Abstract
Significant interlaboratory variability is observed in testing the caspofungin susceptibility of Candida species by both the CLSI and EUCAST broth microdilution methodologies. We evaluated the influence of treated versus untreated polystyrene microtiter trays on caspofungin MICs using 209 isolates of four Candida species, including 16 C. albicans and 11 C. glabrata isolates with defined FKS mutations. Caspofungin MICs were also determined using the commercially available YeastOne and Etest assays and 102 isolates. All C. glabrata isolates had caspofungin MICs of ≥0.5 μg/ml, the clinical breakpoint for caspofungin resistance in this species, measured using trays made of treated polystyrene, regardless of the FKS status. In contrast, susceptible isolates could readily be distinguished from resistant/non-wild-type isolates when caspofungin MICs were measured using untreated polystyrene trays and both the YeastOne and Etest assays. Similar results were also observed for C. krusei isolates, as all isolates had caspofungin MICs above the threshold for resistance measured using treated polystyrene trays. In contrast, C. albicans isolates could be correctly identified as susceptible or resistant when caspofungin MICs were measured with treated or untreated trays and with the YeastOne and Etest assays. MICs falsely elevated above the resistance breakpoint were also not observed for C. tropicalis isolates. These results demonstrated that the use of treated polystyrene may be one factor that leads to falsely elevated caspofungin in vitro susceptibility results and that this may also be a greater issue for some Candida species than for others.
INTRODUCTION
Significant interlaboratory variability has been reported for caspofungin susceptibility testing, with some laboratories reporting MIC values elevated above the revised CLSI clinical breakpoints (1). The interlaboratory variability is not without consequences, as it may result in major errors for caspofungin use (i.e., falsely interpreting an isolate as resistant to this echinocandin). In a retrospective review of our antifungal susceptibility database using data from 2008 to 2012, we found that 95.6% of C. glabrata and 74.3% of C. krusei isolates would be classified as resistant to caspofungin on the basis of the revised CLSI echinocandin clinical breakpoints (2). Other laboratories have reported similar results (3). As the factors that lead to interlaboratory variability are unknown, we sought to determine if the choice of plastic used in the microtiter trays, either treated or untreated polystyrene, could affect caspofungin MIC values. These trays are designed for cell cultures, and treatment of the plastic, either by coating or corona discharge to impart an electrical charge to the plastic, allows the cells to adhere. Our laboratory has historically used 96-well microtiter trays made of treated polystyrene, while other laboratories have used those made of untreated plastic. The CLSI M27-A3 standard for yeast antifungal susceptibility testing has not addressed plastic type or recommended either type as the appropriate tray for use to obtain standard results. We also compared the results obtained with treated and untreated polystyrene trays to those obtained using the commercially available YeastOne and Etest assays.
MATERIALS AND METHODS
Isolates.
Clinical isolates (n = 209) of 4 different Candida species, including C. albicans, C. glabrata, C. krusei, and C. tropicalis, that had been sent to the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio between 2008 and 2013 were used in this study. They included 16 C. albicans and 11 C. glabrata isolates with previously determined mutations in hot spot regions of the FKS1 and FKS2 genes known to cause resistance to the echinocandins (4–6). Seven C. albicans challenge strains (strains M1 to M7) provided by Merck (Merck & Co., Whitehouse Station, NJ) were also included. All isolates were maintained as frozen stocks and were subcultured twice onto Sabouraud dextrose agar prior to in vitro susceptibility testing.
Antifungal susceptibility testing.
Antifungal susceptibility testing was performed by broth microdilution methods according to the CLSI M27-A3 reference standard (7). A common lot of caspofungin powder (Merck & Co., Inc., Whitehouse Station, NJ) was used for all prospective MIC determinations, and stock solutions were made using dimethyl sulfoxide (DMSO). Further dilutions were made using RPMI 1640 without bicarbonate and buffered with 0.165 M MOPS (morpholinepropanesulfonic acid; pH 7.0). Microtiter trays were prepared using treated and untreated polystyrene 96-well cell culture trays from the same manufacturer (Corning, Inc., Corning, NY). In addition, untreated polystyrene trays were also obtained from a second source (Evergreen Scientific, Los Angeles, CA) in order to compare differences in MIC values between the trays from the different manufacturers. The final caspofungin concentration range was 0.015 to 8 μg/ml. Caspofungin MICs for each isolate were also measured using the Sensititre YeastOne colorimetric assay (Trek Diagnostic Systems, Inc., Cleveland, OH) and Etest strips (bioMérieux, Marcy l'Etoile, France) according to the instructions provided by the respective manufacturers. Micafungin (Astellas US, Inc., Northbrook, IL) MICs for C. glabrata isolates by broth microdilution methods were also measured according to the CLSI M27-A3 standard.
Data analysis.
Isolates were classified as susceptible, intermediate, or resistant to caspofungin or micafungin measured by broth microdilution on the basis of the drug- and species-specific CLSI clinical breakpoints for these agents as published in the CLSI M27-S4 document (8). The recently reported YeastOne caspofungin epidemiological cutoff values (ECVs) were used to classify isolates as wild-type or non-wild-type isolates in this colorimetric assay (9). The rates of very major errors (VME), major errors (ME), and minor errors (mE) were also determined according to accepted definitions (10, 11). Geometric mean (GM) MICs were calculated, and differences in GM MIC values among multiple groups were assessed for significance by analysis of variance (ANOVA) with Tukey's posttest for multiple comparisons. For comparisons between two groups, a paired t test was used. A P value of <0.05 was considered statistically significant.
RESULTS
Candida glabrata isolates.
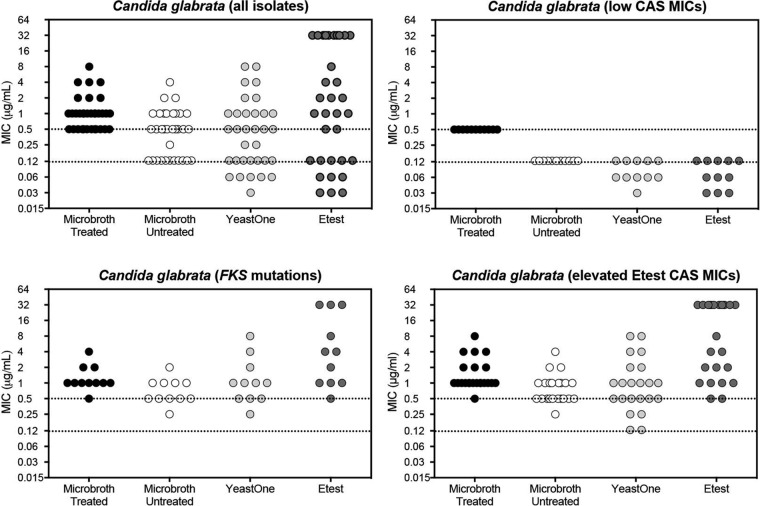
As shown in Fig. 1, there was a dichotomous distribution of caspofungin MIC values for the C. glabrata isolates measured using microtiter trays made of untreated polystyrene, the YeastOne colorimetric assay, or Etest strips (n = 33). The methods using untreated microtiter trays, YeastOne assay, and Etest strips were all found to able to distinguish between susceptible C. glabrata isolates with correspondingly low micafungin MIC values (≤0.03 μg/ml) and those with defined FKS1 or FKS2 mutations associated with echinocandin resistance. One isolate with a defined FKS mutation was classified by the untreated microtiter tray as intermediate to caspofungin and by the YeastOne assay as a wild-type isolate by the use of the YeastOne caspofungin ECV for this species (MIC, 0.25 μg/ml). The Etest caspofungin MICs for all isolates with FKS mutations were >0.5 μg/ml. In contrast, all caspofungin MICs measured using microtiter trays made of treated polystyrene were at or above the CLSI clinical breakpoint for resistance for this species (≥0.5 μg/ml). Thus, tests using microdilution plates prepared with treated polystyrene were unable to distinguish between susceptible and resistant C. glabrata isolates (ME rate of 100%), while those performed with plates made of untreated polystyrene and other commercially available assays were able to distinguish between susceptible/wild-type isolates and resistant/non-wild-type isolates. Interestingly, when three additional isolates with unknown FKS status but with elevated caspofungin MICs by both the Etest assay and in untreated polystyrene plates were tested, all three were classified as wild-type isolates by the YeastOne assay on the basis of the ECV (two with caspofungin MICs of 0.125 μg/ml and one with a caspofungin MIC of 0.25 μg/ml). The MIC determinations were repeated, and identical results were obtained, with each isolate being wild type by YeastOne and both resistant by broth microdilution using untreated polystyrene trays (≥0.5 μg/ml) and Etest strips (>1 μg/ml).
FIG 1.
Caspofungin MIC values for Candida glabrata isolates measured by CLSI broth microdilution methods using treated and untreated polystyrene trays and the commercially available YeastOne and Etest assays. A total of 33 clinical C. glabrata isolates were included, including resistant isolates with defined FKS mutations as well as susceptible isolates that had low caspofungin (CAS) MICs by Etest combined with low micafungin MICs. The upper dotted line represents the threshold for caspofungin resistance in C. glabrata per CLSI clinical breakpoints and the lower line the threshold for susceptibility measured using the CLSI broth microdilution methods.
We also measured caspofungin MICs using untreated polystyrene trays and 107 banked C. glabrata isolates that had been previously tested using treated polystyrene trays, all of which had historical caspofungin MICs of ≥0.5 μg/ml. When the caspofungin MICs were measured using untreated polystyrene trays, the MICs ranged from 0.06 to >8 μg/ml, with a GM MIC of 0.216 μg/ml, and 12.1% of the isolates were resistant, 15.9% intermediate, and 72% susceptible. In contrast, the historical caspofungin MIC ranged from 0.5 to 8 μg/ml, with a GM MIC value of 0.728 μg/ml, and 100% resistance was determined on the basis of the current CSLI breakpoints for caspofungin and C. glabrata. We also measured micafungin MICs for these 107 isolates, and the overall categorical agreement for isolates classified as susceptible/intermediate versus resistant between caspofungin and micafungin was 93.5%, with a 3.7% VME rate, a 2.8% ME rate, and a mE rate of 17.8%. Micafungin resistance was observed in 15.9% of the isolates (MIC, ≥0.25 μg/ml), 3.7% of the isolates were intermediate (0.12 μg/ml), and 79.5% were susceptible (≤0.06 μg/ml).
Candida albicans isolates.
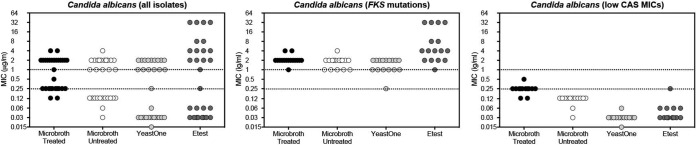
In contrast to what was observed with C. glabrata, each method was able to accurately distinguish between susceptible and resistant C. albicans isolates (n = 29). As shown in Fig. 2, caspofungin MICs measured in microtiter trays made of treated and untreated polystyrene and by the YeastOne and Etest assays clearly separated the susceptible/wild-type and resistant/non-wild-type groups. Isolates with defined FKS mutations clearly had MIC values at or above the CLSI breakpoint for echinocandin resistance for this species (≥1 μg/ml). Each method was also able to correctly distinguish the four echinocandin-resistant challenge strains provided by Merck from the three susceptible strains. In addition, caspofungin MICs obtained using treated polystyrene trays were at or below the breakpoint for susceptibility (0.25 μg/ml) for all but one of the C. albicans isolates that had low MIC values by each of the other methods. However, the GM MIC for the susceptible isolates was higher with treated polystyrene trays (0.237 μg/ml) than with each of the other methods (0.03 to 0.106 μg/ml; P ≤ 0.001).
FIG 2.
Caspofungin MIC values for Candida albicans isolates measured by CLSI broth microdilution methods using treated and untreated polystyrene trays and the commercially available YeastOne and Etest assays. A total of 29 clinical C. albicans isolates were included, including resistant isolates with defined FKS mutations as well as susceptible isolates that had low caspofungin MICs by Etest and YeastOne assays. The upper dotted line represents the threshold for caspofungin resistance in C. albicans per CLSI clinical breakpoints and the lower line the threshold for susceptibility measured using the CLSI broth microdilution methods.
Other Candida species.
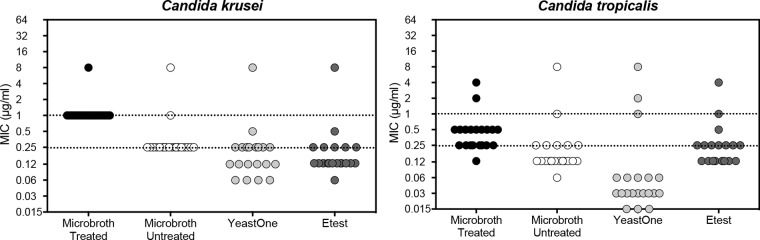
Similarly to what was observed with C. glabrata, the caspofungin MICs for C. krusei isolates that were read using treated polystyrene plates were all ≥1 μg/ml and the isolates were classified as resistant (Fig. 3). In contrast, only two C. krusei isolates were resistant when MICs were measured using untreated polystyrene trays, while only one was a non-wild-type isolate as determined using the YeastOne assay (MIC, 8 μg/ml; ECV, 1 μg/ml). The same isolate also had a caspofungin MIC of 8 μg/ml measured by Etest. Among the C. tropicalis isolates, many of the caspofungin MICs fell into the intermediate range (0.5 μg/ml) measured using treated polystyrene plates, and only two had values greater than the threshold for resistance (≥1 μg/ml). Similarly, only two isolates had caspofungin MIC values that were classified as representing resistance as measured using untreated polystyrene trays, and three isolates were classified as non-wild-type isolates when caspofungin MICs were measured using the YeastOne assay. For both C. krusei and C. tropicalis isolates, caspofungin MICs were lowest by the YeastOne assay (GM MIC of 0.062 μg/ml compared to 0.125 to 0.25 μg/ml for the other methods; P ≤ 0.01 for all comparisons).
FIG 3.
Caspofungin MIC values for clinical isolates of Candida krusei and C. tropicalis measured by CLSI broth microdilution methods using treated and untreated polystyrene trays and the commercially available YeastOne and Etest assays. A total of 20 isolates per species were included. The upper dotted line represents the threshold for caspofungin resistance in each species per CLSI clinical breakpoints and the lower line the threshold for susceptibility measured using the CLSI broth microdilution methods.
Influence of manufacturer of untreated polystyrene plates.
We also evaluated if untreated polystyrene trays from different manufacturers would have different caspofungin MICs. Among the 29 C. albicans isolates that were used to determine differences in caspofungin MICs between treated and untreated polystyrene trays from the same manufacturer (Corning) with YeastOne and Etest assays, there was a significant difference between the GM MIC values when these isolates were tested using untreated polystyrene from one manufacturer (Evergreen, 0.298 μg/ml) versus using untreated polystyrene from another manufacturer (Corning, 0.487 μg/ml; P < 0.0001). This difference was primarily driven by differences in the caspofungin MIC values for susceptible isolates (GM MIC value of 0.046 μg/ml compared to 0.106 μg/ml for Evergreen and Corning untreated polystyrene plates; P < 0.0001) and less so by the resistance status of the isolates (GM MIC 1.354 μg/ml versus 1.682 μg/ml). In addition, the categorical agreement for C. albicans was 100% for each microtiter tray manufacturer. Interestingly, this did not appear to be the case for the 33 C. glabrata isolates that were evaluated, as the GM MICs were not significantly different between the different manufacturers of untreated polystyrene microtiter trays (0.521 μg/ml versus 0.423 μg/ml).
Reproducibility between different observers.
The reproducibility of the caspofungin MIC results was also determined between three different clinical laboratory scientists for both the Corning and Evergreen untreated trays. A total of 25 C. albicans and C. glabrata isolates, including 8 that were resistant to caspofungin, were used and set up on two different days. For both the Corning and Evergreen untreated trays, the essential agreement among the three observers, each of whom was blind to the status of the isolates and the results reported by the two other observers, was 100%. Categorical agreement for the Corning trays was 96%, with one resistant C. glabrata isolate (MIC, 1 μg/ml) being classified as intermediate (MIC, 0.25 μg/ml). For the untreated Evergreen trays, categorical agreement was also 96%. However, the one disagreement resulted in a VME, as one resistant C. albicans isolate with a known FKS mutation (MIC, 1 μg/ml) was classified as susceptible (MIC, 0.25 μg/ml).
DISCUSSION
Significant interlaboratory variability is a concern for caspofungin susceptibility testing performed using the CLSI and EUCAST broth microdilution methodologies (1). Although this variability can be observed among different species, the differences are most pronounced with C. glabrata and C. krusei (1, 2, 12). The issue of variable caspofungin MICs is important to address because of the lowering of the CLSI clinical breakpoints for the echinocandins against most Candida species. Previously, the resistance rates for caspofungin were low when the clinical breakpoint was set at >2 μg/ml for nonsusceptibility. However, the rate of C. glabrata caspofungin resistance for laboratories that measure high caspofungin MICs may be increased now that the clinical breakpoint for resistance for this echinocandin has been set at ≥0.5 μg/ml.
In the current study, we sought to determine if caspofungin MICs measured with trays made of untreated polystyrene would be different from those measured with trays made of treated polystyrene. The CLSI M27-A3 reference standard has not previously specified whether treated or untreated polystyrene should be used for broth microdilution susceptibility testing. Historically, our laboratory has used treated plates, while some others have used untreated plates. The results of the current study demonstrate that the use of microtiter trays made of treated polystyrene can lead to falsely elevated caspofungin MICs and that this outcome may be species dependent. We observed a 100% ME rate in C. glabrata isolates for caspofungin MICs measured by broth microdilution using treated polystyrene trays, as all isolates, even those with low caspofungin MICs by other commercially available assays and with low micafungin MICs, were resistant by the revised clinical breakpoints. Similar results were also observed for C. krusei isolates. However, when caspofungin MICs were measured by broth microdilution using untreated polystyrene trays, the percentages of isolates that were classified as susceptible or resistant to caspofungin were very similar to the percentages of those measured using the YeastOne and Etest assays. Against a larger panel of C. glabrata isolates that historically had caspofungin MICs of ≥0.5 μg/ml, the percentages of isolates classified as susceptible or resistant to this echinocandin measured using untreated polystyrene were similar to those observed with micafungin. For C. albicans, the use of treated versus untreated plates did not appear to influence the identification of susceptible or resistant isolates. However, caspofungin MICs measured using treated polystyrene trays were somewhat higher than those measured by other methods.
Our results demonstrate that the choice of treated versus untreated polystyrene trays may be one factor that influences the in vitro potency of caspofungin for Candida species and that the effect may be more pronounced for certain species, such as C. glabrata and C. krusei. The exact mechanism accounting for this difference was not explored. It is known that the type of plastic used for broth microdilution testing can significantly affect MIC results for polymyxins, as these amphiphilic drugs readily adhere to polystyrene (13–16; K. Sei, presented at the meeting of the CLSI Subcommittee on Antimicrobial Susceptibility Testing, Tempe, AZ, January 2012). The degree of adherence is dependent on the coating that is applied to the wells as well as on the corona treatment that is used to treat the plates (16, 17). To overcome this limitation, some laboratories add a small concentration of polysorbate 80, a surfactant, in order to reduce adherence. However, this practice is not currently recommended for susceptibility testing of polymyxins by CLSI.
Other factors may be involved in the interlaboratory variability that is observed with caspofungin for in vitro susceptibility testing, such as potential differences in media and plasticware from obtained from different sources, including the use of different batches, and variability in the reading of the results between different laboratory personnel. A larger study involving multiple laboratories and assessing multiple factors is needed to truly determine which variables may affect in vitro susceptibility testing with caspofungin.
ACKNOWLEDGMENTS
Caspofungin powder was provided by Merck. Caspofungin Etest strips were kindly provided by bioMérieux. Micafungin powder was provided by Astellas.
N.P.W. has received research support from Astellas, Dow, F2G, Merck, Merz, Revolution Medicines, and Viamet and has served on advisory boards for Merck, Astellas, Toyama, and Viamet. The rest of the authors have no conflicts of interest to declare.
REFERENCES
- 1.Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Fothergill A, Fuller J, Gaustad P, Gonzalez GM, Guarro J, Lass-Florl C, Lockhart SR, Meis JF, Moore CB, Ostrosky-Zeichner L, Pelaez T, Pukinskas SR, St-Germain G, Szeszs MW, Turnidge J. 2013. Interlaboratory variability of Caspofungin MICs for Candida spp. using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother 57:5836–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fothergill AW, Sutton DA, McCarthy DI, Wiederhold NP. 2013. The impact of new CLSI breakpoints on antifungal resistance in Candida species, abstr M-1364 Abstr Intersci Conf Antimicrob Agents Chemother, Denver, CO. [Google Scholar]
- 3.Shields RK, Nguyen MH, Press EG, Kwa AL, Cheng S, Du C, Clancy CJ. 2012. The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob Agents Chemother 56:4862–4869. doi: 10.1128/AAC.00027-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiederhold NP, Grabinski JL, Garcia-Effron G, Perlin DS, Lee SA. 2008. Pyrosequencing to detect mutations in FKS1 that confer reduced echinocandin susceptibility in Candida albicans. Antimicrob Agents Chemother 52:4145–4148. doi: 10.1128/AAC.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Effron G, Lee S, Park S, Cleary JD, Perlin DS. 2009. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-beta-d-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob Agents Chemother 53:3690–3699. doi: 10.1128/AAC.00443-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleary JD, Garcia-Effron G, Chapman SW, Perlin DS. 2008. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob Agents Chemother 52:2263–2265. doi: 10.1128/AAC.01568-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—3rd ed CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.CLSI. 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement. CLSI document M27-S4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 9.Espinel-Ingroff A, Alvarez-Fernandez M, Canton E, Carver PL, Chen SC, Eschenauer G, Getsinger DL, Gonzalez GM, Govender NP, Grancini A, Hanson KE, Kidd SE, Klinker K, Kubin CJ, Kus JV, Lockhart SR, Meletiadis J, Morris AJ, Pelaez T, Quindos G, Rodriguez-Iglesias M, Sanchez-Reus F, Shoham S, Wengenack NL, Borrell Sole N, Echeverria J, Esperalba J, Gómez-G de la Pedrosa E, Garcia Garcia I, Linares MJ, Marco F, Merino P, Peman J, Perez Del Molino L, Rosello Mayans E, Rubio Calvo C, Ruiz Pérez de Pipaon M, Yague G, Garcia-Effron G, Guinea J, Perlin DS, Sanguinetti M, Shields R, Turnidge J. 2015. Multicenter study of epidemiological cutoff values and detection of resistance in Candida spp. to anidulafungin, caspofungin, and micafungin using the Sensititre YeastOne colorimetric method. Antimicrob Agents Chemother 59:6725–6732. doi: 10.1128/AAC.01250-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaller MA, Messer SA, Diekema DJ, Jones RN, Castanheira M. 2014. Use of micafungin as a surrogate marker to predict susceptibility and resistance to caspofungin among 3,764 clinical isolates of Candida by use of CLSI methods and interpretive criteria. J Clin Microbiol 52:108–114. doi: 10.1128/JCM.02481-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaller MA, Diekema DJ, Jones RN, Castanheira M. 2014. Use of anidulafungin as a surrogate marker to predict susceptibility and resistance to caspofungin among 4,290 clinical isolates of Candida by using CLSI methods and interpretive criteria. J Clin Microbiol 52:3223–3229. doi: 10.1128/JCM.00782-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eschenauer GA, Nguyen MH, Shoham S, Vazquez JA, Morris AJ, Pasculle WA, Kubin CJ, Klinker KP, Carver PL, Hanson KE, Chen S, Lam SW, Potoski BA, Clarke LG, Shields RK, Clancy CJ. 2014. Real-world experience with echinocandin MICs against Candida species in a multicenter study of hospitals that routinely perform susceptibility testing of bloodstream isolates. Antimicrob Agents Chemother 58:1897–1906. doi: 10.1128/AAC.02163-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hindler JA, Humphries RM. 2013. Colistin MIC variability by method for contemporary clinical isolates of multidrug-resistant Gram-negative bacilli. J Clin Microbiol 51:1678–1684. doi: 10.1128/JCM.03385-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karvanen M, Malmber C, Mohamed A, Lagerback P, Friberg LE, Cars. 2011. Colistin is extensively lost during normal experimental conditions, abstr D-690 Abstr Intersci Conf Antimicrob Agents Chemother, Washington, DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albur M, Noel A, Bowker K, Macgowan A. 2014. Colistin susceptibility testing: time for a review. J Antimicrob Chemother 69:1432–1434. doi: 10.1093/jac/dkt503. [DOI] [PubMed] [Google Scholar]
- 16.Humphries RM. 2015. Susceptibility testing of the polymyxins: where are we now? Pharmacotherapy 35:22–27. doi: 10.1002/phar.1505. [DOI] [PubMed] [Google Scholar]
- 17.Sader HS, Rhomberg PR, Flamm RK, Jones RN. 2012. Use of a surfactant (polysorbate 80) to improve MIC susceptibility testing results for polymyxin B and colistin. Diagn Microbiol Infect Dis 74:412–414. doi: 10.1016/j.diagmicrobio.2012.08.025. [DOI] [PubMed] [Google Scholar]