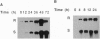Abstract
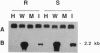
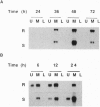
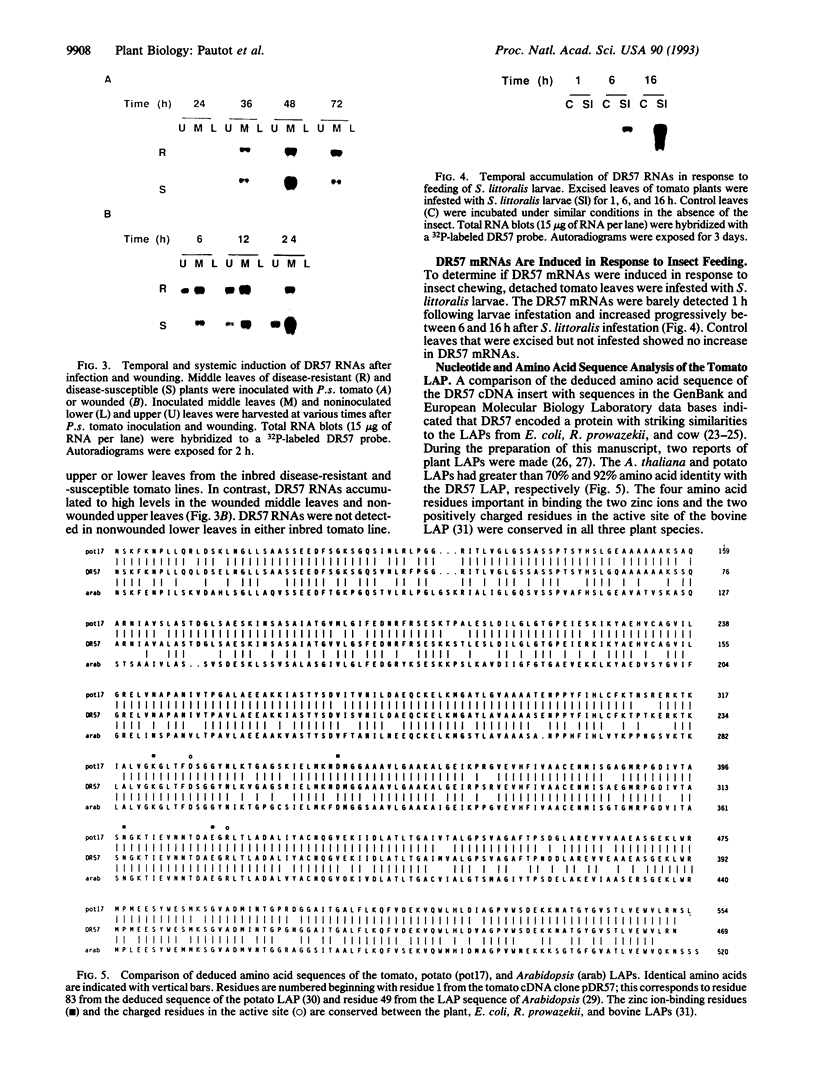
A leucine aminopeptidase (EC 3.4.11.1) cDNA clone (DR57) that was induced in response to Pseudomonas syringae pv. tomato (P.s. tomato) infection was isolated using a subtractive hybridization-enriched cDNA probe. Genomic DNA blot analysis showed that the tomato genome had two leucine aminopeptidase genes. The levels of DR57 mRNAs after P.s. tomato infection and mechanical wounding were determined in two inbred tomato lines that exhibit susceptibility and resistance to P.s. tomato. DR57 mRNAs were detected 12 hours after infection and 4 hours after wounding. Furthermore, DR57 mRNAs were systemically induced in response to wounding. DR57 mRNAs were induced in leaves after Spodoptera littoralis feeding but were not detected in detached leaf controls. Possible roles for the DR57 leucine aminopeptidase in the defense reactions are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmair A., Finley D., Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986 Oct 10;234(4773):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- Bartling D., Weiler E. W. Leucine aminopeptidase from Arabidopsis thaliana. Molecular evidence for a phylogenetically conserved enzyme of protein turnover in higher plants. Eur J Biochem. 1992 Apr 1;205(1):425–431. doi: 10.1111/j.1432-1033.1992.tb16796.x. [DOI] [PubMed] [Google Scholar]
- Bell J. N., Ryder T. B., Wingate V. P., Bailey J. A., Lamb C. J. Differential accumulation of plant defense gene transcripts in a compatible and an incompatible plant-pathogen interaction. Mol Cell Biol. 1986 May;6(5):1615–1623. doi: 10.1128/mcb.6.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bowles D. J. Defense-related proteins in higher plants. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- Burley S. K., David P. R., Lipscomb W. N. Leucine aminopeptidase: bestatin inhibition and a model for enzyme-catalyzed peptide hydrolysis. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6916–6920. doi: 10.1073/pnas.88.16.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers H. T., van Loon-Klaassen L. A., Egberts W. T., de Jong W. W., Bloemendal H. The primary structure of leucine aminopeptidase from bovine eye lens. J Biol Chem. 1982 Jun 25;257(12):7077–7085. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawale S., Souciet G., Kuhn D. N. Increase of chalcone synthase mRNA in pathogen-inoculated soybeans with race-specific resistance is different in leaves and roots. Plant Physiol. 1989 Nov;91(3):911–916. doi: 10.1104/pp.91.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Harrison M. J. Activation, structure, and organization of genes involved in microbial defense in plants. Adv Genet. 1990;28:165–234. doi: 10.1016/s0065-2660(08)60527-1. [DOI] [PubMed] [Google Scholar]
- Enyedi A. J., Yalpani N., Silverman P., Raskin I. Signal molecules in systemic plant resistance to pathogens and pests. Cell. 1992 Sep 18;70(6):879–886. doi: 10.1016/0092-8674(92)90239-9. [DOI] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Octadecanoid Precursors of Jasmonic Acid Activate the Synthesis of Wound-Inducible Proteinase Inhibitors. Plant Cell. 1992 Feb;4(2):129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Graham J. S., Pearce G., Merryweather J., Titani K., Ericsson L. H., Ryan C. A. Wound-induced proteinase inhibitors from tomato leaves. II. The cDNA-deduced primary structure of pre-inhibitor II. J Biol Chem. 1985 Jun 10;260(11):6561–6564. [PubMed] [Google Scholar]
- Graham J. S., Pearce G., Merryweather J., Titani K., Ericsson L., Ryan C. A. Wound-induced proteinase inhibitors from tomato leaves. I. The cDNA-deduced primary structure of pre-inhibitor I and its post-translational processing. J Biol Chem. 1985 Jun 10;260(11):6555–6560. [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Hildmann T., Ebneth M., Peña-Cortés H., Sánchez-Serrano J. J., Willmitzer L., Prat S. General roles of abscisic and jasmonic acids in gene activation as a result of mechanical wounding. Plant Cell. 1992 Sep;4(9):1157–1170. doi: 10.1105/tpc.4.9.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten M. H., De Wit P. J. Identification of Several Pathogenesis-Related Proteins in Tomato Leaves Inoculated with Cladosporium fulvum (syn. Fulvia fulva) as 1,3-beta-Glucanases and Chitinases. Plant Physiol. 1989 Mar;89(3):945–951. doi: 10.1104/pp.89.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. J., Lawton M. A., Dron M., Dixon R. A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989 Jan 27;56(2):215–224. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Lawton K., Ward E., Payne G., Moyer M., Ryals J. Acidic and basic class III chitinase mRNA accumulation in response to TMV infection of tobacco. Plant Mol Biol. 1992 Aug;19(5):735–743. doi: 10.1007/BF00027070. [DOI] [PubMed] [Google Scholar]
- Lincoln J. E., Cordes S., Read E., Fischer R. L. Regulation of gene expression by ethylene during Lycopersicon esculentum (tomato) fruit development. Proc Natl Acad Sci U S A. 1987 May;84(9):2793–2797. doi: 10.1073/pnas.84.9.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvy J. F., Blumberg S. Inactivation and metabolism of neuropeptides. Annu Rev Neurosci. 1986;9:415–434. doi: 10.1146/annurev.ne.09.030186.002215. [DOI] [PubMed] [Google Scholar]
- Pautot V., Holzer F. M., Walling L. L. Differential expression of tomato proteinase inhibitor I and II genes during bacterial pathogen invasion and wounding. Mol Plant Microbe Interact. 1991 May-Jun;4(3):284–292. doi: 10.1094/mpmi-4-284. [DOI] [PubMed] [Google Scholar]
- Pearce G., Strydom D., Johnson S., Ryan C. A. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 1991 Aug 23;253(5022):895–897. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz V., Fluhr R. Ethylene Signal Is Transduced via Protein Phosphorylation Events in Plants. Plant Cell. 1993 May;5(5):523–530. doi: 10.1105/tpc.5.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. Proteinase inhibitor gene families: strategies for transformation to improve plant defenses against herbivores. Bioessays. 1989 Jan;10(1):20–24. doi: 10.1002/bies.950100106. [DOI] [PubMed] [Google Scholar]
- Stirling C. J., Colloms S. D., Collins J. F., Szatmari G., Sherratt D. J. xerB, an Escherichia coli gene required for plasmid ColE1 site-specific recombination, is identical to pepA, encoding aminopeptidase A, a protein with substantial similarity to bovine lens leucine aminopeptidase. EMBO J. 1989 May;8(5):1623–1627. doi: 10.1002/j.1460-2075.1989.tb03547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita M., Manzara T., Pichersky E., Cashmore A., Gruissem W. Genomic organization, sequence analysis and expression of all five genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase from tomato. Mol Gen Genet. 1987 Sep;209(2):247–256. doi: 10.1007/BF00329650. [DOI] [PubMed] [Google Scholar]
- Taylor A. Aminopeptidases: structure and function. FASEB J. 1993 Feb 1;7(2):290–298. doi: 10.1096/fasebj.7.2.8440407. [DOI] [PubMed] [Google Scholar]
- Thayer S. S., Choe H. T., Rausser S., Huffaker R. C. Characterization and subcellular localization of aminopeptidases in senescing barley leaves. Plant Physiol. 1988;87:894–897. doi: 10.1104/pp.87.4.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ness J., Hahn W. E. Physical parameters affecting the rate and completion of RNA driven hybridization of DNA: new measurements relevant to quantitation based on kinetics. Nucleic Acids Res. 1982 Dec 20;10(24):8061–8077. doi: 10.1093/nar/10.24.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Walling L. L., Chang Y. C., Demmin D. S., Holzer F. M. Isolation, characterization and evolutionary relatedness of three members from the soybean multigene family encoding chlorophyll a/b binding proteins. Nucleic Acids Res. 1988 Nov 25;16(22):10477–10492. doi: 10.1093/nar/16.22.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]