Abstract
Dopamine function is disturbed in Parkinson's disease (PD), but whether and how release of dopamine from surviving neurons is altered has long been debated. Nicotinic acetylcholine receptors (nAChRs) on dopamine axons powerfully govern dopamine release and could be critical contributing factors. We revisited whether fundamental properties of dopamine transmission are changed in a parkinsonian brain and tested the potentially profound masking effects of nAChRs. Using real-time detection of dopamine in mouse striatum after a partial 6-hydroxydopamine lesion and under nAChR inhibition, we reveal that dopamine signals show diminished sensitivity to presynaptic activity. This effect manifested as diminished contrast between DA release evoked by the lowest versus highest frequencies. This reduced activity-dependence was underpinned by loss of short-term facilitation of dopamine release, consistent with an increase in release probability (Pr). With nAChRs active, the reduced activity-dependence of dopamine release after a parkinsonian lesion was masked. Consequently, moment-by-moment variation in activity of nAChRs may lead to dynamic co-variation in dopamine signal impairments in PD.
Abbreviations: 6-OHDA, 6-hydroxydopamine; [Ca2 +]o, extracellular calcium concentration; [DA]o, extracellular dopamine concentration; ACh, acetylcholine; ChI, cholinergic interneuron; CFM, carbon-fibre microelectrode; DA, dopamine; DAT, dopamine transporter; DHβE, dihydro-beta-erythroidine; FCV, fast-scan cyclic voltammetry; nAChR, nicotinic acetylcholine receptor; P1 release, DA release at the first pulse; P2 release, DA release at the second pulse; PPR, paired-pulse ratio; Pr, release probability; PD, Parkinson's disease; IPI, interpulse interval; SNc, Substantia nigra pars compacta
Keywords: Parkinsonian, Dopamine, Acetylcholine, Nicotinic receptors, Phasic dopamine, 6-OHDA, Release probability, Pr, Fast-scan cyclic voltammetry, Striatum
Graphical abstract
Highlights
-
•
After a parkinsonian lesion, activity-dependence of DA release is impaired.
-
•
Enhanced Pr and reduced uptake combine to compromise activity-dependence.
-
•
Dopamine Pr only weakly relates to short-term plasticity of release.
-
•
nAChR activation abolishes the difference in activity-dependence of DA release between lesion and non-lesion.
Introduction
In PD, degeneration of midbrain dopamine (DA) neurons causes progressive loss of striatal DA. Motor deficits occur when neurodegeneration exceeds ~ 60% suggesting that compensatory mechanisms maintain DA function (Zigmond et al., 1990). Whether changes in release or reuptake mediate this compensation has been debated. Early studies reported enhanced DA synthesis (tyrosine hydroxylase, TH activity) and turnover (altered brain DA metabolites) (Agid et al., 1973, Altar et al., 1987, Hornykiewicz and Kish, 1987, Stachowiak et al., 1987), hypothesising that increased release from the remaining release sites compensates for reduced axonal density (Garris et al., 1997, Zigmond et al., 1990). By contrast, voltammetry studies which can distinctly resolve release and reuptake find that release is unchanged in the parkinsonian rodent brain but that reduced uptake compensates for the diminished number of DA release sites through a process of ‘passive stabilization’ (Bergstrom and Garris, 2003, Bezard et al., 2000, Garris et al., 1997). Additionally, whilst direct evidence of increased release is lacking in voltammetry studies, one study has found that sustainability of DA release is enhanced after lesion (Bergstrom et al., 2011).
We have recently identified that striatal DA release is under potent control by striatal acetylcholine, which is primarily released from cholinergic interneurons (ChIs) and acts at nAChRs on DA axons. Compared to DA release when nAChRs are active, DA release when nAChRs are inactive shows a reduced probability of release and a stronger sensitivity to the firing frequency of DA neurons (Cragg, 2006, Rice and Cragg, 2004, Zhang and Sulzer, 2004). By contrast, upon activation of nAChRs, DA Pr is apparently increased, and sensitivity of DA release to DA neuron firing frequency is limited (Rice and Cragg, 2004, Cragg, 2006). Furthermore, DA release can even be driven directly by activation of ChIs, without the need for DA neuron activity (Threlfell et al., 2012). Therefore, the dynamic availability of extracellular DA is predicted to be highly variable depending on the activity of both DA neurons and ChIs (Cragg, 2006). In PD, altered DA release might be determined by adaptations in either of these two neuron types. Here, we identify novel adaptations to DA release after a parkinsonian lesion that are distinct from the potent influence of ChIs and which can be masked by nAChR activation.
Materials and methods
Animals and surgery
Male C57BL6J mice (8–18 weeks, Charles River, UK) were kept on a 12 h light:dark cycle (lights on at 7 am) and had free access to lab chow, water and nesting materials. All animal procedures were carried out in accordance with the Animals (Scientific Procedures) Act (1986). Mice were anaesthetised with isoflurane (4 then 1.6–2.5%) and local anaesthesia was administered at the incision site (bupivacaine 0.1 mg/kg s.c.). Unilateral craniotomy was performed and 6-hydroxydopamine solution was injected into the SNc using a graduated micropipette (1 μl of a 0.9% saline solution containing 4 μg/μl 6-OHDA [Sigma-Aldrich] and 0.02% acetic acid over 5–20 min; AP − 3.5; ML ± 1.0 from Bregma, DV − 4.1 mm from brain surface). The pipette was withdrawn after a further 10 min to minimise backflow up the injection tract. Palatable food (forage mix, jelly) and heat were provided routinely during the first 48 h, and as necessary during the remainder of the time after surgery. No gross behavioural changes were observed after 6-OHDA lesion. All further reference of lesion refers to this 6-OHDA-induced lesion.
Slice preparation and fast scan cyclic voltammetry (FCV)
At 21 ± 5 days post-surgery, mice were killed by cervical dislocation and the brain dissected and sliced (300 μm) in ice-cold HEPES ringer (in mM: 120 NaCl, 5 KCl, 20 NaHCO3, 6.7 HEPES acid, 3.3 HEPES salt, 2 CaCl2, 2 MgSO4, 1.2 KH2PO4 and 10 glucose saturated with 95% O2/5% CO2) using a Leica VT1000S vibratome. After ≥ 1 h at room temperature, slices were transferred to a recording chamber and superfused with bicarbonate-buffered artificial CSF (aCSF) at ~ 1.5 ml/min and 30–32 °C (in mM: 124 NaCl, 3.7 KCl, 26 NaHCO3, 2.4 CaCl2, 1.3 MgSO4, 1.3 KH2PO4, and 10 glucose saturated with 95% O2/5% CO2).
All DA recordings were made using a Millar Voltammeter (Julian Millar, Barts and the London School of Medicine and Dentistry, UK) as described previously (e.g., Threlfell et al., 2012). Voltammograms were obtained by scanning with a triangular waveform (− 0.7 to + 1.3 V to − 0.7 V versus Ag/AgCl switching to 0 V between scans; 800 V/s; 8 Hz). Evoked extracellular DA concentration ([DA]o) was measured using FCV with carbon fibre microelectrodes (CFMs) constructed in-house (fibre tip diameter, ~ 7 μm; exposed fibre length, 50–100 μm) and positioned ~ 100 μm into the tissue. Electrode calibrations were performed after recordings for each electrode using 2 μM DA in Ca2 +-appropriate aCSF.
Experiments were designed so that lesioned and non-lesioned experiments were carried out in the same animal (except in [Ca2 +] response experiments). Each non-lesioned recording was carried out in the equivalent anatomical location as a paired lesioned recording from that animal.
Electrical stimulation
A concentric bipolar stimulating electrode (FHC, Bowdoinham, ME) was used to electrically evoke DA release in the striatum. The stimulating electrode was positioned on the tissue surface ~ 50–100 μm from the CFM. Monophasic stimulus pulses (0.2 ms duration; 0.65 mA) were applied out-of-phase with FCV scans. Stimuli were repeated at intervals of 2.5 min, as single pulses and 5-pulse trains at 5, 10, 25 and 100 Hz. Stimulation at 5–25 Hz was chosen to encompass the physiological firing frequencies commonly reported in DA neurons. Stimulation at 100 Hz was chosen as a ‘release-limited frequency’ specifically for probing the short-term plasticity of DA release. This method has previously been published for DA (Cragg, 2003, Rice and Cragg, 2004) and the approach is a well-established method for interrogating short-term plasticity mechanisms at glutamate and GABA synapses (e.g., Regehr, 2012, Thomson, 2000, Thomson, 2003).
Paired pulse experiments
In a subset of experiments, paired-pulse release was explored to assess release probability (Pr). Because release summates during two-pulse trains, release at the second pulse (P2 release) was calculated by subtracting single pulse release (P1 release) from paired-pulse release. P2 release was then divided by P1 release to obtain the paired-pulse ratio (PPR). Previous studies of glutamate neurons have demonstrated that as initial Pr increases (i.e., P1 release), P2 release and PPR decrease. Conversely, when initial Pr is low, P2 release increases and can show facilitation such that P2 release > P1 release and PPR > 1. Note that PPR is here expressed as a percentage to maintain consistency throughout the figures.
Drug application
Experimental drugs were made up in stock solutions of 1000–5000 × final concentration in water and stored in aliquots at − 20 °C until use. Just prior to use, aliquots were diluted into O2/CO2-saturated aCSF and bath-applied. In experiments where extracellular [Ca2 +] was varied, measurements were taken in standard aCSF (2.4 mM Ca2 +), before switching to the lowest [Ca2 +] solution and working up through the concentrations.
High performance liquid chromatography
Tissue punches (1 mm diameter) were taken from the striatum of lesioned and non-lesioned hemispheres of acute slices prepared from 6-OHDA treated mice. Punches were placed into ice-cold perchloric acid (0.1 M) and stored at − 80 °C until analysis. Tissue was homogenised using a sonicating probe (pulsed for ~ 10 s), centrifuged (25000 ×g; 15 min) and supernatant removed for analysis. Dopamine was separated on a ChromSep C18 reverse-phase column (5 μm; 4.6 × 250 mm; Varian) and detected (Decade SDC; Antec Leyden) using a glassy carbon working electrode (VT03 Antec Leyden; + 0.7 V vs Ag/AgCl reference). Mobile phase consisted of 13% methanol, 0.12 M NaH2PO4, 0.8 mM EDTA, 2.0 mM octane sulphonic acid, pH 3.2. Dopamine concentrations were interpolated from known concentrations of dopamine run alongside samples and normalised to protein content.
Immunohistochemistry
After FCV, acute slices were fixed in 4% paraformaldehyde for several days before re-sectioning (40 μm) and processing for immunohistochemistry. Sections were incubated in 0.5% triton in phosphate buffered saline (PBS) for 30 min at room temperature, washed (3 × 10 min PBS), blocked for 30 min (PBS with 0.5% triton, 10% normal goat serum [NGS], 10% foetal calf serum [FCS]) and incubated overnight in primary antibody (1:1000; rabbit anti-tyrosine hydroxylase, T8700, Sigma; in PBS with 0.5% triton, 1% NGS, 1% FCS). The following day, sections were washed (3 × 10 min PBS), and incubated in secondary antibody (1:500 goat anti-rabbit, PK6101, Vector Labs; in PBS with 0.5% Triton, 1% NGS, 1% FCS) and visualised using the ABC system (PK6101, Vector Labs) and VIP peroxidase substrate (SK4600; 3–4 min incubation). Sections were then mounted on glass slides, dehydrated and delipidated in ascending ethanol concentrations (1–2 min each) followed by xylene (30 min) and glass coverslips were mounted using DePex.
Data analysis and statistics
FCV data were acquired and analysed using Strathclyde Whole Cell Program (University of Strathclyde, Glasgow, Scotland, UK) and Microsoft Excel. DA oxidation currents were measured from background-subtracted voltammograms, converted to concentration using the calibration factor for that CFM in each solution, and plotted against time to give a transient profile of [DA]o. Triplicate measurements for each stimulation type at each recording site were averaged to produce a profile per stimulation type. After confirming that lesion caused a significant reduction to evoked [DA]o, data were expressed as a percentage of [DA]o evoked by a single pulse to allow assessment of frequency-dependence, PPR, train-length dependence and calcium-dependence, independent of absolute levels of [DA]o. Mean peak evoked [DA]o ± SEM were statistically analysed using SPSS 16. Data were tested for normal distribution using the Shapiro–Wilk test, and where non-normally distributed, a Box–Cox transformation (Osborne, 2010) was applied to normalise the distribution (λ values listed next to the statistics where applicable) or, where the data distribution could not be made normal, a Friedman test was used. Transformed data were re-tested for normality and analysed with a two-way ANOVA. Significant main effects were examined and interactions were interrogated further with a post hoc protected Fisher's LSD.
Results
nAChR inhibition unmasks a deficit in dynamic properties of DA release after a partial 6-OHDA lesion
To assess the impact on striatal DA transmission remaining after a substantial but partial DA lesion, we injected 6-OHDA into the substantia nigra pars compacta (SNc). We detected evoked DA release using fast-scan cyclic voltammetry (FCV) in four regions of lesioned caudate putamen (dorsomedial, central, ventrolateral and ventromedial) and showed that evoked [DA]o was reduced to 18–30% of controls (Fig. 1A). In addition, we confirmed that the falling phase of the DA transient was significantly extended (indicative of reduced uptake rate; see Fig. 1B) and tissue DA content was reduced (Fig. 1C), as expected.
Fig. 1.
Partial lesion of striatal DA changes the dynamic range of DA signals.
A, top, A 6-OHDA injection into the SNc reduced mean [DA]o (± SEM) evoked by 1 pulse (Χ2(7) = 58.1, ***p < 0.001 Friedman test; n = 10 experiments from 5 animals; colours correspond to site illustrated in the bottom). Bottom, reduced tyrosine hydroxylase (TH) staining in dorsal striatum, SNc. B, Extended clearance phase for DA after a lesion shows reduced DA uptake rate, representative example. Group statistics (n = 7 experiments from 7 animals) showed significant main effect of time (F(11,154) = 142, p < 0.001) and lesion (F(1,14) = 24.9, p < 0.001) and a lesion ∗ time interaction (F(11,154) = 6.0, p < 0.001). A lower stimulation current was used in non-lesion sites to evoke comparable peak [DA]o to lesion. C, HPLC measurement of reduced tissue DA content after 6-OHDA lesion, t(8) = 7.4, ***p < 0.001 T-test. D, Mean profiles of [DA]o ± SEM vs time evoked by 1p or 5p at 5–100 Hz (vertical lines) in non-lesion control (black) and in the lesion (red) in the presence of nAChR antagonist DHβE (1 μM). E, Mean peak [DA]o ± SEM vs frequency (5p) in non-lesion (unfilled) vs lesion (filled) from D expressed as % of peak [DA]o evoked by 1p in each condition, for each group n = 7 experiments from 7 animals (*p < 0.001 frequency ∗ lesion F(3,18) = 14.2 and p < 0.05 lesion versus non-lesion, Fisher's LSD; data transformed to normality, λ = 0.061). F, Upper, mean profiles of [DA]o ± SEM vs time evoked by 1–9p at 100 Hz (arrows) in non-lesion (left) and in the lesion (right); and lower, mean peak [DA]o ± SEM vs pulse number (100 Hz) in non-lesion (unfilled) vs lesion (filled) expressed as % of peak [DA]o evoked by 1p in each condition. For both groups n = 6 experiments from 6 animals. Lesion reduces the pulse number dependence of evoked [DA]o (**p < 0.005 lesion F(2,10) = 14.6, ANOVA). Data from D, E and F were collected in DHβE (1 μM).
Striatal electrical stimulation will activate both dopaminergic axons and cholinergic neurons, meaning that DA release driven by DA axon activity as well as by nAChR activation will contribute to our [DA]o signal (Threlfell et al., 2012, Wang et al., 2014). Therefore, we inhibited nAChRs to dissociate cholinergic- and dopaminergic-driven DA release and to define the locus of any adaptations in DA release after lesion. DHβE (1 μM) was included in bath solutions unless otherwise stated. Under nAChR inhibition, the frequency dependence of evoked [DA]o during 5-pulse electrical trains at 5–100 Hz was significantly changed by the lesion (Figs. 1D, E). The range of peak [DA]o evoked by the lowest to highest frequencies (5–100 Hz) was decreased from 5.5-fold (non-lesion) to only ~ 2 fold (lesion) suggesting that changes in DA neuron firing rate in the parkinsonian brain could generate reduced moment-by-moment contrast in DA signals.
After lesion, lower frequency trains (5–10 Hz) were relatively better at releasing DA (normalised to a single pulse, p < 0.05), whereas there was a trend for higher frequency trains (100 Hz) to be relatively worse at releasing DA (Figs. 1D, E). Post hoc testing did not find this latter effect to be significant, but because of the large spread of data and degrees of freedom due to the multiple frequencies in the experimental design, we probed this effect further in separate experiments at 100 Hz. Using trains of varying length (2–9 pulses, 100 Hz), we confirmed that at this high frequency, [DA]o was relatively lower in lesioned than non-lesioned sites (Fig. 1F). Overall, these data show that the sensitivity of DA release to dynamic activity in DA axons is impaired in release sites that remain after a lesion.
Paired-pulse studies indicate loss of short-term facilitation and an increase in Pr after 6-OHDA lesion
A relative increase in [DA]o at lower frequencies, often called ‘uptake-limited’ frequencies, is an expected consequence of the reduced densities of DA axons and uptake transporters (DAT) in the striatum following a lesion. A reduction in DAT density will reduce the rate of DA uptake at all substrate concentrations, allowing greater residual [DA]o and summation at longer interpulse intervals (IPIs) (Benoit-Marand et al., 2000, Chergui et al., 1994, Suaud-Chagny et al., 1995). However, at higher frequencies, a relative impairment in [DA]o is not predicted by reduced DAT density alone, suggesting that the DA release process is also altered in these neurons (note that such changes in release machinery would be expected to impact all frequencies but the effects will be more visible at ‘release-limited’ frequencies where effects of reduced uptake are less of a confound). To separate the contributions of altered release from altered summation/uptake, we used paired-pulse stimulations to explore the dynamics of DA release and assess Pr. At classical, amino acid synapses when paired pulses are delivered with short inter-pulse intervals (typically < 50 ms), the initial Pr determines release by the first pulse (P1 release) and is inversely related to release at the second pulse (P2 release). Therefore by determining P2 release as a function of P1 release (known as the paired-pulse ratio, PPR), insights into initial Pr for DA can be gained.
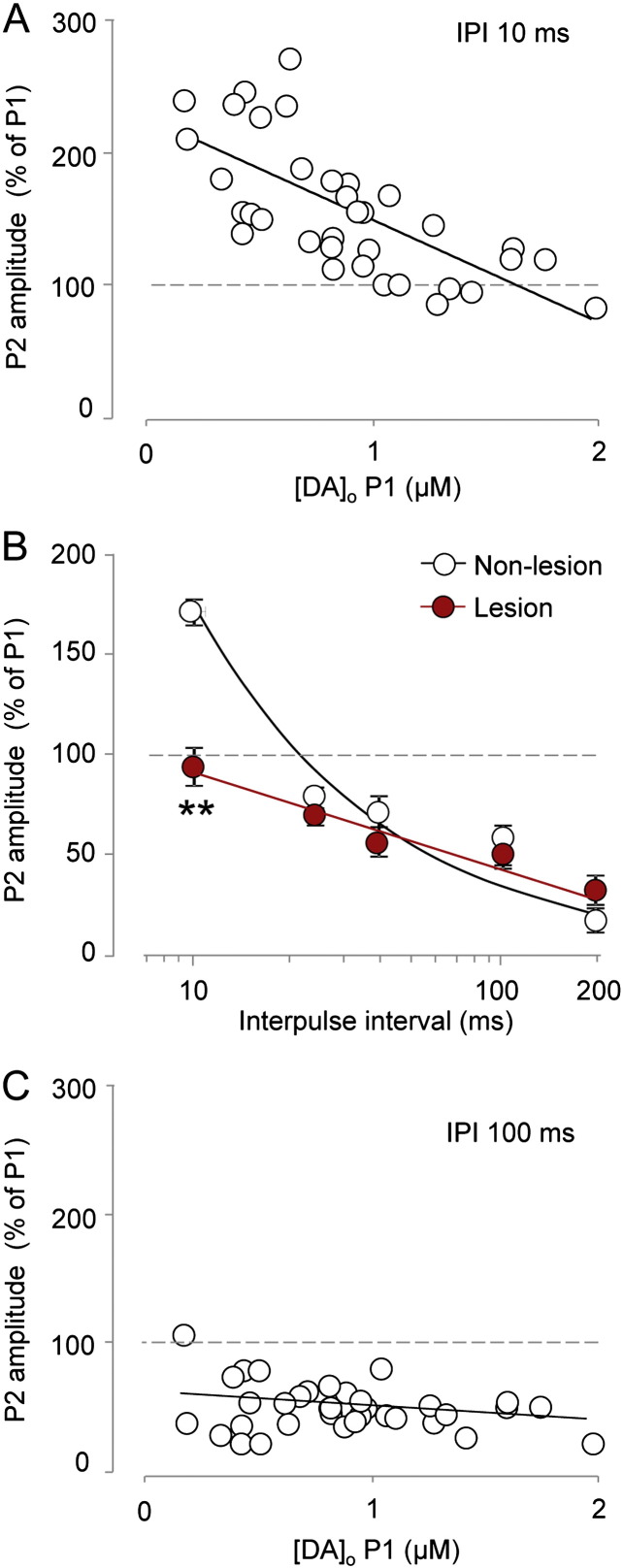
We first identified the relationship between P2 and P1 for DA release at a range of release sites in intact striatum (IPI 10 ms). P2 release (expressed as a percentage of P1) varied inversely with P1 release across sites (Fig. 2A), confirming that DA release at a subsequent pulse is an inverse predictor of initial Pr. We next explored the effect of the lesion, using a range of inter-pulse intervals (10–200 ms) to explore dynamic changes in Pr for a range of frequencies (5–100 Hz). For amino acid transmitters and DA, P2 release normally equals or exceeds P1 release (short-term facilitation) at intervals of 50 ms or less and decreases as interpulse interval increases (100–200 ms) when facilitation gives way to short-term depression (Dobrunz and Stevens, 1997, Regehr, 2012, Rice and Cragg, 2004, Thomson, 2000). We confirmed a similar relationship here for [DA]o in non-lesioned striatum (Fig. 2B). However, lesioned striatum differed significantly: there was a significant loss of paired-pulse facilitation at the shortest IPI (10 ms) (Fig. 2B) suggestive of an underlying increase in initial Pr. Loss of facilitation would account for the reduced [DA]o observed during high frequency trains seen in Fig. 1.
Fig. 2.
Paired-pulse ratios at 10 ms reveal increased Pr.
A, Plot of [DA]o evoked by the second (P2) versus the first (P1) of two pulses in a pair, at an IPI of 10 ms, shows an inverse relationship indicating that P2 is inversely related to initial Pr (linear regression R2 = 0.48; F(1,33) = 30.9, p < 0.001; n = 35 sites from 35 animals). B, Mean P2 ± SEM decreases as inter-pulse interval increases (two-way ANOVA, data transformed to normality λ = 0.67: F(4,24) = 48.3, p < 0.001) but P2 at 10 ms is reduced in lesion (IPI ∗ lesion F(4,24) = 5.5, p < 0.005; **p < 0.005 lesion versus non-lesion Fisher's LSD test at 10 ms, non-lesion n = 3 experiments from 3 animals; lesion n = 5 experiments from 5 animals). C, At an IPI of 100 ms, [DA]o at P2 is not related to P1 (linear regression, R2 = 0.07; F(1,33) = 2.5, p > 0.1; n = 35 sites from 35 animals). All data are in the presence of nAChR antagonist DHβE (1 μM).
Although there was a marked loss of facilitation/increase in Pr at an IPI of 10 ms, there was no difference between lesioned and non-lesioned striata in P2 release seen at IPIs longer than 10 ms. These findings could be explained by two possibilities: either that P2 is inversely related to Pr at very short IPIs only (~ 10 ms) – and can therefore only be used to predict Pr accurately at this IPI – or that a loss of facilitation of P2 is not reflective of increased Pr, but of some other change (e.g., loss of short-term facilitatory mechanisms). To address the former possibility we explored the relationship between P1 and P2 across a range of release sites in intact striatum with a longer IPI of 100 ms. Our data show that although P2 varies inversely with P1 at release sites for short intervals of 10 ms (see Fig. 2A), by contrast P2 does not vary with P1 at the longer interval of 100 ms (Fig. 2C). These data identify that P2 delivered only at very short IPI is an index of DA Pr, and support the hypothesis that loss of short-term facilitation in a lesion is due to an increase in Pr. These data suggest that a lesion leads to changes in dynamic DA signals through a combination of reduced uptake, enhanced Pr and loss of short-term facilitation.
DAT inhibition recapitulates aspects of altered DA release caused by 6-OHDA lesion
To further verify whether simultaneous changes in uptake and Pr described above could combine to cause the observed changes in dynamic availability of [DA]o, we compared the effects of a lesion to those of cocaine since this drug has been shown to both inhibit DA uptake and enhance DA releasability (Kile et al., 2010, Venton et al., 2006). Similarly to the lesion, cocaine (5 μM) modified the frequency dependence of evoked [DA]o: low frequencies were relatively better at evoking [DA]o (normalised to single pulse release) whilst higher frequencies released relatively less [DA]o (Fig. 3A). During trains of 2–9 pulses at 100 Hz, cocaine reduced evoked [DA]o (normalised to a single pulse) compared to cocaine-free sites and the resulting relationship between train length and [DA]o was indistinguishable from that induced by a lesion (Fig. 3B). In paired-pulse experiments, a similar trend was observed, with cocaine causing a loss of facilitation comparable to lesion at an IPI of 10 ms (data not illustrated: mean amplitude P2 (% of P1) ± SEM: 174 ± 8%, non-lesion without cocaine; 94 ± 2%, non-lesion with cocaine; 94 ± 10% lesion without cocaine; IPI ∗ drug ∗ lesion F(4,24) = 5.7, p < 0.005; lesion versus non-lesion with cocaine p > 0.19; non-lesion with cocaine versus without p < 0.005; data transformed to normality λ = 0.67). However, despite these similarities between the effects of cocaine and lesion, their effects appeared to be mediated by different mechanisms. Cocaine promotes DA release via a synapsin-dependent mechanism, and knockout of synapsins changes the Ca2 +-dependence of DA release (Kile et al., 2010) suggesting that cocaine might act to change the Ca2 +-dependence of [DA]o. We showed this to be the case (Fig. 3C). However, lesion by contrast did not change the Ca2 +-dependence of DA release (Fig. 3C) from that seen in cocaine-free, non-lesioned sites.
Fig. 3.
Cocaine recapitulates the altered DA release profile seen in a lesion but through a different mechanism.
A, Mean peak [DA]o ± SEM vs frequency (5p) in cocaine (chequered) vs control (filled) expressed as % of peak [DA]o evoked by 1p in each condition, n = 6 experiments from 3 animals. Note similarity to Fig. 1E. Two-way ANOVA: drug ∗ frequency F(3,15) = 65.3, p < 0.001; *p < 0.05 naïve versus cocaine, Fisher's LSD test. B, Mean peak [DA]o ± SEM vs pulse number (100 Hz) in non-lesion, lesion and cocaine-treated non-lesioned striatum expressed as % of peak [DA]o evoked by 1p in each condition. Two-way ANOVA: F(2,10) = 14.6, Treatment p < 0.01; #p < 0.05 main effect of cocaine versus non-lesion; **p < 0.005 main effects lesion versus non-lesion. For each group, n = 5 experiments from 5 animals C, Mean peak [DA]o ± SEM vs [Ca2 +]o in lesion, non-lesion and cocaine-treated non-lesioned sites. [Ca2 +] F(3,42) = 2.7, p < 0.001, [Ca2 +] ∗ treatment F(1,14) = 4.7, p < 0.05; #p < 0.05 main effect of cocaine versus both lesion and non-lesion, *p < 0.05, lesion n = 5 experiments from 5 animals; for all other groups n = 6 experiments from 6 animals.
nAChR activity masks the DA release abnormalities caused by 6-OHDA lesion
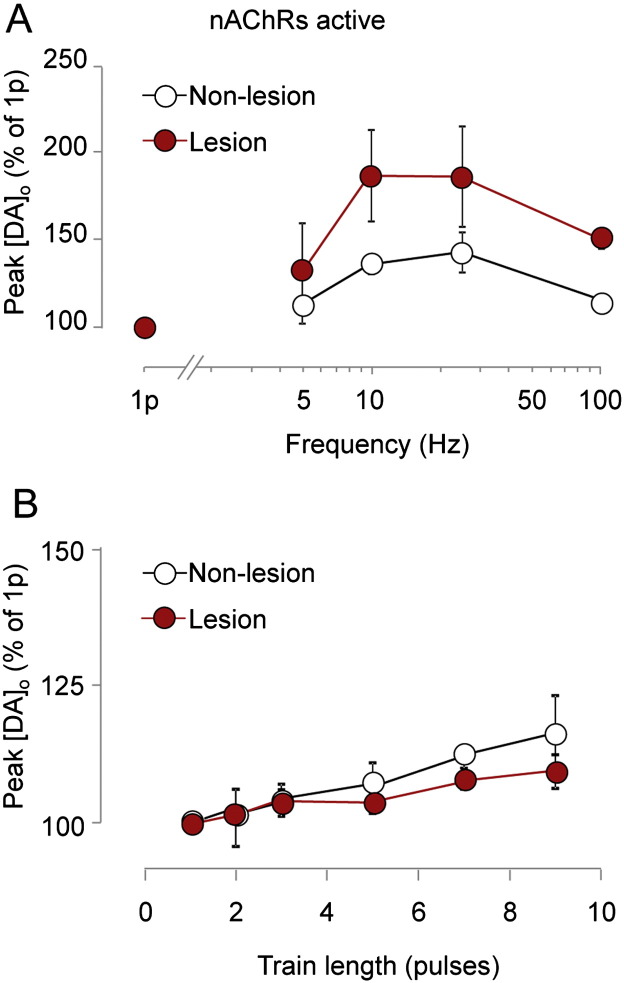
All observations so far were made in the absence of the confounding effects of nAChR activation, under nAChR blockade. Therefore, we explored finally whether our observed changes to the activity-dependence of DA signals after a lesion were exacerbated or offset by concurrent activation of nAChRs. When nAChRs were permitted to be activated by ACh released by local electrical stimulation (Exley et al., 2008, Rice and Cragg, 2004, Threlfell et al., 2012), [DA]o in non-lesioned sites showed only weak dependence on frequency (Fig. 4A) or pulse number (Fig. 4B) compared to when nAChRs are inactive (cf. Fig. 1). Furthermore, these relationships did not differ significantly between lesioned and non-lesioned sites (Fig. 4), indicating that the underlying changes to DA release resulting from a lesion are masked during concurrent nAChR activation.
Fig. 4.
nAChR activity masks effects of a lesion.
A, Mean peak [DA]o ± SEM vs frequency (5p) in the absence of nAChR inhibitors, in non-lesioned (unfilled) vs lesioned striatum (filled) expressed as % of peak [DA]o evoked by 1p in each condition. Two-way ANOVA: Frequency, F(3,18) = 16.1; p < 0.001; data transformed for normality λ = − 1.55, for each group n = 7 experiments from 7 animals B, Mean peak [DA]o ± SEM vs pulse number (100 Hz) in the absence of nAChR inhibitors in non-lesioned (unfilled) vs lesioned striatum (filled) expressed as % of peak [DA]o evoked by 1p in each condition. Two-way ANOVA: no significant difference, for each group n = 6 experiments from 6 animals.
Discussion
We show that DA release remaining in the parkinsonian brain is dysregulated, with decreased short-term facilitation reflecting an increased Pr. This altered release combines with reduced uptake to lessen the contrast between DA signals evoked by different firing frequencies. Furthermore, activation of nAChRs by ACh masks these adaptations, with no significant difference in the activity-dependence of DA signals between lesioned and non-lesioned striata when nAChRs are active.
Impaired DA signal contrast after parkinsonian lesion
Our data indicate that, when nAChRs are inactive, the contrast between DA signals evoked by low versus high frequencies of DA neuron activity will be reduced in PD. Our findings corroborate and build further on the previous finding that DA release is impaired after a parkinsonian lesion during high frequency stimulus trains in vivo (Bergstrom and Garris, 2003), and suggest a novel mechanism to explain this impairment. We show a dysregulation of DA signals that is expected to be particularly prevalent during periods of nAChR inactivity. Such periods of inactivity could feasibly arise during the pauses in ChI activity that are seen in vivo (Aosaki et al., 1994, Aosaki et al., 1995, Cragg, 2006, Morris et al., 2004). Pauses in ChI activity are acquired during motor learning and show coincidence with phasic activity in DA neurons (e.g., Morris et al., 2004), which could have relevance for action selection and initiation. Our data predict that reduced nAChR activation due to a ChI pause will impair most profoundly the DA signals released by high frequency activity in DA neurons.
High frequency activity is observed in most dopamine neurons in VTA and SNc (Schultz, 1998). Traditionally, high frequency activity in dopamine neurons has been associated exclusively with reward-processing and decision-making. However, recent evidence has shown that high frequency activity in SNc neurons in particular correlates with the onset and offset of learned action sequences (Jin and Costa, 2010) and may be important for habit formation, a process that is dorsal striatum-mediated (Wang et al., 2011). Therefore, our observation of altered activity-dependence of dopamine may have significance for both decision-making processes and for motor function, most importantly, for the movement deficits seen in PD. It will be important to establish empirically whether the behavioural consequences of DA released by higher DA neuron firing frequencies are impaired in PD and if so, whether such impairments can be rescued by changing ChI and/or nAChR activity.
Enhanced DA Pr after 6-OHDA lesion
Historically, studies have typically assumed that DA release is invariant with pulses within a train and have not resolved subsecond changes to DA release in models of PD (Bezard and Gross, 1998, Bezard et al., 2000, Garris et al., 1997). However, DA release is not constant during a train of action potentials but is subject to moment-by-moment short-term plasticity of release probability (Cragg, 2003, Montague et al., 2004, Rice and Cragg, 2004). We revealed a relationship between DA release probability and short-term plasticity of release that is visible over short timescales only (on the order of 10 ms) and not over longer timescales (on the order of 100 ms). Furthermore, using subsecond trains and pairs of pulses we reveal for the first time that a DA lesion causes loss of facilitation of DA release at short inter-pulse intervals, which indicates an increased Pr from surviving DA release sites in dorsal striatum. Note that whilst our data suggest that loss of facilitation is likely only to be evident at the high firing frequencies not commonly reported for DA neurons, the mechanisms underpinning this and enhanced Pr are likely to have consequences for DA release at all spontaneous firing frequencies.
Experiments with cocaine showed that DAT inhibition was followed by changes to frequency dependence and DA Pr that are distinct from simple increases in extracellular summation of DA. These findings corroborate previous findings that cocaine increase DA release in addition to reducing uptake (Kile et al., 2010, Venton et al., 2006). However, although cocaine and a DA lesion caused similar effects here, the mechanisms were not the same. Cocaine modified the dependence of DA release on [Ca2 +] but lesion did not. Changes in the distribution of vesicle pools can regulate Pr at CNS synapses (e.g., Regehr, 2012) and we have recently shown that changes to vesicle clustering accompany changes to DA Pr in an alpha-synuclein-overexpressing mouse model of PD (Janezic et al., 2013). Altered vesicular regulation is a candidate mechanism for altered DA Pr here in a late stage DA lesion that should be addressed in future studies.
One previous study found evidence of altered release in a PD model, reporting enhanced sustainability of release during long, fatiguing stimuli (Bergstrom et al., 2011). The authors suggested that loss of a long-term depression (LTD) component (Montague et al., 2004) underpinned these observations. We show here rather that Pr is increased and short-term facilitation is impaired. This mechanism might preserve release pools and sustain release over longer time frames. In support of this relationship between our findings and those of enhanced sustainability of DA release, are the findings that inhibition of DA uptake recapitulated both the long-term sustainability of release (Bergstrom et al., 2011) and the short-term impairment in high frequency DA release shown here. It is not yet evident however whether the most desirable outcome of an improved therapy for PD would be to reverse the deficit in high frequency DA signalling or to promote the long term maintenance of DA release, but the potential competition between these two factors is important to keep in mind.
nAChRs reverse activity dependence deficits after a 6-OHDA lesion?
The changes we observed in activity-dependence of DA release after a partial 6-OHDA lesion were exposed in the absence of nAChR-regulation of DA. However, when nAChRs were not blocked, and were activated by local electrical stimulation of DA axons and ChIs, the activity-dependence of DA release was not different between lesioned and non-lesioned striata. Thus, abnormalities in DA Pr could be masked by nAChR activation which restores DA activity-dependence to a pre-lesion state. This is most likely because nAChRs very powerfully increase the apparent DA Pr by ~ 100–300% (Rice and Cragg, 2004, Threlfell et al., 2012, Zhang and Sulzer, 2004, Zhou et al., 2001). Such a powerful effect is expected to dominate and eclipse other mechanisms located in DA axons that regulate DA Pr, increasing Pr maximally and causing a ceiling effect which ensures that no difference in signalling is detectable between healthy and parkinsonian states. Activity in the ChI network activity might therefore determine the extent of impairment to DA release at any given moment. Our data suggest that activity-dependence will be most impaired when nAChR activation is minimal, which should occur when ChIs pause. Our findings may appear on first sight to be contradictory to the widely held view that anti-cholinergic drugs are beneficial for PD. However, it should be noted that anti-cholinergic therapies act at muscarinic receptors, whilst the data presented here provide further support for nicotinic receptor activation as therapeutic targets in PD (Quik et al., 2009). Future experiments should explore our results during in vivo patterns of activity and determine whether activating nAChRs is desirable for behaviour.
Conclusions
In summary, we show for the first time that besides the profound deficit in DA levels in PD, a parkinsonian lesion causes a blunted dynamic range of DA release, which is underpinned by the loss of short-term facilitation and increased Pr of remaining DA. This difference in dynamic availability of DA is minimised by nAChR activation. These findings reveal that high frequency DA function in particular is impaired in the parkinsonian brain and suggest that striatal nAChRs might be targets for future PD therapies.
Acknowledgments
This work was funded by a Parkinson's UK project grant (G0803). There are no conflicts of interest. The authors would like to thank Mrs Debbie Cobley and Ms Carol Broadbent and their staff for providing animal care during this study.
References
- Agid Y., Javoy F., Glowinski J. Hyperactivity of remaining dopaminergic neurones after partial destruction of the nigro-striatal dopaminergic system in the rat. Nat. New Biol. 1973;245:150–151. doi: 10.1038/newbio245150a0. [DOI] [PubMed] [Google Scholar]
- Altar C.A., Marien M.R., Marshall J.F. Time course of adaptations in dopamine biosynthesis, metabolism, and release following nigrostriatal lesions: implications for behavioral recovery from brain injury. J. Neurochem. 1987;48:390–399. doi: 10.1111/j.1471-4159.1987.tb04106.x. [DOI] [PubMed] [Google Scholar]
- Aosaki T., Tsubokawa H., Ishida A., Watanabe K., Graybiel A.M., Kimura M. Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. J. Neurosci. 1994;14:3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki T., Kimura M., Graybiel A.M. Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J. Neurophysiol. 1995;73:1234–1252. doi: 10.1152/jn.1995.73.3.1234. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M., Jaber M., Gonon F. Release and elimination of dopamine in vivo in mice lacking the dopamine transporter: functional consequences. Eur. J. Neurosci. 2000;12:2985–2992. doi: 10.1046/j.1460-9568.2000.00155.x. [DOI] [PubMed] [Google Scholar]
- Bergstrom B.P., Garris P.A. “Passive stabilization” of striatal extracellular dopamine across the lesion spectrum encompassing the presymptomatic phase of Parkinson's disease: a voltammetric study in the 6-OHDA-lesioned rat. J. Neurochem. 2003;87:1224–1236. doi: 10.1046/j.1471-4159.2003.02104.x. [DOI] [PubMed] [Google Scholar]
- Bergstrom B.P., Sanberg S.G., Andersson M., Mithyantha J., Carroll F.I., Garris P.a. Functional reorganization of the presynaptic dopaminergic terminal in parkinsonism. Neuroscience. 2011;193:310–322. doi: 10.1016/j.neuroscience.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E., Gross C.E. Compensatory mechanisms in experimental and human Parkinsonism: towards a dynamic approach. Prog. Neurobiol. 1998;55:93–116. doi: 10.1016/s0301-0082(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Bezard E., Jaber M., Gonon F., Boireau A., Bloch B., Gross C.E., Gonon Ë., Segalen Â.V., Saignat Â., Cedex B. Adaptive changes in the nigrostriatal pathway in response to increased 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration in the mouse. Neuroscience. 2000;12:2892–2900. doi: 10.1046/j.1460-9568.2000.00180.x. [DOI] [PubMed] [Google Scholar]
- Chergui K., Suaud-Chagny M.F., Gonon F. Nonlinear relationship between impulse flow, dopamine release and dopamine elimination in the rat brain in vivo. Neuroscience. 1994;62:641–645. doi: 10.1016/0306-4522(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Cragg S.J. Variable dopamine release probability and short-term plasticity between functional domains of the primate striatum. J. Neurosci. 2003;23:4378–4385. doi: 10.1523/JNEUROSCI.23-10-04378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg S.J. Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 2006;29:125–131. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Dobrunz L.E., Stevens C.F. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Exley R., Clements M.A., Hartung H., McIntosh J.M., Cragg S.J. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- Garris P.A., Walker Q.D., Wightman R.M. Dopamine release and uptake rates both decrease in the partially denervated striatum in proportion to the loss of dopamine terminals. Brain Res. 1997;753:225–234. doi: 10.1016/s0006-8993(97)00003-6. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O., Kish S. Biochemical pathophysiology of Parkinson's disease. Adv. Neurol. 1987;45:19–34. [PubMed] [Google Scholar]
- Janezic S., Threlfell S., Dodson P.D., Dowie M.J., Taylor T.N., Potgieter D., Parkkinen L., Senior S.L., Anwar S., Ryan B. Deficits in dopaminergic transmission precede neuron loss and dysfunction in a new Parkinson model. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E4016–E4025. doi: 10.1073/pnas.1309143110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Costa R.M. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature. 2010;466:457–462. doi: 10.1038/nature09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile B.M., Guillot T.S., Venton B.J., Wetsel W.C., Augustine G.J., Wightman R.M. Synapsins differentially control dopamine and serotonin release. J. Neurosci. 2010;30:9762–9770. doi: 10.1523/JNEUROSCI.2071-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague P.R., McClure S.M., Baldwin P.R., Phillips P.E.M., Budygin E., a, Stuber G.D., Kilpatrick M.R., Wightman R.M. Dynamic gain control of dopamine delivery in freely moving animals. J. Neurosci. 2004;24:1754–1759. doi: 10.1523/JNEUROSCI.4279-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G., Arkadir D., Nevet A., Vaadia E., Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Osborne J.W. Improving your data transformations: applying the Box–Cox transformation. Pract. Assess. Res. Eval. 2010;15:1–9. [Google Scholar]
- Quik M., Huang L.Z., Parameswaran N., Bordia T., Campos C., Perez X.a. Multiple roles for nicotine in Parkinson's disease. Biochem. Pharmacol. 2009;78:677–685. doi: 10.1016/j.bcp.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr W.G. Short-term presynaptic plasticity. Cold Spring Harb. Perspect. Biol. 2012;4:a005702. doi: 10.1101/cshperspect.a005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice M.E., Cragg S.J. Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Stachowiak K., Keller W., Stricker M., Zigmond J., Stachowiak M.K., Keller R.W., Stricker E.M., Zigmond M.J. Increased dopamine efflux from striatal slices during development and after nigrostriatal bundle damage. J. Neurosci. 1987;7:1648–1654. doi: 10.1523/JNEUROSCI.07-06-01648.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaud-Chagny M.F., Dugast C., Chergui K., Msghina M., Gonon F., Dugxst C., Msgbina M. Uptake of dopamine released by impulse flow in the rat mesolimbic and striatal systems in vivo. J. Neurochem. 1995;65:2603–2611. doi: 10.1046/j.1471-4159.1995.65062603.x. [DOI] [PubMed] [Google Scholar]
- Thomson A.M. Facilitation, augmentation and potentiation at central synapses. Trends Neurosci. 2000;23:305–312. doi: 10.1016/s0166-2236(00)01580-0. [DOI] [PubMed] [Google Scholar]
- Thomson A.M. Presynaptic frequency- and pattern-dependent filtering. J. Comput. Neurosci. 2003;15:159–202. doi: 10.1023/a:1025812808362. [DOI] [PubMed] [Google Scholar]
- Threlfell S., Lalic T., Platt N.J., Jennings K.A., Deisseroth K., Cragg S.J. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Venton B.J., Seipel A.T., Phillips P.E.M., Wetsel W.C., Gitler D., Greengard P., Augustine G.J., Wightman R.M. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J. Neurosci. 2006;26:3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.P., Li F., Wang D., Xie K., Wang D., Shen X., Tsien J.Z. NMDA receptors in dopaminergic neurons are crucial for habit learning. Neuron. 2011;72:1055–1066. doi: 10.1016/j.neuron.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhang X., Xu H., Zhou L., Jiao R., Liu W., Zhu F., Kang X., Liu B., Teng S. Temporal components of cholinergic terminal to dopaminergic terminal transmission in dorsal striatum slices of mice. J. Physiol. 2014;592:3559–3576. doi: 10.1113/jphysiol.2014.271825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat. Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhou F.M., Liang Y., Dani J.A. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat. Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- Zigmond M.J., Abercrombie E.D., Berger T.W., Grace A.A., Stricker E.M. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]