Abstract
Galactomannan (GM) testing of urine specimens may provide important advantages, compared to serum testing, such as easy noninvasive sample collection. We evaluated a total of 632 serial urine samples from 71 patients with underlying hematological malignancies and found that the urine GM/creatinine ratio, i.e., (urine GM level × 100)/urine creatinine level, which takes urine dilution into account, reliably detected invasive aspergillosis and may be a promising diagnostic tool for patients with hematological malignancies. (This study has been registered at ClinicalTrials.gov under registration no. NCT01576653.)
TEXT
Galactomannan (GM) is a polysaccharide component of the cell wall of filamentous fungi, including Aspergillus species. GM is hematogenously released by fungal hyphae during invasive growth. Serum GM detection has proved to be useful for the diagnosis of invasive aspergillosis (IA) in patients with hematological malignancies (1–3).
In contrast, data on the performance of GM testing with urine samples are limited. After promising results in an animal model nearly 3 decades ago (4), only very recent pilot studies have indicated that GM detection in urine may be promising for IA screening in humans (5–8). Besides important advantages, one of the potential major limitations of urine specimen testing is that GM dilutions in urine vary, thus influencing GM concentrations. Interestingly, previous studies on the diagnostic performance of urine GM determinations for IA did not take into account urine dilution, which may explain the inconsistent results (5–8). Urine creatinine concentrations vary depending on water intake and are used to determine reliably the concentrations of various analytes in urine when 24-hour urine collection is not feasible. Similar to serum creatinine findings, elevated urine creatinine concentrations can be found in conditions such as kidney failure, rhabdomyolyses, and pyelonephritis, as well as with a high-meat diet. The objective of this study was to evaluate urine GM testing and to determine whether the diagnostic performance of urine GM testing can be improved by the use of a creatinine-based urine GM ratio.
(Data in the manuscript were presented, in part, at the 55th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, 2015, and the 7th Trends in Medical Mycology, Lisbon, Portugal, 2015.)
This prospective single-center study was conducted at the Medical University Hospital of Graz (Graz, Austria) between September 2014 and August 2015. Adult patients (>18 years of age) who were admitted to the hematology or bone marrow transplantation ward and were at risk for IA (e.g., patients with prolonged neutropenia secondary to high-dose induction/myeloablative chemotherapy or patients with graft-versus-host disease) (9, 10) were identified and, after informed consent was obtained, were monitored prospectively using clinical rounds, chart reviews, and surveys of electronic documents, including microbiological test results. Patients' medical records were reviewed individually using a standardized data collection template, in order to obtain demographic information, clinical data on therapy outcomes and adverse events, and mycological laboratory test results.
IA was defined according to the consensus definitions provided by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the Mycoses Study Group (10). For each time point, samples were obtained and study patients were classified as having no evidence for IA, possible IA, probable IA, or proven IA. In addition, patients without hematological malignancies and with no evidence for IA who presented to an outpatient department were recruited as a validation cohort.
Specimen collection, processing, and analysis.
For all hematological malignancy patients who participated in this study, twice-weekly routine serum GM screening was performed until diagnosis of IA, resolution of risks for developing IA (e.g., recovery of neutropenia), or discharge. On the mornings of routine serum GM screening, 6 ml of midstream urine was collected into closed sterile urine tubes. Urine samples were stored at −70°C within 2 h after sample collection, and urine GM optical density index (ODI) values and urine creatinine levels were retrospectively analyzed, always within 6 months after sample collection. Determination of GM levels in urine was performed after vortex-mixing, using the Platelia enzyme immunoassay (EIA) (Bio-Rad Laboratories, Marnes-la-Coquette, France). Urine creatinine levels were determined with a Cobas 8000 automated analyzer (Roche Diagnostics, Rotkreuz, Switzerland), using the Jaffé Generation 2 assay for urine.
Prior to sample testing, serial dilution (1:10, 1:25, 1:50, 1:75, and 1:100) of a highly GM-positive urine sample was performed, and determination of GM and creatinine levels showed directly proportional reductions in the concentrations of the two analytes (Fig. 1). Therefore, urine GM levels could be normalized against corresponding urine creatinine levels (in milligrams per deciliter) using the urine GM/creatinine ratio, i.e., (urine GM ODI × 100)/urine creatinine level.
FIG 1.
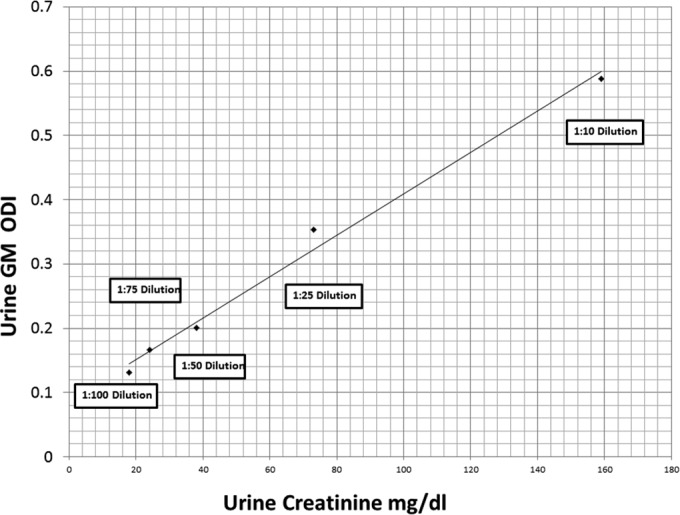
Serial dilution (1:10, 1:25, 1:50, 1:75, and 1:100) of a urine sample highly positive for galactomannan (GM). Determination of GM and creatinine levels showed directly proportional reductions of the levels of the two analytes.
For statistical analysis, IBM SPSS Statistics (version 22; IBM Corp., Amrok, NY, USA) and R 3.2.0 (www.r-project.org) were used. Clinical performance was analyzed for the urine GM/creatinine ratio and the conventional GM ODI by comparing results obtained from IA (i.e., proven, probable, or possible IA) samples versus no-evidence-for-IA samples and IA samples versus negative controls (i.e., validation cohort). Diagnostic cutoff values for the samples were determined using the Youden index, and the sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were calculated. Receiver operating characteristic (ROC) curve analyses were performed to evaluate diagnostic accuracies, and area under the curve (AUC) values are presented. Correlations between urine GM/creatinine ratios and GM ODI values for urine and serum samples were calculated using Spearman rho correlation analysis. P values of <0.05 were considered statistically significant. The study protocol was approved by the local ethics committee at the Medical University of Graz (Graz, Austria) (EC no. 23-343) and was registered at ClinicalTrials.gov (registration no. NCT01576653).
Patients with hematological malignancies.
A total of 71 hematological malignancy patients at risk for IA were included; 37 (52.1%) were male and 34 (47.9%) were female, with a median age of 55 years (interquartile range [IQR], 46 to 62 years). The most frequently observed underlying disease was acute myeloid leukemia (30 patients [42.3%]), followed by acute lymphoblastic leukemia (9 patients [12.7%]), myelodysplastic syndrome (8 patients [11.3%]), non-Hodgkin lymphoma and multiple myeloma (each 7 patients [9.8%]), and others (10 patients [14.1%]). Thirty-four patients (47.9%) had undergone allogeneic stem cell transplantation (SCT) before or during collection of study samples, and 12 (16.9%) had undergone autologous SCT.
From those 71 patients, a total of 632 urine samples were included in this study. Three urine samples were obtained from 1 patient with proven IA, 16 urine samples were obtained from 5 patients with probable IA, and 15 urine samples were obtained from 4 patients with possible IA. Therefore, a total of 34 samples were classified as IA samples (19 of those as probable/proven IA samples). The remaining 598 samples from 61 patients (85.9%) without evidence for IA were classified as no-IA samples. The majority of samples (32/34 [94%] IA samples and 419/598 [70%] no-IA samples) were obtained from patients receiving mold-active antifungal prophylaxis or therapy.
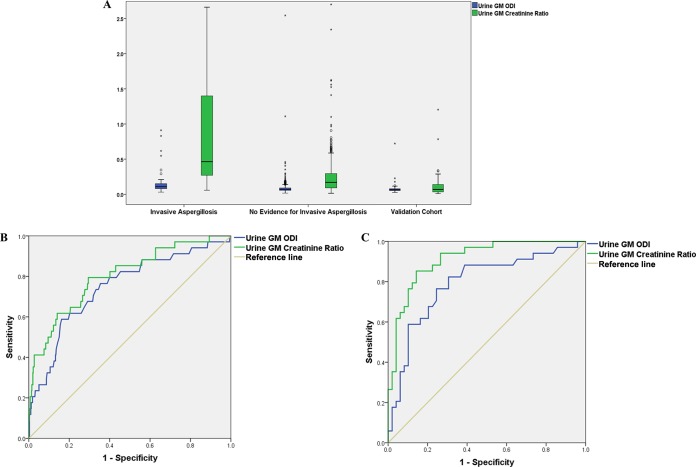
Median urine GM/creatinine ratios were nearly 3 times higher in IA samples than in no-IA samples (median ratio of 0.46 [IQR, 0.27 to 1.41] versus 0.17 [IQR, 0.09 to 0.30]; P < 0.001), while the difference was less pronounced for conventional urine GM ODI values (median ODI of 0.11 [IQR, 0.08 to 0.16] versus 0.07 [IQR, 0.06 to 0.09]; P < 0.001). Boxplots are presented in Fig. 2.
FIG 2.
Boxplots (A) and ROC curves (B and C) comparing urine GM/creatinine ratios and conventional urine GM ODI values. (A) Circles represent mild outliers, and asterisks represent extreme outliers. (B) Analysis restricted to patients with hematological malignancies, i.e., the IA cohort (n = 34) versus the no-evidence-for-IA cohort (n = 598). (C) Analysis comparing the IA cohort (n = 34) with the validation cohort (n = 48), without hematological malignancies.
ROC curve analysis revealed AUC values of 0.801 (95% confidence interval [CI], 0.719 to 0.882; P < 0.001) for urine GM/creatinine ratios, 0.746 (95% CI, 0.655 to 0.837) for conventional urine GM ODI values, and 0.749 (95% CI, 0.657 to 0.841) for serum GM levels in differentiating IA samples (n = 34) from samples with no evidence for IA (n = 598). ROC curves for urine tests are presented in Fig. 2.
The Youden index determined a cutoff value of 0.26 for the urine GM/creatinine ratio. Per-sample performance was as follows: (i) possible/probable/proven IA versus no evidence for IA: sensitivity, 79%; specificity, 70%; NPV, 98.4%; PPV, 13%; (ii) probable/proven IA versus no evidence for IA: sensitivity, 84%; specificity, 70%; NPV, 99.3%; PPV, 8%. Spearman rho correlation analysis revealed a strong correlation between urine GM/creatinine ratios and GM ODI values from urine (r = 0.712, P < 0.001) and weak correlations between GM ODI values from serum and both urine GM/creatinine ratios (r = 0.157, P < 0.001) and conventional urine GM ODI values (r = 0.180, P < 0.001).
Validation cohort.
Forty-eight urine samples, collected using the same preanalytical precautions, were obtained from 48 patients from an outpatient department. Boxplots for urine GM/creatinine ratios and conventional urine GM ODI values for the validation cohort are presented in Fig. 2.
ROC curve analysis revealed AUCs of 0.913 (95% CI, 0.853 to 0.973; P < 0.001) for urine GM/creatinine ratios for differentiating IA samples from the validation cohort and 0.792 (95% CI, 0.689 to 0.895; P < 0.001) for conventional urine GM ODI values. The proposed cutoff value of 0.26 for the urine GM/creatinine ratio had a specificity of 88% in the validation cohort.
We performed a prospective study on GM detection in urine samples and found that determination of the urine GM/creatinine ratio may be a reliable approach for detecting IA in patients with hematological malignancies. This represents the first study to evaluate the confounding factor of urine dilution when testing GM in urine samples.
Testing of urine specimens may provide important advantages. First, noninvasive and easy sample collection may allow for home testing for urine GM in the future, once point-of-care (POC) tests have been developed. Second, urine GM testing, in contrast to serum testing, allows more frequent examination of large volumes, which may increase the sensitivity of the GM test (6, 8, 11). The diagnostic performance of urine GM testing was improved by calculation of the urine GM/creatinine ratio. In particular, the excellent AUC for differentiating IA samples from validation cohort samples may argue for the superiority of the use of this ratio, compared to conventional urine GM determinations.
The calculated urine GM/creatinine cutoff value of 0.26 was associated with 79% sensitivity for possible/probable or proven IA among patients with hematological malignancies. The low PPV of 13% found for the proposed urine GM/creatinine ratio cutoff in this study is in line with PPVs published in previous studies for serum GM screening in patients receiving mold-active prophylaxis (3, 12). The NPV of >98% may, however, be a useful measure for ruling out breakthrough IA and discontinuing empirical antifungal treatment for patients who actually do not have IA. The main limitations of the study include the relatively small number of samples from probable and proven cases. Also, normalization may fail with high-meat diets, rhabdomyolyses, extensive exercise, and other conditions that may be associated with highly elevated urine creatinine levels.
Conclusions.
Our results indicate that the diagnostic value of GM detection in urine samples may be increased when urine dilution is taken into account. Use of the proposed urine GM/creatinine ratio may be a simple reliable approach and warrants further investigations as a diagnostic tool for IA in patients with hematological malignancies.
ACKNOWLEDGMENTS
This work was supported by funds from the Oesterreichische Nationalbank (Anniversary Fund, project 15346). The funders had no role in study design, data collection, analysis, and interpretation, the decision to publish, the writing of the manuscript, or the decision to submit the manuscript for publication.
Reinhard B. Raggam received travel grants from Pfizer and Merck. Albert Wölfler received research grants and speaker honoraria from Merck. Martin Hoenigl received research grants from Merck and Pfizer, served on the speakers' bureaus of Pfizer, Gilead, Astellas, Basilea, and Merck, and received travel grants from Astellas, Merck, Gilead, and Pfizer. All other authors report no conflicts.
REFERENCES
- 1.Hoenigl M, Salzer HJ, Raggam RB, Valentin T, Rohn A, Woelfler A, Seeber K, Linkesch W, Krause R. 2012. Impact of galactomannan testing on the prevalence of invasive aspergillosis in patients with hematological malignancies. Med Mycol 50:266–269. doi: 10.3109/13693786.2011.603102. [DOI] [PubMed] [Google Scholar]
- 2.Hoenigl M, Prattes J, Spiess B, Wagner J, Prueller F, Raggam RB, Posch V, Duettmann W, Hoenigl K, Wolfler A, Koidl C, Buzina W, Reinwald M, Thornton CR, Krause R, Buchheidt D. 2014. Performance of galactomannan, beta-d-glucan, Aspergillus lateral-flow device, conventional culture, and PCR tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J Clin Microbiol 52:2039–2045. doi: 10.1128/JCM.00467-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duarte RF, Sanchez-Ortega I, Cuesta I, Arnan M, Patino B, Fernandez de Sevilla A, Gudiol C, Ayats J, Cuenca-Estrella M. 2014. Serum galactomannan-based early detection of invasive aspergillosis in hematology patients receiving effective antimold prophylaxis. Clin Infect Dis 59:1696–1702. doi: 10.1093/cid/ciu673. [DOI] [PubMed] [Google Scholar]
- 4.Dupont B, Huber M, Kim SJ, Bennett JE. 1987. Galactomannan antigenemia and antigenuria in aspergillosis: studies in patients and experimentally infected rabbits. J Infect Dis 155:1–11. doi: 10.1093/infdis/155.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Duettmann W, Koidl C, Troppan K, Seeber K, Buzina W, Wolfler A, Wagner J, Krause R, Hoenigl M. 2014. Serum and urine galactomannan testing for screening in patients with hematological malignancies. Med Mycol 52:647–652. doi: 10.1093/mmy/myu019. [DOI] [PubMed] [Google Scholar]
- 6.Klont RR, Mennink-Kersten MA, Verweij PE. 2004. Utility of Aspergillus antigen detection in specimens other than serum specimens. Clin Infect Dis 39:1467–1474. doi: 10.1086/425317. [DOI] [PubMed] [Google Scholar]
- 7.Fisher BT, Zaoutis TE, Park JR, Bleakley M, Englund JA, Kane C, Arceci RJ, Guinan E, Smith FO, Luan X, Marr KA. 2012. Galactomannan antigen testing for diagnosis of invasive aspergillosis in pediatric hematology patients. J Pediatric Infect Dis Soc 1:103–111. doi: 10.1093/jpids/pis044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dufresne SF, Datta K, Li X, Dadachova E, Staab JF, Patterson TF, Feldmesser M, Marr KA. 2012. Detection of urinary excreted fungal galactomannan-like antigens for diagnosis of invasive aspergillosis. PLoS One 7:e42736. doi: 10.1371/journal.pone.0042736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoenigl M, Strenger V, Buzina W, Valentin T, Koidl C, Wolfler A, Seeber K, Valentin A, Strohmeier AT, Zollner-Schwetz I, Raggam RB, Urban C, Lass-Florl C, Linkesch W, Krause R. 2012. European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) host factors and invasive fungal infections in patients with haematological malignancies. J Antimicrob Chemother 67:2029–2033. doi: 10.1093/jac/dks155. [DOI] [PubMed] [Google Scholar]
- 10.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raggam RB, Fischbach LM, Prattes J, Duettmann W, Eigl S, Reischies F, Wolfler A, Rabensteiner J, Prueller F, Krause R, Hoenigl M. 2015. Detection of (1→3)-β-D-glucan in same-day urine and serum samples obtained from patients with haematological malignancies. Mycoses 58:394–398. doi: 10.1111/myc.12328. [DOI] [PubMed] [Google Scholar]
- 12.Cornely OA. 2014. Galactomannan testing during mold-active prophylaxis. Clin Infect Dis 59:1703–1704. doi: 10.1093/cid/ciu677. [DOI] [PMC free article] [PubMed] [Google Scholar]