Abstract
Magnetic core shell nanoparticles are composed of a highly magnetic core material surrounded by a thin shell of desired drug, polymer or metal oxide. These magnetic core shell nanoparticles have a wide range of applications in biomedical research, more specifically in tissue imaging, drug delivery and therapeutics. The present review discusses the up-to-date knowledge on the various procedures for synthesis of magnetic core shell nanoparticles along with their applications in cancer imaging, drug delivery and hyperthermia or cancer therapeutics. Literature in this area shows that magnetic core shell nanoparticle-based imaging, drug targeting and therapy through hyperthermia can potentially be a powerful tool for the advanced diagnosis and treatment of various cancers.
Keywords: Magnetic core shell nanoparticles, Magnetic resonance imaging, Cancer therapeutics, Drug delivery, Hyperthermia
Core tip: Magnetic core shell nanoparticles have recently gained a lot of interest due to its excellent design and applicability to various fields of research including biomedical sciences. The core shell particle contains a highly magnetic core surrounded by a thin shell of desired material, the choice being dependent on the application. The applicability of core shell nanoparticles in the area of in vivo imaging, drug delivery and therapeutics in the form of hyperthermia have been discussed along with the various procedures for synthesis of these useful nanoprobes.
INTRODUCTION
Nanoparticles are emerging as a prospective biomedical tool with applications in diagnostics, specific drug delivery and therapeutics of diseases. Among these, magnetic nanomaterials with a core shell structure has been of interest for a long time primarily owing to their vast applications for example in magnetic data recording devices, in sensors, in catalytic reactions and in various biomedical applications encompassing tissue imaging for diagnosis, drug delivery and therapeutic management of diseases[1-4]. Attachment of biomolecular markers to the surface of magnetic nanoparticles has been used to demonstrate that magnetic nanoparticles have the potential to deliver noxious drugs at a specific location in the body accurately[5], which in effect can lead to design of highly specialized bio-probes for diagnostic imaging[6,7]. These advances have encouraged the development of biocompatible magnetic nanomaterials endowed with ultra-sensitive imaging potential in order to find wide applications in targeted as well as non-invasive in vivo medical imaging.
One such example could be Au coated magnetic nanoparticles, a complex system that has drawn a lot of attention[8]. Au coating is responsible for more effective stabilization of the magnetic nanoparticles, particularly metallic nanoparticles with high magnetic moment under toxic biological environment. The magnetic nanoparticles can readily be functionalized by Au because of its well-developed Au-S chemistry; the gold coating provides plasmonic properties to the magnetic nanoparticles. This renders the composite core/shell nanoparticles exceedingly attractive for multidisciplinary applications comprising of magnetic, optical, and biomedical applications. Another example could be arsenicals which have been known for thousands of years to be carcinogenic or poisonous despite their therapeutic or beneficial effects which are observed at a low dose. The United States Food and Drug Administration has granted approval of arsenic trioxide (ATO) as an effective leading edge treatment for relapsed and/or refractory APL patients. Further, several reports emphasize the success of arsenic in a diverse hematological malignancies for example promonocytic leukemia, chronic myelogenous leukemia, multiple myeloma, T-cell leukemia, and a wide array of cancers derived from solid tumors such as renal cell carcinoma, neuroblastoma, glioblastoma, gastric, hepatocellular, head and neck, cervical, prostate and breast cancers[9-15]. ATO either alone or in combination with other drugs has been used for each disease. The soluble most toxic and naturally prevalent form of arsenic (NaAsO2) has been shown to induce apoptosis in human malignant melanoma cells (A375) in vitro. Enhanced generation of reactive oxygen species, mitochondrial membrane potential damage and caspase activation are the critical mediators of apoptosis[16]. Extensive clinical application has been limited by: (1) diverse sensitivity of tumor cells to a higher dose of arsenic; and (2) less sensitive cell sensitization needing up to 10 times higher concentration of arsenic entailing the risk of arsenic-induced side effects[17-19] (illustrated in Figure 1). Cancers, especially solid tumours, at their advanced stage are therefore difficult to manage by arsenic administration in its present form. Nanotechnology, especially the core shell magnetic nanoparticles, has been shown to have advantages over others in terms of using effective lower dose of arsenite with minimal side effect and higher efficacy[20]. In this context, the present review highlights the synthesis and applications of diverse magnetic core shell nanoparticles in diagnostics especially imaging and in therapeutics.
Figure 1.
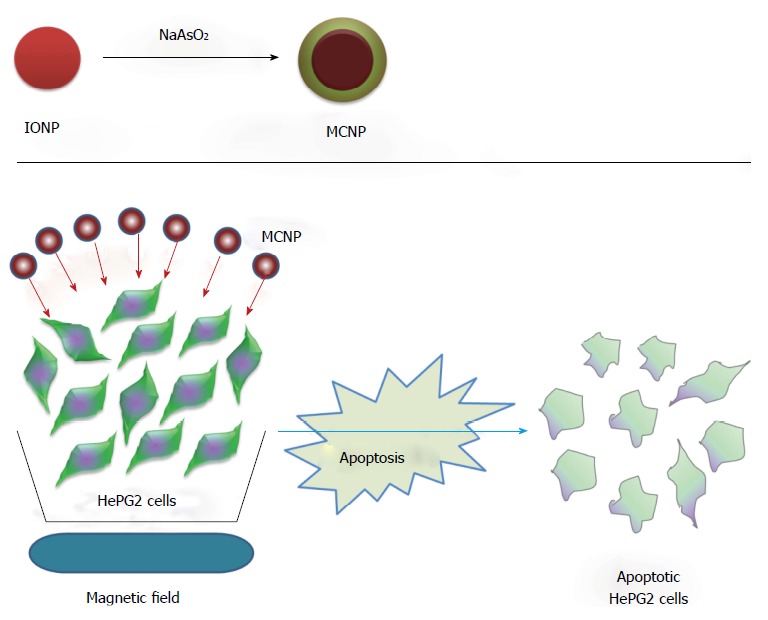
Schematic showing synthesis of magnetic core shell nanoparticle and their application towards targeting the cancer cells. Upper panel shows generation of MCNPs and lower panel represents magnetically facilitated arsenite delivery using MCNPs to kill cancerous cells through apoptosis. MCNP: Magnetic core shell nanoparticle; IONP: Iron oxide nanoparticle.
TYPES OF MAGNETIC CORE-SHELL NANOPARTICLES
The core shell nanoparticle is a type of nanoparticle consisting of a core or inner matter and a shell or outer coating material. Various types of core shell nanoparticles with different combinations in close interaction have been prepared with distinctive use. The combinations can be inorganic/inorganic, inorganic/organic and organic/inorganic materials. Generally magnetic core-shell nanoparticles can be classified into two following types.
Magnetic oxide core shell
The maghemite or magnetite magnetic nanoparticle has a relatively inert surface composition which generally does not permit strong covalent bond formation with functional molecules. The use of shell of silica onto the surface of magnetic nanoparticles has been shown to enhance the reactivity of magnetic nanoparticles[21]. The silica shell can be readily customized through the formation of covalent bonds with a variety of surface functional groups[22]. These silica-functionalized magnetic nanoparticles can be used to conjugate with some fluorescent dye molecules through the formation of covalent bond[23].
Ferrite nanoparticle clusters, comprising of about 80 maghemite super paramagnetic oxide nanoparticles per bead with silica shell, have a number of benefits over metallic nanoparticles. These can be summed up as: (1) greater chemical and thermodynamic stability; (2) precise size distribution; (3) greater colloidal stability; (4) adjustability of the magnetic moment with the nanoparticle cluster size; (5) retention of super paramagnetic properties irrespective of the cluster size of nanoparticles; and (6) straightforward covalent functionalization by the silica surface.
Metallic magnet core shell
The core material of magnetic naoparticles may be deactivated by mild oxidation, use of surfactants and/or polymers[24]. In an oxidative surroundings, anti-ferromagnetic CoO layer was formed onto the surface of the Co nanoparticle. Lately, the exchange bias synthetic protocol has been used to generate Co core CoO shell nanoparticles having gold outer shell[25]. Also, much interest has been generated in nanoparticles with a magnetic core consisting either of elementary iron or cobalt with a nonreactive shell made of grapheme, which have been synthesized recently[26]. The advantages compared to ferrite or elemental nanoparticles are: (1) higher magnetization; and (2) higher stability in acidic and basic solutions as well as organic solvents.
Synthesis of magnetic core shell nanoparticles
Magnetic core shell nanoparticle synthesis involves mainly two steps: First is the synthesis of magnetic nanomaterials and after that coating of the magnetic nanomaterials with desired organic or inorganic materials according to the requirement. The synthesis can be affected by a variety of combinations including inorganic/organic, organic/inorganic and inorganic/inorganic materials in close interaction. The selection of shell materials in core-shell nanoparticles is largely decided by the end application and utility. Generally silica, different types of metal and nonmetallic oxides, polymer and drug molecules are employed as coating materials. Thus here the main important task is the synthesis of magnetic nanomaterials (core). These core nanomaterials can be synthesized by the following four methodologies.
CO-PRECIPITATION TECHNIQUE
Iron oxide (Fe3O4 or γ-Fe2O3) nanoparticles could be prepared by the slow addition of a base into a mixture of aqueous Fe2+/Fe3+ salt solutions under an inert atmosphere at elevated temperature or room temperature depending upon requirement. This method is termed as co-precipitation technique. The composition and morphology of the magnetic nanoparticles prepared by co-precipitation method is highly dependent on the precursor salt and reaction conditions such as temperature, pH and ionic strength of reaction system. The quality of the magnetite nanoparticles is fully reproducible if the synthetic conditions are fixed. The experiment showed that the magnetic saturation values of magnetite nanoparticles are lower than bulk value (90 emu/g). Under ambient conditions, magnetite nanoparticles are not very stable. These nanoparticles are readily oxidized to a more stable maghemite form or dissolved in an acidic pH. Being a ferrimagnet, oxidation process is less problematic. The conversation of magnetite to maghemite is accomplished by forming acidic dispersion, followed by iron (III) nitrate addition. Now this maghemite particle shows chemical stability through a wide range of pH.
Although the magnetite particles are transformed into maghemite nanoparticles at the initial stage, the experimental challenge in the formation of Fe3O4 involving co-precipitation technique lies in controlling narrow particle size distribution. As particle size and morphology largely depend on blocking temperature, a broad range of particle size distribution would be due to the fluctuation of blocking temperature, which results in irregular magnetic activity. Nanoparticles obtained by co-precipitation technique are polydisperse in nature.
Recently, considerable approaches in synthesizing monodisperse magnetite nanoparticles having diverse sizes and morphology have been finished by the exploitation of various organic stabilizers and reducing agents. For instance, the stabilization of magnetite nanoparticles having dimensions of 3-11 nm was done by applying an aqueous solution of 1 wt% polyvinlyalcohol (PVA).
On the other hand chainlike clusters precipitate of magnetite nanoparticles could be formed by the application of PVA having 0.1 mol% carboxyl groups as stabilizing agent[27]. The above fact shows that the appropriate surfactant choice is a key matter to stabilize the particle. Formation of magnetite by the use of trisodium citrate in an alkaline pH, followed by successive oxidation at 90 °C for 30 min with iron (III) nitrate produces size-tunable maghemite nanoparticles. The adjustment of the molar ratio of metal ions (Fe2+/Fe3+) and citrate ions is the main key to vary the particle size from 3 to 8 nm[28]. The special effect of carboxylate and hydroxy carboxylate ions on the preparation of iron oxides or oxyhydroxides has been investigated elaborately[29]. Deprotonated carboxy and deprotonated α-hydroxy groups are essentials for the formation of surface complexes[30]. The advanced investigations have revealed that the best stabilization of magnetic Fe3O4 nanoparticles was done by oleic acid[31,32].
Thermal decomposition
Synthesis of magnetic particles with desired shape and size generated from the idea of quality semiconductor nanocrystals and oxide-nanoparticles synthesis involving thermal decomposition technique using non-aqueous media[33-35]. Organometallic compounds on thermal decomposition in high-boiling organic solvents with stabilizing surfactants produce monodisperse magnetic nanocrystals with smaller size[36,37]. In this process, the frequently used surfactants are fatty acids, oleic acid[38], and hexadecylamine[39]. Metal acetylacetonates [M(acac)n] (M = Fe, Mn, Co, Ni, Cr; n = 2 or 3, acac = acetylacetonate), metal cupferronates (MxCupx) [M = metal ion; Cup = N-nitrosophenylhydroxylamine, C6H5N(NO)O-] or carbonyls[40] can be used as organometallic precursors. The size and shape of magnetic nanocomposite can be regulated by varying the ratio of the starting reagents including organometallic compounds, surfactant, and solvent. The reaction temperature, time and aging period are also vital for the precise control of size and shape of magnetic nanoparticles. In case of the metal in zero valent state, for example in carbonyls, thermal decomposition primarily tends to the creation of the metal, however a two-step procedure is often used to make oxide nanoparticles. For example, at 100 °C, iron pentacarbonyl is capable of decomposing into a mixture of oleic acid and octyl ether, followed by the addition of a mild oxidant like trimethylamine oxide (CH3)3NO at a higher temperature, produces monodisperse γ-Fe2O3 nanocrystals of approximately 12 nm in size[41]. A precursor with cationic metal centers on decomposition produces oxide nano. For example, Fe3O4 is formed on decomposition of Fe(acac)3 in the presence of oleoylamine, 1,2-hexadecanediol and oleic acid in phenol ether[37,42]. The pyrolysis of metal fatty acid salts (such as salts of decanoic acid, lauric acid, myristic acid, palmitic acid, oleic acid, stearic acid) in a non-aqueous solution (octadecene, n-eicosane, tetracosane, or a mixture of octadecene and tetracosane) generated size and shape controlled magnetic oxide nanocrystal[43]. This method provides nearly monodisperse Fe3O4 nanocrystals having a wide range of size adjustable capacity (4-50 nm) with controlled shapes, including dots and cubes. This method has successfully employed for the synthesis of Cr2O3, MnO, Co3O4, and NiO magnetic nanocrystals. Variation of the reactivity and concentration of the precursors is the key factor to control the size and shape of the nanocrystals. Variation of concentration and chain length of the fatty acids determine the reactivity of the materials. Generally faster reaction rate associates with the shorter chain length. Alcohols or primary amines are often employed to speed up the reaction rate and decrease the reaction temperature.
Hyeon et al[41] employed a similar thermal decomposition procedure for the synthesis of monodisperse iron oxide nanoparticles[27]. They have generated an iron oleate complex in situ by using iron (III) chloride and sodium oleate which were then decomposed between 240 °C and 320 °C in different solvent systems like 1-hexadecene, 1-octadecene, 1-eicosene, octyl ether or trioctylamine. In this process particle size is determined by the temperature of decomposition and period of aging. Here aging was an important and necessary step for the generation of iron oxide nanoparticles. The nanoparticles prepared by this root can be dispersed in a variety of organic solvents alongside hexane and toluene. Iron pentacarbonyl and the iron oleate complex on decomposition at different temperatures produce monodisperse iron nanoparticles (6-15 nm) which can again be oxidized to magnetite[44]. This process is comparable with seed-mediated growth and explained by the classical LaMer mechanism. Hyeon synthesis involves thermal decomposition of iron pentacarbonyl at a moderately low temperature and the decomposition of the iron oleate complex at a higher temperature leading to the formation of iron oxide nanoparticles which disperse easily in organic solvents.
In biotechnology application, magnetic nanoparticles which are water soluble are more advantageous. This requirement led to the preparation of water soluble Fe3O4 nanocrystals with FeCl3·6H2O as an iron source and 2-pyrrolidone as a coordinating solvent under refluxing condition (245 °C)[45]. In this method, the mean particle size is controlled at 4, 12, and 60 nm, respectively, when the reflux times are 1, 10, and 24 h. With increasing reflux time, change of shapes of the particles from spherical to cubic morphologies was observed. Recently, water-soluble magnetite nanoparticles have been synthesized using one-pot synthesis under analogous reaction states with the addition of a surface capping agent like α,ω-dicarboxyl-terminated poly (ethylene glycol)[46]. These nanoparticles are exploited as magnetic resonance imaging (MRI) contrast agents for the diagnosis of cancer.
Metallic nanoparticles can also be prepared by thermal-decomposition method. The metallic nanoparticles have a large number of advantages over other metal oxide nanoparticles owing to their larger magnetization. Thermal breakdown of [Fe(CO)5] in the presence of polyisobutene in decalin in a nitrogen atmosphere at 170 °C produces metallic iron nanoparticles[47]. Depending on the Fe(CO)5/polyisobutene ratio, the size of the particle can be adjusted from 3 to 10 nm, with a polydispersity of approximately 10%. Susceptibility measurements revealed that the iron nanoparticles prepared by this way can be easily oxidized by exposure to air. This oxidation can generate a marginal increase of particle sizes approximately by a factor of 1.3. Iron nanocubes can be synthesized by the breakdown of Fe[N(Si(CH3)3)2]2 with H2 in the presence of hexadecylammonium chloride or hexadecylamine and oleic acid at 150 °C[48]. The edge-length of the nanocubes varied from 7 nm to 8.3 nm along with the varying relative concentrations of amine and acid ligand. These nanocubes can accumulate into expanded crystalline superlattices by way of their crystallographic axes aligned.
Cobalt nanoparticles can also be prepared by the thermal-decomposition method. Their shape and morphology both can be controlled by this method[49]. Cobalt nanodisks can also be prepared by thermal-decomposition of a cobalt carbonyl precursor[50,51]. The high-temperature reduction of noncarbonyl organometallic complexes produces cobalt nanorods[52,53] and nickel nanorods[54]. For example, decomposition of [Co(H3-C8H13)(h4-C8H12)] with H2 in anisole at 150 °C in the presence of a combination of hexadecylamine and a fatty acid (lauric, octanoic, or stearic acid) produces monodisperse ferromagnetic cobalt nanorods. The variation of diameter and length of the cobalt nanorods largly depends upon different acids used[53].
For easy handling and application under oxidizing conditions, air-stable magnetic nanoparticles are very important. The thermolysis of Co2(CO)8 in the presence of alkyl-aluminum compounds produces monodisperse colloidal cobalt nanoparticles[54]. The Co particles can be regulated in the size-range of 3-11 nm, by changing the alkyl chain length of these organo aluminum complexes. Air-stable particles can be synthesized by mild surface oxidation of the cobalt nanoparticles with synthetic air. The oxidation step is necessary as saturation magnetization of the CoO particles decays rapidly while exposed to air subsequent to the peptization with the surfactant KorantinSH.
Magnetic alloy nanoparticles have many benefits over other magnetic nanoparticles owing to their high magnetic anisotropy, enhanced magnetic susceptibility and large coercivities[55]. Currently metal phosphides have generated a lot of scientific interest in nanotechnology and chemistry beside CoPt3 and FePt[56-59]. For ferromagnetism, magnetoresistance, and magnetocaloric effects, hexagonal iron phosphide and allied materials have been rigorously studied[60,61]. Recently FeP and MnP nanoparticles have been synthesized from the reaction of iron(III) acetylacetonate and manganese carbonyl, respectively, with tris(trimethylsilyl)phosphane at elevated temperatures[62,63]. Antiferromagnetic FeP nanorods were prepared by the thermal decomposition of a precursor/surfactant mixture solution[64]. Thermal decomposition of permanently supplied iron pentacarbonyl in trioctylphosphane using a syringe pump produces discrete iron phosphide (Fe2P) nanorods.
Microemulsion techniques
Thermodynamically stable isotropic liquid mixture, where the micro-domain of either or both liquids is stabilized by an interfacial surfactant film, is called microemulsion[64]. In case of water-in-oil microemulsions, the aqueous phase is dispersed as micro size droplets bounded by a monolayer of surfactant molecules in the continuous hydrocarbon phase. The molar ratio of water to surfactant determined the size of the reverse micelle[65]. Mixing of two identical water-in-oil microemulsions including the preferred reactants results in continuous collision, and break again, finally forming a precipitate in the micelles[66]. Addition of solvent like acetone or ethanol to the microemulsions produces a precipitate, which can be collected by filtration or centrifugation. In this concept, a microemulsion served as a nanoreactor for the generation of nanoparticles.
Metallic Co, Co/Pt alloy, and gold-coated Co/Pt nanostructures have been prepared using this microemulsion technique in reverse micelles of cetyltrimethlyammonium bromide, with 1-butanol as co-surfactant in octane oil phase[66]. Using microemulsion technique, spinel ferrites can be synthesized. Water-in-toluene inverse micelles and sodium dodecylbenzenesulfonate (NaDBS) as surfactant were employed to prepare MnFe2O4 nanoparticles of sizes 4-15 nm[67]. A clear aqueous solution of Mn(NO3)2 and Fe(NO3)3 has been employed for this synthesis. An NaDBS aqueous solution is administered to the metal salt solution, followed by addition of a huge volume of toluene when reverse micelles are formed. The size of the resulting MnFe2O4 nanoparticles was determined by the volume ratio of water and toluene. A sol-gel reaction was employed to prepare iron oxide nanorods through reverse micelle formation from oleic acid and benzyl ether, using FeCl3·6H2O as iron precursor and propylene oxide as a proton scavenger[68]. The shape of nanorods was controlled by the fluctuation of reaction temperature, environmental conditions, and hydration state of the gels during reflux or heating in tetralin.
The reaction of methylamine and in situ formation of cobalt and iron dodecyl sulfate (formed by combination of aqueous sodium dodecyl sulfate with iron chloride or cobalt acetate solution) produces cobalt ferrite fluid[69]. An increase of sodium dodecyl sulfate concentration and a decrease of total reactant concentration decrease the size of the cobalt ferrite particles.
Although the microemulsion procedure has been used to prepare magnetic nanoparticles in a controlled fashion, the size and shape of the products vary over a wide range. Furthermore, compared to other methods the yield of nanoparticles is low in microemulsion technique. For the synthesis of material, large amounts of solvent are necessary. Moreover, the efficiency of the process is low and it is rather difficult to scale up.
Hydrothermal synthesis
A wide range of nanostructures can be synthesized by applying hydrothermal conditions. A liquid-solid-solution reaction has been employed for the synthesis of a diverse array of nanocrystals. The system employed for the synthesis consists of a solid- liquid-solution matrix containing metal linoleate (solid), an ethanol linoleic acid (liquid) and a water-ethanol solution at different reaction temperatures under hydrothermal conditions[70]. This approach is based on phase transfer and separation mechanism occurring at the liquid-solid-solution interfaces present during the synthesis. Using hydrothermal reduction, monodisperse, hydrophilic, single crystalline ferrite microspheres were synthesized. According to this process, a mixture of FeCl3, sodium acetate, ethylene glycol, and PEG was stirred vigorously till a clear solution is formed, followed by sealing in a Teflon-coated stainless-steel autoclave, and heated to 200 °C for 8-72 h.
The above four synthetic methods have some advantages and disadvantages. Among the four roots co-precipitation is the preferred route in terms of simplicity of the synthesis. Thermal decomposition method can be considered the best in terms of control of size and morphology of the nanoparticles. As a substitute, microemulsions can also be employed to synthesize monodispersed nanoparticles having various morphologies. However, a large amount of solvent is needed in this method. Hydrothermal synthesis, although generates superior quality nanoparticles, is a comparatively little investigated method for the synthesis of magnetic nanoparticles. To date, magnetic nanoparticles are synthesized on a large scale by use of co-precipitation and thermal decomposition procedures.
MRI AND CANCER THERAPY
MRI, magnetic resonance tomography or nuclear MRI (NMRI) is an important non-invasive imaging technique to visualize internal structures of the body in detail by using the magnetic property of the various interacting magnetic nuclei present inside the body. When a person is kept under a powerful static magnetic field, the average magnetic moment of the magnetic nuclei present inside the body becomes aligned with the direction of that magnetic field. These magnetic nuclei upon excitation with another external electromagnetic field having the correct frequency (known as the resonance frequency) can flip the spin to the reverse direction by absorbing this radiation. This resonance frequency is generated by turning on the radio frequency current for a very short period. As soon as the electromagnetic field is turned off, the nuclei return to the original thermodynamic equilibrium position and the bulk magnetization re-aligns along the static magnetic field. During this relaxation phase, an electromagnetic radiation in the radio frequency range is emitted which is measured with receiver coils.
MRI signal strengths are influenced by the factors T1 (spin-lattice/longitudinal relaxation time), T2 (transverse relaxation time) and r (spin energy). To enhance the tissue contrast, several exogenous contrast agents like complexes of gadolinium(III) and magnetic nanoparticles are injected intravenously. However, Gd(III) complex system has some serious drawbacks regarding Gd(III) ion exchange using endogenous metals like Zn, Cu and uptake of complexes in extravascular space. This problem can be overcome by employing monodisperse, cross-linked iron oxide (CLIO) nanoparticles as an MRI contrasting agent[71,72]. Owing to highly stable, non-toxic and high cellular uptake, CLIO has been widely used as an exogenous contrast agent[71,73,74].
Advancement of MRI contrast agents which can be frequently applied for biomedical imaging is a demanding assignment. This is primarily due to the prospect of a suitable MRI agent with: (1) ability to be synthesized in huge amount; (2) long shelf life; (3) proper biocompatibility; (4) hindrance to its aggregation in biological fluids; and (5) large relaxivity, which will bring about better contrast in biological imaging. FePt@Fe2O3 core-shell magnetic nanoparticles, for example, act as a T2 MRI contrast agent and at the same time as a carrier of drug and can therefore be employed in cancer management applications[75]. For the latter application FePt@Fe2O3 core-shell nanoparticles are at first synthesized followed by functionalization using polyethylene glycol (PEG). Further, efficient targeting of folate receptor positive tumor cells is mediated by using folic acid conjugated FePt@Fe2O3-PEG nanoparticles. The chemotherapy drug, doxorubicin (DOX), is finally attached to these nanoparticles through hydrophobic adsorption, to deliver the drug in a selective manner for killing of cancer cells. Use of these FePt@Fe2O3-PEG nanoparticles has been done for in vivo MRI, to generate tumor MR contrasts, which can be accumulated in a passive tumor or can be utilized for active tumor targeting. Moreover, FePt@Fe2O3-PEG did not reveal any noticeable toxicity in both in vitro and in vivo experiments. Therefore, PEGylated FePt@Fe2O3 core-shell nanoparticles can be exploited as a potential multimodal integrated therapeutic and diagnostic nanoplatform.
Recently DOX and magnetic nanoparticles were incorporated into antibody-conjugated poly-(D,L-lactide-co-glycolide) (PLGA) nanoparticles where DOX serves as an anticarcinogenic drug and Fe2O3 nanoparticles utilized as an imaging agent (Figure 2). This nanocomposite has been employed for the concurrent targeted iagnosis and management of breast cancer[76].
Figure 2.
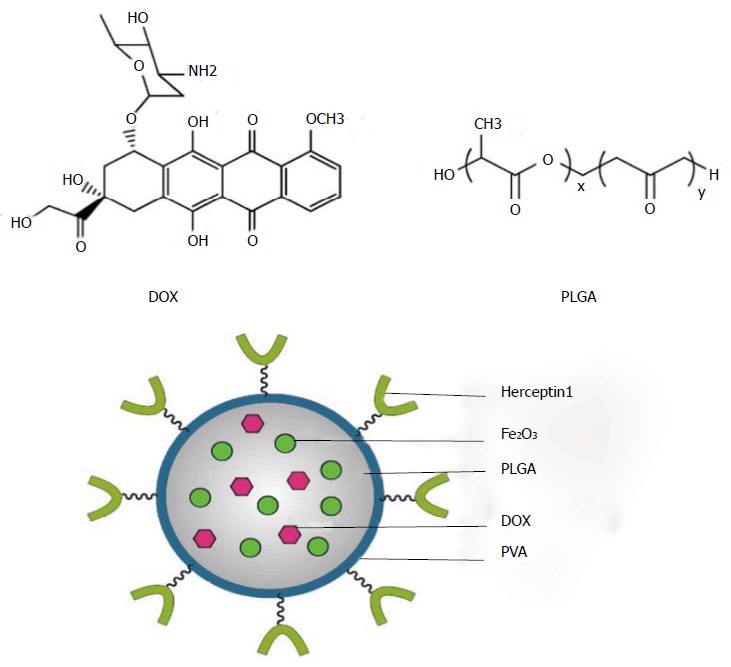
Use of magnetic nanoparticles embedded into poly-(D,L-lactide-co-glycolide) nanoparticles for diagnosis and treatment of cancer. DOX: Doxorubicin; PLGA: Poly-(D,L-lactide-co-glycolide); PVA: Polyvinlyalcohol.
Recently, multimodal nano-materials have been exploited for immediate diagnosis and therapy. In this situation, simultaneous bio-imaging and drug delivery could be done by forming homogeneous core-shell composite particles through the integration of mesoporous silica with superparamagnetic monodisperse nanocomposite. For example, distinct, monodisperse, and perfectly size regulated core-shell mesoporous silica nanoparticles having a size < 100 nm with single Fe3O4 nanocrystals as cores (designated as Fe3O4@mSiO2) were employed for synchronized MR/fluorescence imaging as well as drug delivery[77]. Multimodal imaging agents like magneto-fluorescent nanoparticles have been developed by creating optical imaging and MRI property simultaneously in the nanoparticles[78-81]. For example, glycine functionalized CLIO-Cy5.5 (CLIOGly) had a great affinity for activated macrophages, while the 3,3’,4,4’-benzophenontetracarboxylic dianhydride attached CLIO-Cy5.5 (CLIO-bentri) selectively interacted with latent macrophages.
APPLICATION OF MAGNETIC CORE-SHELL NANOPARTICLES IN DRUG DELIVERY
A frontline application of magnetic nanomaterials is as drug carriers wherein magnetic field induced drug delivery popularly known as “magnetic drug delivery” is the method of choice. The idea of magnetic drug delivery involves the injection of drug-loaded magnetic nanomaterials which are guided to the specific site by the influence of magnetic field gradient. These nanomaterials are held at the targeted site until the therapy is done, after which these are removed. By this process high local concentration of desired drug could be created, thus avoiding toxicity and other undesirable side effects on normal cells in other parts of the body. Although significant success has been achieved in in vivo experiments, so far, definite clinical studies are still lacking. Many basic concerns over magnetic drug delivery methods are yet to be deciphered, some of which are: (1) size regulated preparation and shelf-life of magnetic nanoparticles; (2) compatibility of the covering layers (polymer or silica); (3) binding of drug molecules; and (4) the physiological considerations[66,82].
APPLICATION OF MAGNETIC CORE-SHELL NANOPARTICLES IN HYPERTHERMIA
Magnetic nanoparticles can be used in the management of hyperthermia. This method is regarded as a complementary treatment to chemotherapy, radiotherapy and surgery in cancer[6,83]. Magnetic materials when subjected to exposure to an oscillating magnetic field, lead to heat production through magnetic hysteresis loss, Neel-relaxation and Brown-relaxation mechanisms. This principle is employed in magnetic induction hyperthermia[47]. Induced currents are generated in metallic objects during the application of alternating magnetic field, leading to the generation of heat. Due to the collective magnetic behaviour of metals, this phenomenon is greatly enhanced in case of metals. As a result, magnetic fluid containing magnetic nanoparticles, upon exposure to an alternating magnetic field, become a powerful heat sources. The heat, generated by this way, destroys cancer cells due to its higher sensitivity to temperatures in excess of 41 °C compared to the normal cells.
Frictional forces due to the rotation of the particles in a medium of low viscosity (Brown-relaxation) or loss processes during the reorientation of the magnetization (Neel-relaxation) are responsible for the heating of magnetic oxide substances having poor electrical conductivity. Magnetic anisotropy is used to determine the losses from the reorientation of magnetization by means of the measurements of intrinsic magnetic properties. Due to the thermal fluctuations, re-magnetization process may occur for single domain particles. As the particle dimension is less, the barrier energy would also be less. The external energy may influence magnetic moment in exceeding the energy barrier.
Another loss type may arise in the case of ferro fluids in addition to the losses caused by magnetization rotation inside the particles. This is associated with the rotational Brownian motion of the magnetic elements. For this process, rotational friction within the suspension fluid of a definite viscosity dictates the energy barrier. The structural properties of the nanoparticles are mainly responsible for the amount of heat generation. Therefore, water soluble metal oxide synthesis in water dispersion medium with regulated shape and size is the challenging task in the advanced research.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest related to the manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 23, 2015
First decision: September 17, 2015
Article in press: December 4, 2015
P- Reviewer: Freire-De-Lima CG, Yeligar SM S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Jiao XK
References
- 1.Seo WS, Lee JH, Sun X, Suzuki Y, Mann D, Liu Z, Terashima M, Yang PC, McConnell MV, Nishimura DG, et al. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat Mater. 2006;5:971–976. doi: 10.1038/nmat1775. [DOI] [PubMed] [Google Scholar]
- 2.Son SJ, Reichel J, He B, Schuchman M, Lee SB. Magnetic nanotubes for magnetic-field-assisted bioseparation, biointeraction, and drug delivery. J Am Chem Soc. 2005;127:7316–7317. doi: 10.1021/ja0517365. [DOI] [PubMed] [Google Scholar]
- 3.Huang HS, Hainfeld JF. Intravenous magnetic nanoparticle cancer hyperthermia. Int J Nanomedicine. 2013;8:2521–2532. doi: 10.2147/IJN.S43770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandal S, Hossain M, Devi PS, Kumar GS, Chaudhuri K. Interaction of carbon nanoparticles to serum albumin: elucidation of the extent of perturbation of serum albumin conformations and thermodynamical parameters. J Hazard Mater. 2013;248-249:238–245. doi: 10.1016/j.jhazmat.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Talelli M, Rijcken CJ, Lammers T, Seevinck PR, Storm G, van Nostrum CF, Hennink WE. Superparamagnetic iron oxide nanoparticles encapsulated in biodegradable thermosensitive polymeric micelles: toward a targeted nanomedicine suitable for image-guided drug delivery. Langmuir. 2009;25:2060–2067. doi: 10.1021/la8036499. [DOI] [PubMed] [Google Scholar]
- 6.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Chen TJ, Cheng TH, Chen CY, Hsu SC, Cheng TL, Liu GC, Wang YM. Targeted Herceptin-dextran iron oxide nanoparticles for noninvasive imaging of HER2/neu receptors using MRI. J Biol Inorg Chem. 2009;14:253–260. doi: 10.1007/s00775-008-0445-9. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Luo J, Fan Q, Suzuki M, Suzuki IS, Engelhard MH, Lin Y, Kim N, Wang JQ, Zhong CJ. Monodispersed core-shell Fe3O4@Au nanoparticles. J Phys Chem B. 2005;109:21593–21601. doi: 10.1021/jp0543429. [DOI] [PubMed] [Google Scholar]
- 9.Hu XM, Hirano T, Oka K. Arsenic trioxide induces apoptosis equally in T lymphoblastoid leukemia MOLT-4 cells and P-gp-expressing daunorubicin-resistant MOLT-4 cells. Cancer Chemother Pharmacol. 2003;51:119–126. doi: 10.1007/s00280-002-0543-2. [DOI] [PubMed] [Google Scholar]
- 10.Jiang JY, Li AL, Wang GM, Ma JB, Hao J, Guan ZQ, Xie SS. [The improving effect of bone marrow stromal cell transfected with IL-3 gene on hematopoietic reconstitution in bone marrow transplantation of mice] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2003;11:633–638. [PubMed] [Google Scholar]
- 11.Larochette N, Decaudin D, Jacotot E, Brenner C, Marzo I, Susin SA, Zamzami N, Xie Z, Reed J, Kroemer G. Arsenite induces apoptosis via a direct effect on the mitochondrial permeability transition pore. Exp Cell Res. 1999;249:413–421. doi: 10.1006/excr.1999.4519. [DOI] [PubMed] [Google Scholar]
- 12.Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, Kim BK, Lee YY. Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis. Cancer Res. 2000;60:3065–3071. [PubMed] [Google Scholar]
- 13.Li JJ, Tang Q, Li Y, Hu BR, Ming ZY, Fu Q, Qian JQ, Xiang JZ. Role of oxidative stress in the apoptosis of hepatocellular carcinoma induced by combination of arsenic trioxide and ascorbic acid. Acta Pharmacol Sin. 2006;27:1078–1084. doi: 10.1111/j.1745-7254.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 14.Perkins C, Kim CN, Fang G, Bhalla KN. Arsenic induces apoptosis of multidrug-resistant human myeloid leukemia cells that express Bcr-Abl or overexpress MDR, MRP, Bcl-2, or Bcl-x(L) Blood. 2000;95:1014–1022. [PubMed] [Google Scholar]
- 15.Shen ZY, Shen J, Cai WJ, Hong C, Zheng MH. The alteration of mitochondria is an early event of arsenic trioxide induced apoptosis in esophageal carcinoma cells. Int J Mol Med. 2000;5:155–158. doi: 10.3892/ijmm.5.2.155. [DOI] [PubMed] [Google Scholar]
- 16.Chowdhury R, Chowdhury S, Roychoudhury P, Mandal C, Chaudhuri K. Arsenic induced apoptosis in malignant melanoma cells is enhanced by menadione through ROS generation, p38 signaling and p53 activation. Apoptosis. 2009;14:108–123. doi: 10.1007/s10495-008-0284-8. [DOI] [PubMed] [Google Scholar]
- 17.Huang RQ, Gao SF, Wang WL, Staunton S, Wang G. Soil arsenic availability and the transfer of soil arsenic to crops in suburban areas in Fujian Province, southeast China. Sci Total Environ. 2006;368:531–541. doi: 10.1016/j.scitotenv.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Bode AM, Dong Z. The paradox of arsenic: molecular mechanisms of cell transformation and chemotherapeutic effects. Crit Rev Oncol Hematol. 2002;42:5–24. doi: 10.1016/s1040-8428(01)00215-3. [DOI] [PubMed] [Google Scholar]
- 19.Mandal S, Hossain M, Muruganandan T, Kumar GS, Chaudhuri K. Gold nanoparticles alter Taq DNA polymerase activity during polymerase chain reaction. RSC Adv. 2013;3:20793–20799. [Google Scholar]
- 20.Mandal S, Chatterjee N, Das S, Saha KD, Chaudhuri K. Magnetic core-shell nanoprobe for sensitive killing of cancer cells via induction with a strong external magnetic field. RSC Adv. 2014;4:20077–20085. [Google Scholar]
- 21.Kralj S, Makovec D, Œampelj S, Drofenik M. Producing ultra-thin silica coatings on iron-oxide nanoparticles to improve their surface reactivity. J Magn Magn Mater. 2010;322:1847–1853. [Google Scholar]
- 22.Kralj S, Drofenik M, Makovec D. Controlled surface functionalization of silica-coated magnetic nanoparticles with terminal amino and carboxyl groups. J Nanopart Res. 2011;13:2829–2841. [Google Scholar]
- 23.Kralj S, Rojnik M, Romih R, Jagodi M, Kos J, Makovec D. Effect of surface charge on the cellular uptake of fluorescent magnetic nanoparticles. J Nanopart Res. 2012;14:1–14. [Google Scholar]
- 24.Lu AH, Salabas EL, Schüth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed Engl. 2007;46:1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 25.Johnson SH, Johnson CL, May SJ, Hirsch S, Cole MW, Spanier JE. Co@CoO@Au core-multi-shell nanocrystals. J Mater Chem. 2010;20:439–443. [Google Scholar]
- 26.Grass RN, Stark WJ. Gas phase synthesis of fcc-cobalt nanoparticles. J Mater Chem. 2006;16:1825–1830. [Google Scholar]
- 27.Li Z, Kawashita M, Araki N, Mitsumori M, Hiraoka M, Doi M. Preparation of magnetic iron oxide nanoparticles for hyperthermia of cancer in a FeCl2-NaNO3-NaOH aqueous system. J Biomater Appl. 2011;25:643–661. doi: 10.1177/0885328209351136. [DOI] [PubMed] [Google Scholar]
- 28.Bee A, Massart R, Neveu S. Synthesis of very fine maghemite particles. J Magn Mater. 1995;149:6–9. [Google Scholar]
- 29.Ishikawa T, Kataoka S, Kandori K. The influence of carboxylate ions on the growth of β-FeOOH particles. J Mater Sci. 1993;28:2693–2698. [Google Scholar]
- 30.Kandori K, Kawashima Y, Ishikawa T. Effects of citrate ions on the formation of monodispersed cubic hematite particles. J Colloid Interface Sci. 1992;152:284–288. [Google Scholar]
- 31.Willis AL, Turro NJ, O’Brien S. Spectroscopic Characterization of the Surface of Iron Oxide Nanocrystals. Chem Mater. 2005;17:5970–5975. [Google Scholar]
- 32.Cushing BL, Kolesnichenko VL, O’Connor CJ. Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem Rev. 2004;104:3893–3946. doi: 10.1021/cr030027b. [DOI] [PubMed] [Google Scholar]
- 33.Murray CB, Norris DJ, Bawendi MG. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J Am Chem Soc. 1993;115:8706–8715. [Google Scholar]
- 34.Peng X, Wickham J, Alivisatos AP. Kinetics of II-VI and III-V Colloidal Semiconductor Nanocrystal Growth: Focusing of Size Distributions. J Am Chem Soc. 1998;120:5343–5344. [Google Scholar]
- 35.Qi JQ, Peng T, Hu YM, Sun L, Wang Y, Chen WP, Li LT, Nan CW, Chan HL. Direct synthesis of ultrafine tetragonal BaTiO3 nanoparticles at room temperature. Nanoscale Res Lett. 2011;6:466. doi: 10.1186/1556-276X-6-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park J, An K, Hwang Y, Park JG, Noh HJ, Kim JY, Park JH, Hwang NM, Hyeon T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat Mater. 2004;3:891–895. doi: 10.1038/nmat1251. [DOI] [PubMed] [Google Scholar]
- 37.Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li G. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J Am Chem Soc. 2004;126:273–279. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- 38.Samia AC, Hyzer K, Schlueter JA, Qin CJ, Jiang JS, Bader SD, Lin XM. Ligand effect on the growth and the digestion of Co nanocrystals. J Am Chem Soc. 2005;127:4126–4127. doi: 10.1021/ja044419r. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Afzaal M, O’Brien P. The synthesis of amine-capped magnetic (Fe, Mn, Co, Ni) oxide nanocrystals and their surface modification for aqueous dispersibility. J Mater Chem. 2006;16:2175–2180. [Google Scholar]
- 40.Farrell D, Majetich SA, Wilcoxon JP. Preparation and Characterization of Monodisperse Fe Nanoparticles. J Phys Chem B. 2003;107:11022–11030. [Google Scholar]
- 41.Hyeon T, Lee SS, Park J, Chung Y, Na HB. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J Am Chem Soc. 2001;123:12798–12801. doi: 10.1021/ja016812s. [DOI] [PubMed] [Google Scholar]
- 42.Sun S, Zeng H. Size-controlled synthesis of magnetite nanoparticles. J Am Chem Soc. 2002;124:8204–8205. doi: 10.1021/ja026501x. [DOI] [PubMed] [Google Scholar]
- 43.Jana NR, Chen Y, Peng X. Size- and Shape-Controlled Magnetic (Cr, Mn, Fe, Co, Ni) Oxide Nanocrystals via a Simple and General Approach. Chem Mater. 2004;16:3931–3935. [Google Scholar]
- 44.Park J, Lee E, Hwang NM, Kang M, Kim SC, Hwang Y, Park JG, Noh HJ, Kim JY, Park JH, et al. One-nanometer-scale size-controlled synthesis of monodisperse magnetic iron oxide nanoparticles. Angew Chem Int Ed Engl. 2005;44:2873–2877. doi: 10.1002/anie.200461665. [DOI] [PubMed] [Google Scholar]
- 45.Li Z, Sun Q, Gao M. Preparation of water-soluble magnetite nanocrystals from hydrated ferric salts in 2-pyrrolidone: mechanism leading to Fe3O4. Angew Chem Int Ed Engl. 2004;44:123–126. doi: 10.1002/anie.200460715. [DOI] [PubMed] [Google Scholar]
- 46.Hu FQ, Wei L, Zhou Z, Ran YL, Li Z, Gao MY. Preparation of Biocompatible Magnetite Nanocrystals for In Vivo Magnetic Resonance Detection of Cancer. Adv Mater. 2006;18:2553–2556. [Google Scholar]
- 47.Rosensweig RE. Heating magnetic fluid with alternating magnetic field. J Magn Magn Mater. 2002;252:370–374. [Google Scholar]
- 48.Dumestre F, Chaudret B, Amiens C, Renaud P, Fejes P. Superlattices of iron nanocubes synthesized from Fe[N(SiMe3)2]2. Science. 2004;303:821–823. doi: 10.1126/science.1092641. [DOI] [PubMed] [Google Scholar]
- 49.Song Q, Zhang ZJ. Shape control and associated magnetic properties of spinel cobalt ferrite nanocrystals. J Am Chem Soc. 2004;126:6164–6168. doi: 10.1021/ja049931r. [DOI] [PubMed] [Google Scholar]
- 50.Puntes VF, Krishnan KM, Alivisatos AP. Colloidal nanocrystal shape and size control: the case of cobalt. Science. 2001;291:2115–2117. doi: 10.1126/science.1057553. [DOI] [PubMed] [Google Scholar]
- 51.Puntes VF, Zanchet D, Erdonmez CK, Alivisatos AP. Synthesis of hcp-Co Nanodisks. J Am Chem Soc. 2002;124:12874–12880. doi: 10.1021/ja027262g. [DOI] [PubMed] [Google Scholar]
- 52.Dumestre F, Chaudret B, Amiens C, Fromen MC, Casanove MJ, Renaud P, Zurcher P. Shape control of thermodynamically stable cobalt nanorods through organometallic chemistry. Angew. Angew Chem Int Ed Engl. 2002;144:4462–4465. doi: 10.1002/1521-3773(20021115)41:22<4286::AID-ANIE4286>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 53.Dumestre F, Chaudret B, Amiens C, Respaud M, Fejes P, Renaud P, Zurcher P. Unprecedented crystalline super-lattices of monodisperse cobalt nanorods. Angew Chem Int Ed Engl. 2003;42:5213–5216. doi: 10.1002/anie.200352090. [DOI] [PubMed] [Google Scholar]
- 54.Cordente N, Respaud M, Senocq Fo, Casanove M-J, Amiens C, Chaudret B. Synthesis and magnetic properties of nickel nanorods. Nano Lett. 2001;1:565–568. [Google Scholar]
- 55.Yamada Y, Suzuki T, Abarra EN. Magnetic properties of electron beam evaporated CoPt alloy thin films. IEEE. 1998;34:343–345. [Google Scholar]
- 56.Sun S, Murray CB, Weller D, Folks L, Moser A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science. 2000;287:1989–1992. doi: 10.1126/science.287.5460.1989. [DOI] [PubMed] [Google Scholar]
- 57.Shevchenko EV, Talapin DV, Rogach AL, Kornowski A, Haase M, Weller H. Colloidal synthesis and self-assembly of CoPt(3) nanocrystals. J Am Chem Soc. 2002;124:11480–11485. doi: 10.1021/ja025976l. [DOI] [PubMed] [Google Scholar]
- 58.Lukehart CM, Milne SB, Stock SR. Formation of crystalline nanoclusters of Fe2P, RuP, Co2P, Rh2P, Ni2P, Pd5P2, or PtP2 in a silica xerogel matrix from single-source molecular precursors. Chem Mater. 1998;10:903–908. [Google Scholar]
- 59.Stinner C, Prins R, Weber T. Binary and ternary transition-metal phosphides as HDN catalysts. J Catal. 2001;202:187–194. [Google Scholar]
- 60.Luo F, Su H-L, Song W, Wang Z-M, Yan Z-G, Yan C-H. Magnetic and magnetotransport properties of Fe2P nanocrystallites via a solvothermal route. J Mater Chem. 2004;14:111–115. [Google Scholar]
- 61.Tegus O, Brück E, Buschow KH, de Boer FR. Transition-metal-based magnetic refrigerants for room-temperature applications. Nature. 2002;415:150–152. doi: 10.1038/415150a. [DOI] [PubMed] [Google Scholar]
- 62.Stamm KL, Garno JC, Liu GY, Brock SL. A general methodology for the synthesis of transition metal pnictide nanoparticles from pnictate precursors and its application to iron-phosphorus phases. J Am Chem Soc. 2003;125:4038–4039. doi: 10.1021/ja028180v. [DOI] [PubMed] [Google Scholar]
- 63.Perera SC, Tsoi G, Wenger LE, Brock SL. Synthesis of MnP nanocrystals by treatment of metal carbonyl complexes with phosphines: a new, versatile route to nanoscale transition metal phosphides. J Am Chem Soc. 2003;125:13960–13961. doi: 10.1021/ja038037h. [DOI] [PubMed] [Google Scholar]
- 64.Qian C, Kim F, Ma L, Tsui F, Yang P, Liu J. Solution-phase synthesis of single-crystalline iron phosphide nanorods/nanowires. J Am Chem Soc. 2004;126:1195–1198. doi: 10.1021/ja038401c. [DOI] [PubMed] [Google Scholar]
- 65.Paul BK, Moulik SP. Uses and applications of microemulsions. Current Sci Assoc/Indian academy Sci. 2001;80:990–100. [Google Scholar]
- 66.Grass RN, Athanassiou EK, Stark WJ. Covalently functionalized cobalt nanoparticles as a platform for magnetic separations in organic synthesis. Angew Chem Int Ed Engl. 2007;46:4909–4912. doi: 10.1002/anie.200700613. [DOI] [PubMed] [Google Scholar]
- 67.Liu C, Zou B, Rondinone AJ, Zhang ZJ. Reverse micelle synthesis and characterization of superparamagnetic MnFe2O4 spinel ferrite nanocrystallites. J Phys Chem B. 2000;104:1141–1145. [Google Scholar]
- 68.Woo K, Lee HJ, Ahn JP, Park YS. Sol-gel mediated synthesis of Fe2O3 nanorods. Adv Mater. 2003;15:1761–1764. [Google Scholar]
- 69.Moumen N, Pileni MP. Control of the size of cobalt ferrite magnetic fluid. J Phys Chem. 1996;100:1867–1873. [Google Scholar]
- 70.Wang X, Zhuang J, Peng Q, Li Y. A general strategy for nanocrystal synthesis. Nature. 2005;437:121–124. doi: 10.1038/nature03968. [DOI] [PubMed] [Google Scholar]
- 71.Wunderbaldinger P, Josephson L, Weissleder R. Crosslinked iron oxides (CLIO): a new platform for the development of targeted MR contrast agents. Acad Radiol. 2002;9 Suppl 2:S304–S306. doi: 10.1016/s1076-6332(03)80210-6. [DOI] [PubMed] [Google Scholar]
- 72.Wunderbaldinger P, Josephson L, Weissleder R. Tat peptide directs enhanced clearance and hepatic permeability of magnetic nanoparticles. Bioconjug Chem. 2002;13:264–268. doi: 10.1021/bc015563u. [DOI] [PubMed] [Google Scholar]
- 73.Sun EY, Josephson L, Weissleder R. “Clickable” nanoparticles for targeted imaging. Mol Imaging. 2006;5:122–128. [PubMed] [Google Scholar]
- 74.Sosnovik DE, Nahrendorf M, Weissleder R. Molecular magnetic resonance imaging in cardiovascular medicine. Circulation. 2007;115:2076–2086. doi: 10.1161/CIRCULATIONAHA.106.658930. [DOI] [PubMed] [Google Scholar]
- 75.Liu Y, Yang K, Cheng L, Zhu J, Ma X, Xu H, Li Y, Guo L, Gu H, Liu Z. PEGylated FePt@Fe2O3 core-shell magnetic nanoparticles: potential theranostic applications and in vivo toxicity studies. Nanomedicine. 2013;9:1077–1088. doi: 10.1016/j.nano.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 76.Yang J, Lee C-H, Park J, Seo S, Lim E-K, Song YJ, Suh J-S, Yoon H-G, Huh Y-M, Haam S. Antibody conjugated magnetic PLGA nanoparticles for diagnosis and treatment of breast cancer. J Mater Chem. 2007;17:2695–2699. [Google Scholar]
- 77.Kim J, Kim HS, Lee N, Kim T, Kim H, Yu T, Song IC, Moon WK, Hyeon T. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew Chem Int Ed Engl. 2008;47:8438–8441. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- 78.Santra S, Bagwe RP, Dutta D, Stanley JT, Walter GA, Tan W, Moudgil BM, Mericle RA. Synthesis and Characterization of Fluorescent, Radio-Opaque, and Paramagnetic Silica Nanoparticles for Multimodal Bioimaging Applications. Adv Mater. 2005;17:2165–2169. [Google Scholar]
- 79.Gerion D, Herberg J, Bok R, Gjersing E, Ramon E, Maxwell R, Kurhanewicz J, Budinger TF, Gray JW, Shuman MA, et al. Paramagnetic Silica-Coated Nanocrystals as an Advanced MRI Contrast Agent. J Physical Chem C. 2007;111:12542–12551. [Google Scholar]
- 80.Mulder WJ, Griffioen AW, Strijkers GJ, Cormode DP, Nicolay K, Fayad ZA. Magnetic and fluorescent nanoparticles for multimodality imaging. Nanomedicine (Lond) 2007;2:307–324. doi: 10.2217/17435889.2.3.307. [DOI] [PubMed] [Google Scholar]
- 81.Insin N, Tracy JB, Lee H, Zimmer JP, Westervelt RM, Bawendi MG. Incorporation of iron oxide nanoparticles and quantum dots into silica microspheres. ACS Nano. 2008;2:197–202. doi: 10.1021/nn700344x. [DOI] [PubMed] [Google Scholar]
- 82.Dobson J. Magnetic nanoparticles for drug delivery. Nanotoday. 2006;2:55–60. [Google Scholar]
- 83.Berry CC, Curtis ASG. Functionalisation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys. 2003;36:R198. [Google Scholar]


