Abstract
This study compared a manual workup of urine clinical samples with fully automated WASPLab processing. As a first step, two different inocula (1 and 10 μl) and different streaking patterns were compared using WASP and InoqulA BT instrumentation. Significantly more single colonies were produced with the10-μl inoculum than with the 1-μl inoculum, and automated streaking yielded significantly more single colonies than manual streaking on whole plates (P < 0.001). In a second step, 379 clinical urine samples were evaluated using WASP and the manual workup. Average numbers of detected morphologies, recovered species, and CFUs per milliliter of all 379 urine samples showed excellent agreement between WASPLab and the manual workup. The percentage of urine samples clinically categorized as positive or negative did not differ between the automated and manual workflow, but within the positive samples, automated processing by WASPLab resulted in the detection of more potential pathogens. In summary, the present study demonstrates that (i) the streaking pattern, i.e., primarily the number of zigzags/length of streaking lines, is critical for optimizing the number of single colonies yielded from primary cultures of urine samples; (ii) automated streaking by the WASP instrument is superior to manual streaking regarding the number of single colonies yielded (for 32.2% of the samples); and (iii) automated streaking leads to higher numbers of detected morphologies (for 47.5% of the samples), species (for 17.4% of the samples), and pathogens (for 3.4% of the samples). The results of this study point to an improved quality of microbiological analyses and laboratory reports when using automated sample processing by WASP and WASPLab.
INTRODUCTION
In recent years, clinical microbiology has been faced with dramatic changes, as full laboratory automation (FLA) has started to enter diagnostic laboratories. This trend toward automation will affect economic efficiency, standardization, and time to result of laboratory procedures to various extents (1). To date, clinical microbiology is still predominately based on manual sample processing. Compared to clinical chemistry, microbiological specimens display a significantly higher degree of complexity (2). For many years, the general perception was that clinical microbiology would be far too complex to allow for automated processing and that robots would not be able to replace human operators. However, it has been demonstrated that automated inoculation of samples can be superior to manual processing and that automated reading of disk diffusion agar plates significantly increases the precision of results (3–6). Thus, automated sample processing promises an improved standardization of sample processing, incubation times, and plate reading protocols.
FLA systems have been developed by several companies. Currently, two FLA solutions are available, i.e., the BD Kiestra total laboratory automation (BD Kiestra B.V., Drachten, Netherlands) and WASPLab (Copan Italia S.p.A., Brescia, Italy). These systems use robotic systems, specifically the Kiestra InoqulA and the Copan WASP, for handling specimen containers and primary culture inoculation. The inoculated media are moved to automated incubators with integrated camera systems by conveyor belts to capture plate images at given time points, henceforth referred to as WASPLab. Currently, digital plate reading (DPR) still depends on highly skilled technologists, who can read plates “virtually,” without physical interaction (7). The first studies on FLA solutions suggest that productivity indicators can be improved and diagnostic processes can be accelerated (8–11).
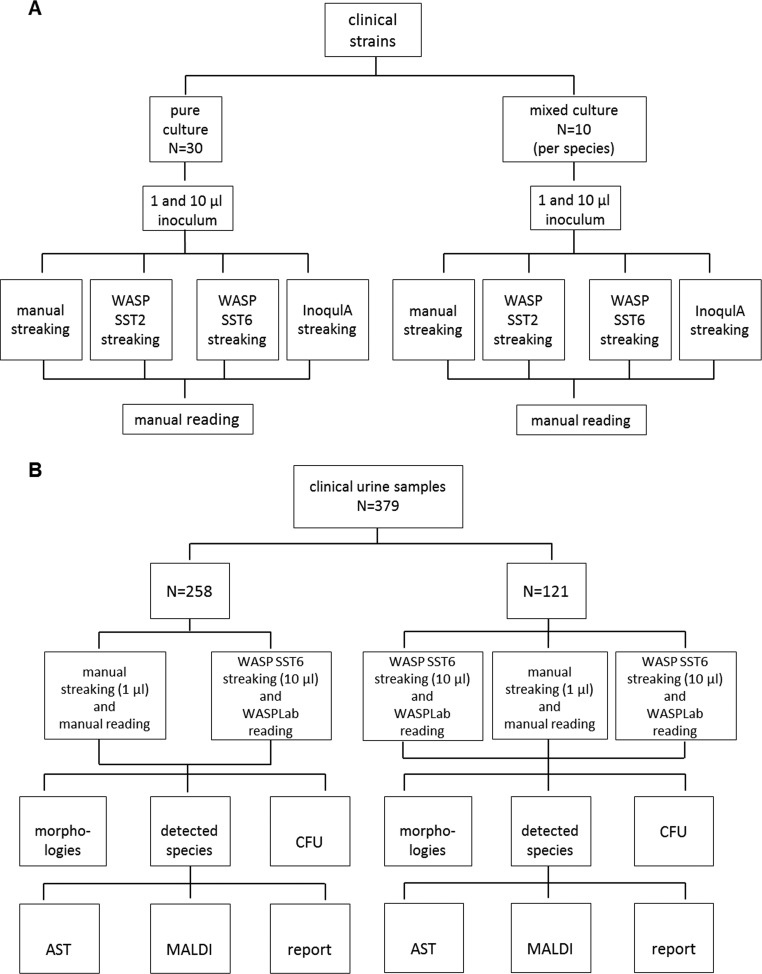
In the present study, we compared workflows for urine sample processing using the fully automated WASP and WASPLab systems and manual standard procedures. Inoculation procedures critically influence further procedures, such as specimen identification and antimicrobial susceptibility testing, mainly by the ability to generate single colonies suitable for further processing. Therefore, the streaking pattern and inoculation volume should be well evaluated before comparing total manual and automated workflows. Different guidelines exist for urine specimen inoculation; e.g., U.S. and Canadian laboratories routinely plate 1 μl of urine specimens, whereas European guidelines suggest 10 μl (4, 12). Therefore, this study was subdivided into two parts (Fig. 1). In part one, the ability to generate single colonies was compared for (i) manual and automated workflows and (ii) different automated streaking patterns using pure and mixed cultures and different inocula (1 and 10 μl) in order to determine the optimal streaking pattern. Part two of the study compared total manual and automated workflows regarding (i) the number of detected CFUs, (ii) the number of detected morphologies, (iii) the number of recovered species, and (iv) the number of follow-up tests after initial plate reading, i.e., the number of identifications and susceptibility tests, in 379 clinical urine samples.
FIG 1.
Study design and evaluation steps.(A) Part 1: evaluation of the optimal inoculation volume and streaking pattern. (B) Part 2: head-to-head comparison.
MATERIALS AND METHODS
Bacterial strains, clinical samples, and growth conditions.
Urine samples and bacterial strains isolated from patient specimens used in this study were collected from January until April 2015 in the clinical laboratory of the Institute of Medical Microbiology, University of Zurich. If not stated otherwise, bacterial cultures were incubated at 35°C ± 2°C and 7.5% CO2 for 16 to 20 h.
Quantitative analysis of streaking pattern/single-colony count.
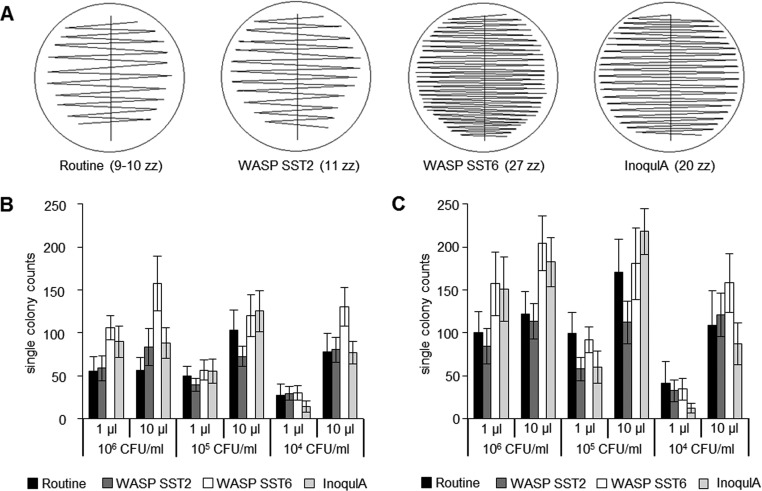
Manual, semiautomated, and fully automated quantitative streaking patterns were evaluated by counting the number of single colonies produced on whole plates. Manual streaking was always performed by the same investigator with 1-μl and 10-μl calibrated plastic inoculation loops (Copan Italia S.p.A., Brescia, Italy) resulting in 9 to 10 zigzag lines (Fig. 2A). For the semiautomated streaking, a ball-based bench-top InoqulA BT (BD Kiestra, Drachten, Netherlands) was used with 1- and 10-μl inocula and the zigzag liquid streaking pattern resulted in 20 zigzag lines (Fig. 2A). In this study, 1- and 10-μl inocula were manually pipetted for InoqulA BT. Furthermore, a 1-μl inoculum was manually pipetted for the semiautomated benchtop InoqulA BT to assure an adequate comparison; however, it has to be noted that the BD Kiestra fully automated instrument cannot handle 1 μl. The fully automated streaking was performed by the loop-based WASP (Copan Italia S.p.A.) with two different streaking patterns: the single streak type 2 (SST2; 11 zigzag lines) and the single streak type 6 (SST6; 27 zigzag lines) (Fig. 2A). Bacterial suspensions of McFarland 0.5 standard, corresponding to ∼1.5 × 108 CFU/ml, were serial diluted in sodium borate/formate containing BD Vacutainer (BD, Franklin Lakes, NJ, USA) and plated onto Columbia 5% sheep blood agar (COS; bioMérieux SA, Marcy l'Etoile, France) for monomicrobial cultures or chromogenic UriSelect 4 agar (URI4; Bio-Rad Laboratories, Hercules, CA, USA) for polymicrobial cultures. Pure Escherichia coli and enterococci cultures were diluted to 104 to 106 CFU/ml, and mixed cultures were prepared with E. coli ranging from 104 to 106 CFU/ml together with 106 enterococci and 106 coagulase-negative staphylococci (CoNS). The single-colony counting was done manually (visually) by the same investigator.
FIG 2.
Streaking pattern details and resulting numbers of single-colony counts. (A) One manual (routine) and three automated quantitative streaking patterns, with varied numbers of zigzag (zz) lines shown in brackets: WASP single streak type 2 (SST2), WASP single streak type 6 (SST6), and InoqulA. Comparison of single colonies obtained by manual and automated streaking. One and 10 μl E. coli (B) and enterococci (C) bacterial suspensions were used with CFUs ranging from 104 to 106. The mean values of 30 clinical strains per species are shown with standard deviations.
Head-to-head comparison of urine samples.
In total, 379 clinical urine samples were processed in parallel by the fully automated WASPLab (Copan Italia S.p.A.) workflow (inoculation, incubation, image acquisition, image analysis) and the manual routine procedures of our clinical laboratory. One microliter of sample inoculum used in the manual routine workflow was compared to 1-μl (121 samples) and 10-μl (379 samples) loops of the WASP. Urine cultures were plated onto COS, URI4, and Columbia colistin-nalidixic acid (CNA) 5% sheep blood agar (bioMérieux SA).
Following manual inoculation, agar plates were analyzed after 1 and 2 days of incubation, and morphologies, CFU counts, and recovered species were recorded. Material was reported negative when there was no growth or growth of a nonrelevant pathogen after 2 days of incubation. Materials were reported positive when there was a relevant pathogen in a relevant quantity and treatment was indicated. The sample evaluation algorithm used resembles the diagnostic approach as suggested by the Cumitech guidelines (13). Classification of the relevant pathogens was done as reported elsewhere (14).
For WASP inoculation, 1- and 10-μl volumes of the urine sample were streaked with the SST6 streaking pattern. Images were taken using WASPLab imaging after 16 and 38 h of incubation at 35°C ± 2°C and 7.5% CO2 and analyzed by the same laboratory personnel. After 16 h, photos were read and morphologies (i.e., visually different colony types) and CFU counts were noted. Plates were reread after 38 h of incubation. Colonies with additional morphology were identified. Samples were categorized as negative and positive according to the same criteria as for the manual reading procedure. Besides the number of different morphologies, CFU counts, and recovered species (i.e., taxonomic entities as reported by matrix-assisted laser desorption ionization-time of flight mass spectrometry [MALDI-TOF MS] identification), the numbers of follow-up tests, such as susceptibility tests and MALDI-TOF MS (Bruker Daltonics, Bremen, Germany) identifications, were recorded for comparison.
Plate reading of manually examined specimens was performed by different laboratory personnel by direct visual inspection; plates of WASP-prepared samples were read by a single technician with the WASPLab, who also did the head-to-head comparison of the final data set.
Technical settings of WASPLab.
Automated images of COS and CNA plates were recorded using three different light settings (top light, dark field, and a combination of top and back light). URI4 images were recorded with top-light settings only. The WASPLab installation used WASPCore version 3.1.0. The settings were conserved throughout the study.
Statistical analysis.
Statistical analysis was performed using IBM SPSS statistical software, version 20 (SPSS Inc., Chicago, IL, USA). The normality of the data were assessed with the Kolmogorov-Smirnov and Shapiro-Wilk tests, followed by the paired t test and nonparametric Wilcoxon signed-rank test for comparison of loop size and streaking pattern for pure cultures. A cutoff value of 0.05 was considered statistically significant. For the mixed cultures, overall differences were tested using the Kruskal-Wallis test and one-way analysis of variance, with the Bonferroni post hoc test to control for alpha inflation. Nonparametric follow-up tests were conducted using the Mann-Whitney U test for pairwise comparison, with Bonferroni correction applied for multiple comparisons with an alpha level of 0.05 divided by the possible number of comparisons, i.e., 0.0083 (= 0.05/6) for streaking pattern and 0.0167 (= 0.05/3), for concentration as independent variables. To compare the proportion between manual and WASP processing in the head-to-head comparison, the Wilcoxon signed-rank test and a crosstab chi-square test of independence were performed. Proportions differ significantly from each other at a P value of 0.05.
RESULTS
As a first step, two different inocula (1 μl and 10 μl) and different streaking patterns (one manual, two fully automated WASP/loop based, and one semiautomated InoqulA/ball based) were evaluated with pure cultures of E. coli and enterococci using CFU counts of 104/ml, 105/ml, and 106/ml, to identify the loop-streaking pattern combination that yields the highest number of isolated single colonies (Fig. 2; see also Fig. S1 and S2 in the supplemental material).
We also studied mixed inocula containing 106 CFU/ml enterococci, 106 CFU/ml CoNS, and E. coli with 104 to 106 CFU/ml to mimic mixed clinical samples of a leading pathogen and residual flora.
Evaluation of the optimal inoculation volume.
Significantly more single colonies were produced with the 10-μl inoculum than with the 1-μl inoculum on whole plates for both E. coli and enterococci when using CFU counts between 104/ml and 105/ml. This finding was independent of the streaking pattern (P < 0.001) (Fig. 2B and C). In contrast, no significantly higher recovery of single colonies was observed for E. coli CFU counts of 106/ml when using a 10-μl versus a 1-μl inoculum and when comparing manual streaking and InoqulA (P = 0.820 and 0.713, respectively) (Fig. 2B and C). However, WASP inoculation retained a significantly higher recovery of single colonies when applying the 10-μl inoculum and manual streaking (P < 0.001) (Fig. 2B and C).
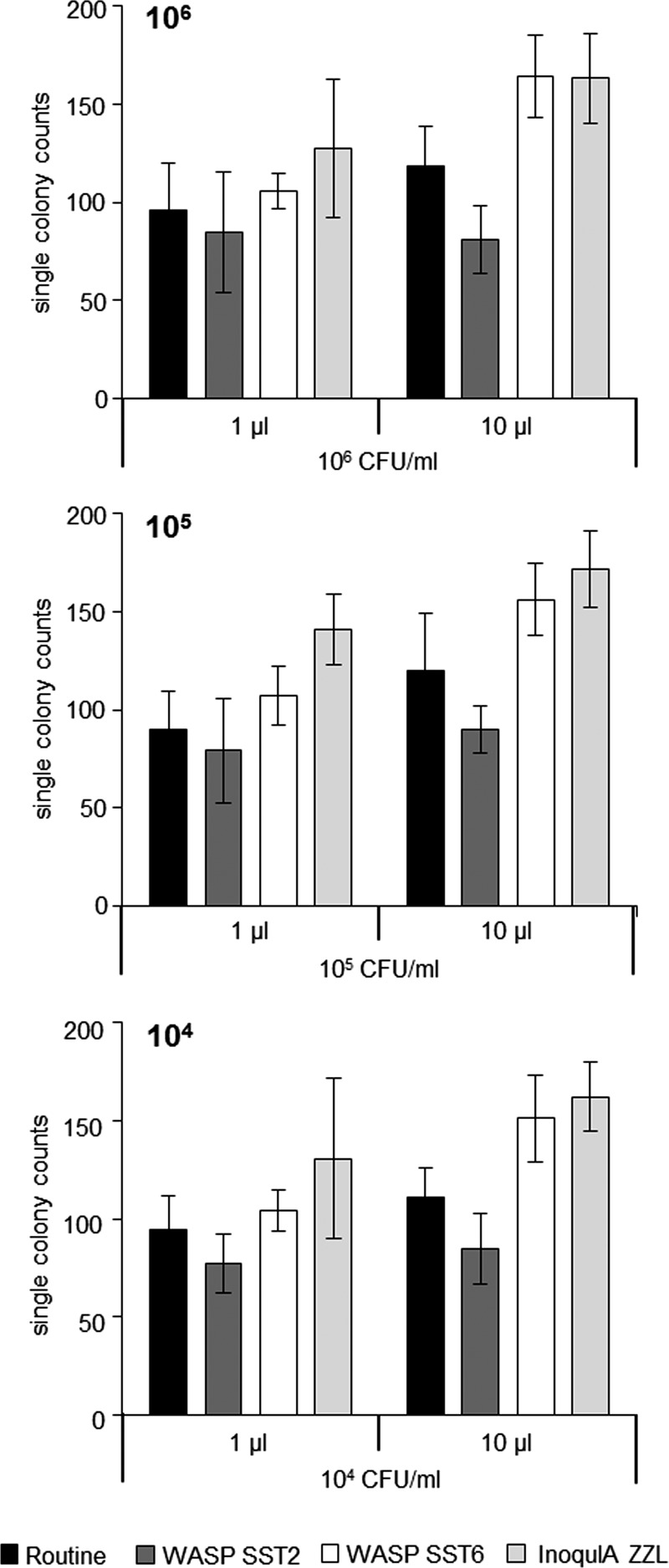
Analysis of mixed cultures of E. coli, enterococci, and CoNS basically resembled the results of pure cultures (Fig. 3). Significantly more single colonies were recovered with the 10-μl inoculum than with the 1-μl inoculum for manual streaking, WASP SST6, and InoqulA (P < 0.001).
FIG 3.
Cumulated numbers of single colonies recovered from mixed cultures containing variable CFU counts of E. coli (104 to 106/ml as indicated) plus fixed CFU counts of 106/ml of both enterococci and coagulase-negative staphylococci. Mean values of 10 mixed cultures prepared with individual clinical strains are shown with standard deviations.
Evaluation of the optimal streaking pattern.
For pure cultures of E. coli and enterococci, the WASP streaking pattern SST2 (11 zigzags) produced fewer single colonies than manual streaking for 6 of the 12 inoculum-CFU combinations, 5 of them significantly (P < 0.001) (Fig. 2B and C). In contrast, WASP streaking pattern SST6 (27 zigzags) and InoqulA (20 zigzags) produced significantly more single colonies than manual streaking for the majority of inoculum-CFU combinations (P < 0.001) (Fig. 2B and C). For 8 out of 12 inoculum-CFU combinations, the WASP SST6 streaking pattern produced significantly more single colonies than InoqulA, WASP SST2, or manual streaking (P < 0.001) (Fig. 2B and C).
For mixed cultures of E. coli, enterococci, and CoNS, both WASP SST6 and InoqulA yielded significantly more single colonies than manual streaking (P < 0.001). No significant difference in the recovery of single colonies was found for mixed inocula when comparing WASP SST6 and InoqulA (P = 0.071) for the 10-μl inoculum, whereas InoqulA BT produced significantly more colonies with the 1-μl inoculum (P = 0.001).
Head-to-head comparison of clinical samples.
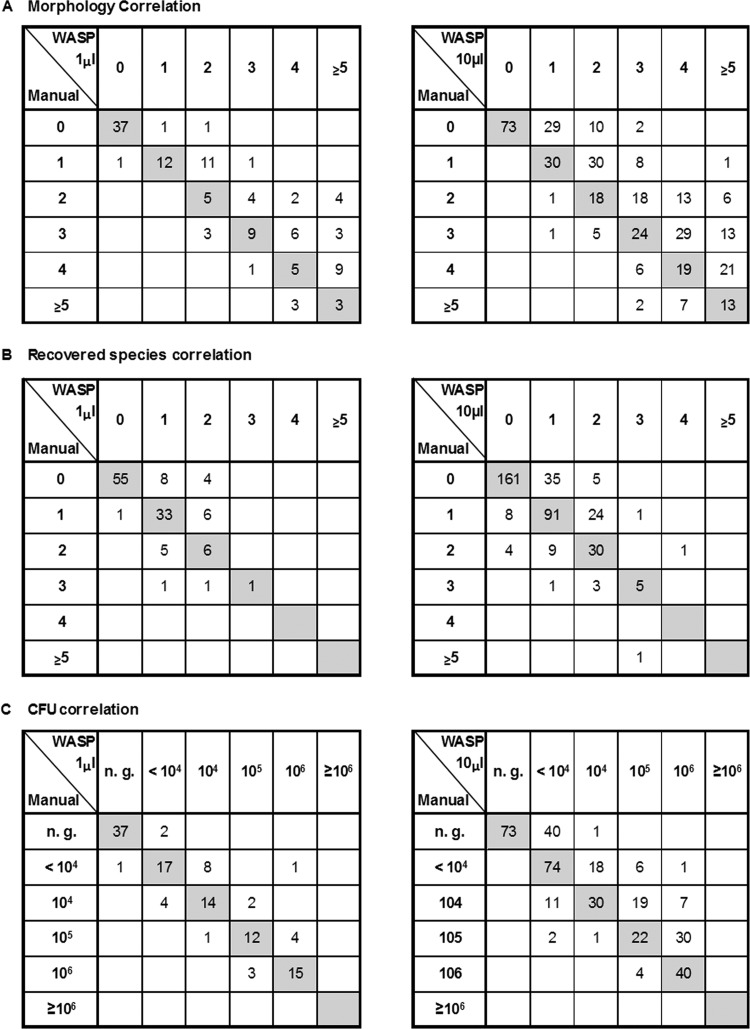
To study the performance of WASP and WASPLab in the routine microbiological workup, we compared fully automated WASP processing (streaking, incubation, and reading) with manual processing for 379 clinical urine samples. Based on the evaluation of optimal inoculation and streaking pattern (see above), a 10-μl inoculum combined with the SST6 streaking pattern (27 zigzags) was chosen for this comparison. We found agreement between the automated WASP processing and the manual workup for detected morphologies, recovered species, and CFUs per milliliter in 177 (46.7%), 287 (75.7%), and 239 (63.1%) of the samples, respectively. However, WASP yielded significantly higher numbers of morphologies and recovered species than manual processing in 180 (47.5%) and 66 (17.4%) of the 379 samples, respectively (P < 0.001) (Table 1; Fig. 4A and B). For 122 (32.2%) of the samples, the WASP workup (10-μl inoculum) indicated a higher CFU count than manual handling (mean increase, 1 log10).
TABLE 1.
Results of the head-to-head comparison of clinical samples (10-μl inocula)
| Parameter | No. (%) of samples with indicated parameter (n = 379)a |
P value | ||||
|---|---|---|---|---|---|---|
| Manual | WASP | Identical | Nonidentical |
|||
| Manual > WASP | WASP > manual | |||||
| Morphologies | 177 (46.7) | 22 (5.8) | 180 (47.5) | 0.000 | ||
| CFU/ml | 239 (63.1) | 14 (4.8) | 122 (32.2) | 0.000 | ||
| Recovered species | 287 (75.7) | 26 (6.9) | 66 (17.4) | 0.000 | ||
| Total no. of MALDI-TOF identifications | 253 | 313 | 0.000 | |||
| Total no. of susceptibility tests | 149 | 163 | 0.337 | |||
| Clinical report | 199 (52.5) | 180 (47.5)c | ||||
| Positive result | 141 (37.2) | 153 (40.4) | ||||
| Negative result | 238 (62.8) | 226 (59.6) | ||||
| Possible pathogensb | 159 (42) | 172 (45.4) | ||||
| Contamination | 10 (2.6) | 10 (2.6) | ||||
Manual inoculation versus WASP inoculation (10-μl loop). Significant differences are indicated in bold (nonparametric Wilcoxon signed-rank test, P < 0.05; crosstab chi-square test of independence, P < 0.05).
Possible pathogens: Gram-negative rods; Staphylococcus aureus; Staphylococcus lugdunensis; Staphylococcus saprophyticus; Streptococcus group B, C, or G; enterococci; yeasts; Corynebacterium glucuronolyticum; Corynebacterium urealyticum.
Pooled number of nonidentical clinical reports comparing manual and WASP inoculation.
FIG 4.
Correlation of morphologies (A), recovered species (B), and CFUs (C) resulting from the head-to-head comparison of 121 and 379 clinical samples, respectively. Data indicate a shift to higher numbers for all three parameters for automated versus manual inoculation.
To exclude a bias due to the higher inoculum used in WASP processing compared to manual processing, a subset of 121 samples were WASP inoculated using a 1-μl loop. No significant difference in CFUs per milliliter was detected between the automated and manual workups when the inoculum was equally set to 1 μl (P = 0.176) (Table 2; Fig. 4C). However, the percentage of samples that displayed higher numbers of morphologies and higher numbers of recovered species was still significantly higher when using automated sample processing versus the manual workup (42/121 [34.7%] and 18/121 [14.9%], respectively; P < 0.001) (Table 2; Fig. 4A and B).
TABLE 2.
Results of the head to head comparison of clinical samples (1-μl inocula)
| Parameter | No. (%) of samples with indicated parameter (n = 121)a |
P value | ||||
|---|---|---|---|---|---|---|
| Manual | WASP | Identical | Nonidentical |
|||
| Manual > WASP | WASP > Manual | |||||
| Morphologies | 71 (58.7) | 8 (6.6) | 42 (34.7) | 0.000 | ||
| CFU/ml | 95 (78.5) | 9 (7.4) | 17 (14.1) | 0.176 | ||
| Recovered species | 95 (78.5) | 8 (6.6) | 18 (14.9) | 0.043 | ||
| Total no. of MALDI-TOF identifications | 76 | 100 | 0.001 | |||
| Total no. of susceptibility tests | 47 | 52 | 0.601 | |||
| Clinical report | 79 (65.3) | 42 (34.7)c | ||||
| Positive result | 47 (39.7) | 51 (42.1) | ||||
| Negative result | 73 (60.3) | 70 (57.9) | ||||
| Possible pathogensb | 48 (39.7) | 59 (48.8) | ||||
| Contamination | 6 (5) | 2 (1.7) | ||||
Manual inoculation versus WASP inoculation (1-μl loop). Significant differences are indicated in bold (nonparametric Wilcoxon signed-rank test, P < 0.05; crosstab chi-square test of independence, P < 0.05).
Possible pathogens: Gram-negative rods; Staphylococcus aureus, Staphylococcus lugdunensis, Staphylococcus saprophyticus; Streptococcus group B, C, or G; enterococci; yeasts; Corynebacterium glucuronolyticum; Corynebacterium urealyticum.
Pooled number of nonidentical clinical reports comparing manual and WASP inoculation.
Automated processing led to the detection of more potential pathogens with both the 10-μl and 1-μl inocula (3.4% and 9.1% of 379 and 121 urine samples processed with the corresponding inoculum, respectively) (Table 2).
The percentage of urine samples categorized in clinical reports as positive, i.e., treatment indicated, or negative, i.e., no treatment indicated, did differ between manual and automatic handling but not statistically significantly. Overall, 37.2% and 40.4% of 379 samples were categorized positive for the manual and automated workup, respectively, while 62.8% and 59.6% were categorized negative (Table 1). This ratio was not affected by using different loop sizes for inoculation (cf. Table 2).
The significantly higher number of morphologies detected by WASPLab processing led to significantly more MALDI-TOF-based identifications. However, the number of subsequent antibiotic susceptibility tests performed was not significantly different between the automated and manual workups, irrespective of whether inoculation was done using a 10-μl or 1-μl loop (Tables 1 and 2).
DISCUSSION
Evaluation of the optimal inoculum.
Traditionally, North American clinical laboratories use a 1-μl inoculum for urine specimens, while European laboratories use 10 μl as the primary inoculum, according to various guidelines (12, 13, 15–17). The primary differences between the two inoculation volumes are a potentially decreased sensitivity using a 1-μl inoculum and the possibility of missing pathogens due to a potential lack of single colonies using a 10-μl inoculum for high CFU counts. We aimed at determining the optimal inoculum for the majority of CFU-pathogen combinations that are most frequently encountered in the clinical laboratory. E. coli was chosen as the most prevalent uropathogen (18). Enterococci and CoNS were selected to represent the most frequently encountered Gram-positive species in urine specimens (14). On whole plates, the 10-μl inoculum was demonstrated to be superior to the 1-μl inoculum in order to maximize the frequency of single-colony recovery, particularly for CFUs of ≤106/ml. As positivity rates and the number of potential pathogens did not differ between the 1-μl and 10-μl inocula, the above-mentioned concerns regarding decreased sensitivity (1-μl inoculum) and missing of pathogens (10-μl inoculum) were not confirmed. A higher frequency of single-colony recovery using automated inoculation may have contributed to the increased detection of potential pathogens (see also “Head-to-head comparison of clinical samples” below). However, the effect of single-colony recovery and improved visual inspection by high-resolution imaging on sample categorization was not distinguished in this study.
Evaluation of the optimal streaking pattern.
For pure cultures, WASP SST6 streaking was superior to WASP SST2 and InoqulA streaking with respect to maximized recovery of single colonies, independent of the inoculum volume. For mixed cultures, single-colony counts generated by WASP SST6 and InoqulA streaking did not differ significantly using a 10-μl inoculum (P = 0.071). However, the number of samples investigated was limited, and a larger follow-up study may reveal statistically significant results given the borderline P value. The bench-top InoqulA combined with manual pipetting produced more colonies than the SST6 for the 1-μl inoculum (P = 0.001). However, the fully automated InoqulA cannot handle inoculation volumes of <10 μl. In part, these findings are in contrast to a recent study that reported the InoqulA to be superior to WASP streaking with respect to the recovery of single colonies (3). However, this effect was shown mainly for exceptionally high CFU counts, i.e., 107 to 108 CFU/ml, and the vast majority of clinical specimens (228/306 [74.5%] in this study) contain up to 105 CFU/ml. Here, for pure cultures, WASP streaking was even superior to the InoqulA method, if SST6 streaking with 27 zigzags was applied. WASP SST2 streaking using 11 zigzags was significantly inferior in generating single colonies. Thus, the critical parameter was the streaking pattern selected, i.e., the number of zigzags, rather than the instrument used for automated inoculation. Differences in streaking patterns may be the reason for discrepancies between the results of this study and the results of the studies of Croxatto et al. (3), Froment et al. (4), and Mischnik et al. (6), as discussed above.
WASP SST6 and InoqulA were superior to manual streaking in maximizing single-colony recovery. These findings are in agreement with those of another study (3). That study found a significant improvement of automation over manual processing mainly for high CFU counts (>106/ml) (3). Our study demonstrates a significant increase in recovery of single colonies using automated inoculation for CFU counts down to104 CFU/ml.
Head-to-head comparison of clinical samples.
WASP processing of clinical specimens yielded significantly more morphologies and recovered species than yielded by manual processing, independent from the number of CFU counts present in the sample. This finding parallels those of a previous study that analyzed urine specimens processed with the BD Kiestra system versus manual handling (4, 12). Indeed, next to a higher number of morphologies and isolated species, automated processing by WASP led to detection of more potential pathogens. Other authors evaluating the BD Kiestra system with urine specimens did not find more pathogens; however, plate reading was not done by camera but rather by the unaided eye (12). The high resolution of the WASPLab imaging system may essentially contribute to the higher number of morphologies detected, resulting in a higher number of identified species and pathogens.
The percentage of categorization of urine samples in clinical reports as positive, i.e., treatment indicated, or negative, i.e., no treatment indicated, differed but not statistically significantly between automated and manual handling. These results are in agreement with data from other studies evaluating the BD Kiestra system (12).
The results of the head-to-head comparison of clinical samples demonstrated that automation in urine microbiology is feasible. However, despite a higher number of potential pathogens within positive samples in the automated workup, the overall categorization of samples as positive or negative was not significantly different between automated and manual processing.
A limitation of this study is that a reliable evaluation of the time to result was not possible, because administrative and organizational lab procedures (e.g., no 24-h/7-day service) must be adapted during the process of automated workflow implementation as the influence of these factors on time to result is significant. As the system was primarily evaluated technically, and WASPLab was not yet part of the diagnostic routine workflow, reliable conclusions regarding the time to result were not drawn from this study and should be the subject of follow-up work. Three main conclusions can be drawn from the present study: (i) the streaking pattern, in particular the number of zigzags/length of streaking lines, is of critical importance for increasing the number of single colonies recovered from primary cultures; (ii) automated streaking by WASP can significantly increase the number of single colonies recovered; and (iii) automated WASPLab processing results in a higher number of detected morphologies, species, and pathogens. In summary, our findings demonstrate that automated sample processing by WASPLab is going to improve the quality of urine microbiological analysis.
Supplementary Material
ACKNOWLEDGMENT
E.C.B. is a consultant of Copan Italia, S.p.A.
Funding Statement
This study was partially supported by the University of Zurich.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02577-15.
REFERENCES
- 1.Sautter RL, Thomson RB Jr. 2015. Consolidated clinical microbiology laboratories. J Clin Microbiol 53:1467–1472. doi: 10.1128/JCM.02569-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourbeau PP, Ledeboer NA. 2013. Automation in clinical microbiology. J Clin Microbiol 51:1658–1665. doi: 10.1128/JCM.00301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croxatto A, Dijkstra K, Prod'hom G, Greub G. 2015. Comparison of the InoqulA and the WASP automated systems with manual inoculation. J Clin Microbiol 53:2298–2307. 10.1128/JCM.03076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Froment P, Marchandin H, Perre PV, Lamy B. 2014. Automated versus manual sample inoculations in routine clinical microbiology: a performance evaluation of the fully automated InoqulA instrument. J Clin Microbiol 52:796–802. doi: 10.1128/JCM.02341-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hombach M, Zbinden R, Bottger EC. 2013. Standardisation of disk diffusion results for antibiotic susceptibility testing using the sirscan automated zone reader. BMC Microbiol 13:225. doi: 10.1186/1471-2180-13-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mischnik A, Mieth M, Busch CJ, Hofer S, Zimmermann S. 2012. First evaluation of automated specimen inoculation for wound swab samples by use of the Previ Isola system compared to manual inoculation in a routine laboratory: finding a cost-effective and accurate approach. J Clin Microbiol 50:2732–2736. doi: 10.1128/JCM.05501-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhoads D, Novak S, Pantanowitz L. 2015. A review of the current state of digital plate reading of cultures in clinical microbiology. J Pathol Inform 6:23. doi: 10.4103/2153-3539.157789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourbeau PP, Swartz BL. 2009. First evaluation of the WASP, a new automated microbiology plating instrument. J Clin Microbiol 47:1101–1106. doi: 10.1128/JCM.01963-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greub G, Prod'hom G. 2011. Automation in clinical bacteriology: what system to choose? Clin Microbiol Infect 17:655–660. doi: 10.1111/j.1469-0691.2011.03513.x. [DOI] [PubMed] [Google Scholar]
- 10.Grohs P, Tillecovidin B, Caumont-Prim A, Carbonnelle E, Day N, Podglajen I, Gutmann L. 2013. Comparison of five media for detection of extended-spectrum Beta-lactamase by use of the wasp instrument for automated specimen processing. J Clin Microbiol 51:2713–2716. doi: 10.1128/JCM.00077-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mutters NT, Hodiamont CJ, de Jong MD, Overmeijer HP, van den Boogaard M, Visser CE. 2014. Performance of Kiestra total laboratory automation combined with MS in clinical microbiology practice. Annals of laboratory medicine 34:111–117. doi: 10.3343/alm.2014.34.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strauss S, Bourbeau PP. 2015. Impact of introduction of the BD Kiestra InoqulA on urine culture results in a hospital clinical microbiology laboratory. J Clin Microbiol 53:1736–1740. doi: 10.1128/JCM.00417-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarter Y, Burd E, Hall G, Zervos M. 2009. Cumitech 2C: laboratory diagnosis of urinary tract infections. American Society for Microbiology, Washington, DC. [Google Scholar]
- 14.Hooton TM. 2012. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med 366:1028–1037. doi: 10.1056/NEJMcp1104429. [DOI] [PubMed] [Google Scholar]
- 15.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW. 2015. Manual of clinical microbiology, 11th ed American Society of Microbiology, Washington, DC. [Google Scholar]
- 17.Sobel JD, Kaye D. 2015. Urinary tract infections, p 886–913.e883. In Bennett JE, Dolan R, Blaser MJ (ed), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 8th ed Saunders, Philadelphia, Pennsylvania. [Google Scholar]
- 18.Barber AE, Norton JP, Spivak AM, Mulvey MA. 2013. Urinary tract infections: current and emerging management strategies. Clin Infect Dis 57:719–724. doi: 10.1093/cid/cit284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.