lines connecting this set of points form a boundary, or “frontier,” in the cost-effectiveness plane.
(iii) Expected cost.
The expected cost is a weighted average of the cost of all outcomes weighted by the probability of each outcome, calculated by , where c ¯ is the expected cost, ci and pi are the cost and probability, respectively, of outcome i, and n is the number of possible outcomes.
(iv) Input parameter.
The input parameter is an argument for a function. For example, x is a parameter of y = f(x). The value of the function, or output, is specified by the value of the parameter, x.
(v) ICER.
The ICER provides the ratio of the incremental change in cost to the incremental change in effectiveness (Eff) between two alternatives: .
(vi) One-way sensitivity analysis.
The one-way sensitivity analysis is one in which one parameter is varied at a time to determine the effect on the outcome. For example, consider the function, . A one-way sensitivity analysis for x would examine the change in z that results from varying x while holding y constant at some fixed value. A one-way sensitivity analysis for y would examine the change in z due to a change in y while holding x constant. In this analysis, the outcome variable is the incremental cost-effectiveness ratio, and there are many input parameters, such as the prevalence, mortality rate, length of stay, drug costs, etc. A one-way sensitivity analysis determines the effect of one input parameter (e.g., drug cost) on the output (ICER) while holding all other input parameters constant.
(vii) Perspective.
Perspective is the vantage point of an economic analysis. The perspective determines which costs and benefits are relevant to the analysis. The payer perspective is the narrowest perspective that accounts only for costs borne by the payer (e.g., an insurance company) over a limited time horizon. Costs to patients and to society (e.g., lost productivity) are not included. The government perspective accounts for all costs borne by the government over a time horizon. Typically, this will include all medical costs associated with an event over a lifetime. The societal perspective includes all costs, including costs to the patient and lost productivity.
(viii) Probabilistic sensitivity analysis.
Probabilistic sensitivity analysis is an approach in which input parameters are sampled from a probability distribution to determine the probability distribution of the output. For example, consider the function, . Assume that x is uniformly distributed between 1 and 3, and that y is uniformly distributed between 5 and 8. The range of the distributions represents the uncertainty or a plausible range for the parameter. In probabilistic sensitivity analysis, values of x and y are randomly selected from their distributions and used to calculate z. The process is repeated thousands of times to determine the distribution of z, given the variations in x and y. Unlike one-way analysis, all input parameters are varied by randomly selection of values from their distributions.
Supplementary Material
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02971-15.
REFERENCES
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in U.S. hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Falagas ME, Apostolou KE, Pappas VD. 2006. Attributable mortality of candidemia: a systematic review of matched cohort and case-control studies. Eur J Clin Microbiol Infect Dis 25:419–425. doi: 10.1007/s10096-006-0159-2. [DOI] [PubMed] [Google Scholar]
- 3.Morrell M, Fraser VJ, Kollef MH. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold HM, Micek ST, Shorr AF, Zilberberg MD, Labelle AJ, Kothari S, Kollef MH. 2010. Hospital resource utilization and costs of inappropriate treatment of candidemia. Pharmacotherapy 30:361–368. [DOI] [PubMed] [Google Scholar]
- 5.Labelle AJ, Micek ST, Roubinian N, Kollef MH. 2008. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med 36:2967–2972. doi: 10.1097/CCM.0b013e31818b3477. [DOI] [PubMed] [Google Scholar]
- 6.Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious Diseases Society of America . 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shields RK, Nguyen MH, Press EG, Updike CL, Clancy CJ. 2013. Caspofungin MICs correlate with treatment outcomes among patients with Candida glabrata invasive candidiasis and prior echinocandin exposure. Antimicrob Agents Chemother 57:3528–3535. doi: 10.1128/AAC.00136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyda ND, John J, Kilic A, Alam MJ, Lasco TM, Garey KW. 2014. FKS mutant Candida glabrata: risk factors and outcomes in patients with candidemia. Clin Infect Dis 59:819–825. doi: 10.1093/cid/ciu407. [DOI] [PubMed] [Google Scholar]
- 9.Pfeiffer CD, Garcia-Effron G, Zaas AK, Perfect JR, Perlin DS, Alexander BD. 2010. Breakthrough invasive candidiasis in patients on micafungin. J Clin Microbiol 48:2373–2380. doi: 10.1128/JCM.02390-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mylonakis E, Clancy CJ, Ostrosky-Zeichner L, Garey KW, Alangaden GJ, Vazquez JA, Groeger JS, Judson MA, Vinagre Y-M, Heard SO, Zervou FN, Zacharioudakis IM, Kontoyiannis DP, Pappas PG. 2015. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clin Infect Dis 60:892–899. doi: 10.1093/cid/ciu959. [DOI] [PubMed] [Google Scholar]
- 12.Ostrosky-Zeichner L, Pappas PG, Shoham S, Reboli A, Barron MA, Sims C, Wood C, Sobel JD. 2011. Improvement of a clinical prediction rule for clinical trials on prophylaxis for invasive candidiasis in the intensive care unit. Mycoses 54:46–51. doi: 10.1111/j.1439-0507.2009.01756.x. [DOI] [PubMed] [Google Scholar]
- 13.Ostrosky-Zeichner L, Sable C, Sobel J, Alexander BD, Donowitz G, Kan V, Kauffman CA, Kett D, Larsen RA, Morrison V, Nucci M, Pappas PG, Bradley ME, Major S, Zimmer L, Wallace D, Dismukes WE, Rex JH. 2007. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis 26:271–276. doi: 10.1007/s10096-007-0270-z. [DOI] [PubMed] [Google Scholar]
- 14.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 15.Pfaller MA, Messer SA, Moet GJ, Jones RN, Castanheira M. 2011. Candida bloodstream infections: comparison of species distribution and resistance to echinocandin and azole antifungal agents in intensive care unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008–2009). Int J Antimicrob Agents 38:65–69. doi: 10.1016/j.ijantimicag.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Cleveland AA, Farley MM, Harrison LH, Stein B, Hollick R, Lockhart SR, Magill SS, Derado G, Park BJ, Chiller TM. 2012. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin Infect Dis 55:1352–1361. doi: 10.1093/cid/cis697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupont BF, Lortholary O, Ostrosky-Zeichner L, Stucker F, Yeldandi V. 2009. Treatment of candidemia and invasive candidiasis in the intensive care unit: post hoc analysis of a randomized, controlled trial comparing micafungin and liposomal amphotericin B. Crit Care 13:R159. doi: 10.1186/cc8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zilberberg MD, Kollef MH, Arnold H, Labelle A, Micek ST, Kothari S, Shorr AF. 2010. Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study. BMC Infect Dis 10:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran C, Grussemeyer CA, Spalding JR, Benjamin DK Jr, Reed SD. 2010. Comparison of costs, length of stay, and mortality associated with Candida glabrata and Candida albicans bloodstream infections. Am J Infect Control 38:78–80. doi: 10.1016/j.ajic.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heimann S, Cornely O, Wisplinghoff H, Kochanek M, Stippel D, Padosch S, Langebartels G, Reuter H, Reiner M, Vierzig A, Seifert H, Vehreschild MJ, Glossmann J, Franke B, Vehreschild JJ. 2014. Candidemia in the intensive care unit: analysis of direct treatment costs and clinical outcome in patients treated with echinocandins or fluconazole. Eur J Clin Microbiol Infect Dis 34:331–338. [DOI] [PubMed] [Google Scholar]
- 21.Pappas PG, Rotstein CMF, Betts RF, Nucci M, Talwar D, De Waele JJ, Vazquez JA, Dupont BF, Horn DL, Ostrosky-Zeichner L, Reboli AC, Suh B, Digumarti R, Wu C, Kovanda LL, Arnold LJ, Buell DN. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis 45:883–893. doi: 10.1086/520980. [DOI] [PubMed] [Google Scholar]
- 22.Kuse ER, Chetchotisakd P, da Cunha CA, Ruhnke M, Barrios C, Raghunadharao D, Sekhon JS, Freire A, Ramasubramanian V, Demeyer I, Nucci M, Leelarasamee A, Jacobs F, Decruyenaere J, Pittet D, Ullmann AJ, Ostrosky-Zeichner L, Lortholary O, Koblinger S, Diekmann-Berndt H, Cornely OA, Micafungin Invasive Candidiasis Working Group . 2007. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 369:1519–1527. doi: 10.1016/S0140-6736(07)60605-9. [DOI] [PubMed] [Google Scholar]
- 23.Altun O, Almuhayawi M, Ullberg M, Ozenci V. 2013. Clinical evaluation of the FilmArray blood culture identification panel in identification of bacteria and yeasts from positive blood culture bottles. J Clin Microbiol 51:4130–4136. doi: 10.1128/JCM.01835-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone NR, Gorton RL, Barker K, Ramnarain P, Kibbler CC. 2013. Evaluation of PNA-FISH yeast traffic light for rapid identification of yeast directly from positive blood cultures and assessment of clinical impact. J Clin Microbiol 51:1301–1302. doi: 10.1128/JCM.00028-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall L, Le Febre KM, Deml SM, Wohlfiel SL, Wengenack NL. 2012. Evaluation of the Yeast Traffic Light PNA FISH probes for identification of Candida species from positive blood cultures. J Clin Microbiol 50:1446–1448. doi: 10.1128/JCM.06148-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castanheira M, Messer SA, Jones RN, Farrell DJ, Pfaller MA. 2014. Activity of echinocandins and triazoles against a contemporary (2012) worldwide collection of yeast and moulds collected from invasive infections. Int J Antimicrob Agents 44:320–326. doi: 10.1016/j.ijantimicag.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. 2013. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J Clin Microbiol 51:2571–2581. doi: 10.1128/JCM.00308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reboli AC, Rotstein C, Kett DH, Maschio M, Cartier S, Chambers R, Tarallo M. 2011. Resource utilization and cost of treatment with anidulafungin or fluconazole for candidaemia and other forms of invasive candidiasis. Pharmacoeconomics 29:705–717. doi: 10.2165/11584810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Department of Veterans Affairs. 2016. National Acquisition Center: pharmaceutical catalog search. U.S. Department of Veterans Affairs, Washington, DC: http://www1.va.gov/nac/index.cfm?template=Search_Pharmaceutical_Catalog. [Google Scholar]
- 30.Drummond M, Sculpher M, Torrance G, O'Brien B, Stoddart G. 2005. Methods for the economic evaluation of health care programmes, 3rd ed Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 31.DiNubile MJ, Lupinacci RJ, Strohmaier KM, Sable CA, Kartsonis NA. 2007. Invasive candidiasis treated in the intensive care unit: observations from a randomized clinical trial. J Crit Care 22:237–244. doi: 10.1016/j.jcrc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Briggs A, Claxton K, Sculpher M. 2006. Decision modelling for health economic evaluation. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 33.Pfaller MA. 2012. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 125:S3–S13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Bilir SP, Ferrufino CP, Pfaller MA, Munakata J. 2015. The economic impact of rapid Candida species identification by T2Candida among high-risk patients. Future Microbiol 29:1–12. doi: 10.2217/fmb15. [DOI] [PubMed] [Google Scholar]
- 35.Pfaller M, Neofytos D, Diekema D, Azie N, Meier-Kriesche HU, Quan SP, Horn D. 2012. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance) registry, 2004–2008. Diagn Microbiol Infect Dis 74:323–331. doi: 10.1016/j.diagmicrobio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Parkins MD, Sabuda DM, Elsayed S, Laupland KB. 2007. Adequacy of empirical antifungal therapy and effect on outcome among patients with invasive Candida species infections. J Antimicrob Chemother 60:613–618. doi: 10.1093/jac/dkm212. [DOI] [PubMed] [Google Scholar]
- 37.Schuster MG, Edwards JE Jr, Sobel JD, Darouiche RO, Karchmer AW, Hadley S, Slotman G, Panzer H, Biswas P, Rex JH. 2008. Empirical fluconazole versus placebo for intensive care unit patients: a randomized trial. Ann Intern Med 149:83–90. doi: 10.7326/0003-4819-149-2-200807150-00004. [DOI] [PubMed] [Google Scholar]
- 38.Blot S, Dimopoulos G, Rello J, Vogelaers D. 2008. Is Candida really a threat in the ICU? Curr Opin Crit Care 14:600–604. doi: 10.1097/MCC.0b013e32830f1dff. [DOI] [PubMed] [Google Scholar]
- 39.Clancy CJ, Nguyen MH. 2013. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis 56:1284–1292. doi: 10.1093/cid/cit006. [DOI] [PubMed] [Google Scholar]
- 40.Lee W, Liew Y, Chlebicki MP, Ong S, Lee P, Kwa A. 2014. An observational study on early empiric versus culture-directed antifungal therapy in critically ill with intra-abdominal sepsis. Crit Care Res Pract 2014:479413. doi: 10.1155/2014/479413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez J, Erstad BL, Petty W, Nix DE. 2009. Time to positive culture and identification for Candida blood stream infections. Diagn Microbiol Infect Dis 64:402–407. doi: 10.1016/j.diagmicrobio.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Cobos-Trigueros N, Kaasch AJ, Soriano A, Torres JL, Vergara A, Morata L, Zboromyrska Y, De La Calle C, Alejo I, Hernandez C, Cardozo C, Marco F, Del Rio A, Almela M, Mensa J, Martinez JA. 2014. Time to positivity and detection of growth in anaerobic blood culture vials predict the presence of Candida glabrata in candidemia: a two-center European cohort study. J Clin Microbiol 52:3082–3084. doi: 10.1128/JCM.01198-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai CC, Wang CY, Liu WL, Huang YT, Hsueh PR. 2012. Time to positivity of blood cultures of different Candida species causing fungaemia. J Med Microbiol 61:701–704. doi: 10.1099/jmm.0.038166-0. [DOI] [PubMed] [Google Scholar]
- 44.Nawrot U, Kowalska-Krochmal B, Sulik-Tyszka B, Kozak M, Świetek K, Pajaczkowska M, Piatkowska E, Rosiak D, Swoboda-Kopeć E. 2015. Evaluation of blood culture media for the detection of fungi. Eur J Clin Microbiol Infect Dis 34:161–167. doi: 10.1007/s10096-014-2218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.