Abstract
AIM: To investigate the influence of ischemia/reperfusion on arctic ground squirrel (AGS) neuronal progenitor cells (NPCs), we subjected these cultured cells to oxygen and glucose deprivation.
METHODS: AGS NPCs were expanded and differentiated into NPCs and as an ischemia vulnerable control, commercially available human NPCs (hNPCs) were seeded from thawed NPCs. NPCs, identified by expression of TUJ1 were seen at 14-21 d in vitro (DIV). Cultures were exposed to control conditions, hypoxia, oxygen and glucose deprivation or glucose deprivation alone or following return to normal conditions to model reperfusion. Cell viability and death were assessed from loss of ATP as well as from measures of alamarBlue® and lactate dehydrogenase in the media and from counts of TUJ1 positive cells using immunocytochemistry. Dividing cells were identified by expression of Ki67 and phenotyped by double labeling with GFAP, MAP2ab or TUJ1.
RESULTS: We report that when cultured in NeuraLife™, AGS cells remain viable out to 21 DIV, continue to express TUJ1 and begin to express MAP2ab. Viability of hNPCs assessed by fluorescence alamarBlue (arbitrary units) depends on both glucose and oxygen availability [viability of hNPCs after 24 h oxygen glucose deprivation (OGD) with return of oxygen and glucose decreased from 48151 ± 4551 in control cultures to 43481 ± 2413 after OGD, P < 0.05]. By contrast, when AGS NPCs are exposed to the same OGD with reperfusion at 14 DIV, cell viability assessed by alamarBlue increased from 165305 ± 11719 in control cultures to 196054 ± 13977 after OGD. Likewise AGS NPCs recovered ATP (92766 ± 6089 in control and 92907 ± 4290 after modeled reperfusion; arbitrary luminescence units), and doubled in the ratio of TUJ1 expressing neurons to total dividing cells (0.11 ± 0.04 in control cultures vs 0.22 ± 0.2 after modeled reperfusion, P < 0.05). Maintaining AGS NPCs for a longer time in culture lowered resistance to injury, however, did not impair proliferation of NPCs relative to other cell lineages after oxygen deprivation followed by re-oxygenation.
CONCLUSION: Ischemic-like insults decrease viability and increase cell death in cultures of human NPCs. Similar conditions have less affect on cell death and promote proliferation in AGS NPCs.
Keywords: Neurogenesis, Neuronal progenitor, Hypoxia tolerance, Hibernation
Core tip: Cultured arctic ground squirrel (AGS) neuronal progenitor cells (NPCs) resist cell death under conditions designed to model ischemia/reperfusion and instead show evidence of proliferation. Persistence of progenitor properties and hence the capacity to divide is a unique characteristic of AGS NPCs. Mechanisms that prolong neuronal progenitor properties may be targets to increase tolerance to cerebral ischemia/reperfusion in humans at risk of stroke and cardiac arrest.
INTRODUCTION
Failed delivery of oxygen and nutrients within the brain leads to neuronal pathology, disability and death in humans[1,2]. Arctic ground squirrels (AGS), a hibernating species, do not show detrimental effects of global cerebral ischemia in vivo that are typical of humans and other mammals[3,4]. Unlike other hibernating species[5], tolerance to modeled ischemia in AGS brain slices does not depend on the hibernating state and persists outside of the hibernation season[6,7]. We thus hypothesized that aspects of resistance to ischemia/reperfusion injury would be evident in neuronal progenitor cells (NPCs) derived from AGS. Because the effects of ischemia/reperfusion injury in NPCs are not well studied, we included human NPCs for comparison.
NPCs are cells derived from neural stem cells (NSCs) that have committed to a neuronal fate, but retain the capacity to divide[8]. Both NSCs and NPCs are found in adult brain and serve as pools of renewable neurons. In the adult brain, traumatic events including cerebral ischemia[9], epileptic seizures[10] and traumatic brain injury[11,12] promote neurogenesis. Though neurogenesis may involve proliferation of NSCs or NPCs evidence suggests that adult neurogenesis in the dentate gyrus of the hippocampus originates from restricted NPCs[13]. The fate of NPCs following ischemia/reperfusion is therefore significant to recovery from stroke and cardiac arrest.
Here we compared human and AGS NPCs, identified as cells that are nestin negative and TUJ1 positive, for vulnerability to oxygen and glucose deprivation in vitro. We report that hypoxic and ischemic-like insults decrease viability and increase cell death in cultures of human NPCs. Similar conditions fail to induce cell death in AGS NPCs.
MATERIALS AND METHODS
Cell culture
AGS NPCs were prepared as described previously[14] by thawing and seeding AGS NSCs onto a T-75 flask treated with poly-L-ornithine. Cells were allowed to proliferate using a DMEM/F12 growth media in the presence of bovine serum and bFGF (10 ng/mL) until 75% confluence. Cells were passaged and seeded onto 96-well plates (Biocoat, poly-L-Lysine coated, Becton Dickenson) at a low density (10000-20000 cells/well) and grown in differentiation media (lacking bFGF) for four days before switching to Neurobasal™ (Invitrogen) or NeuraLife™ (Lifeline Cell Technology) neuron maintenance media. Human NPCs were seeded from thawed NPCs (Clonexpress, Gaithersburg, MD) that originated from whole first trimester fetal brains. Cells were maintained for up to 14 d in vitro (DIV) in Neurobasal™ or up to 21 DIV in NeuraLife™ then fixed with 4% paraformaldehyde.
Hypoxia and oxygen glucose deprivation
Hypoxia with reoxygenation (O2 dep w/reOx) or modeled ischemia with reperfusion [oxygen glucose deprivation (OGD) w/rep] was achieved as follows. Media (80%) was changed from maintenance media containing 25 mmol/L glucose to maintenance media containing 5 mmol/L glucose 24 h prior to substrate deprivation to better approximate in vivo glucose concentrations[15]. Substrate deprivation was initiated by removing 80% of media and replacing it with normoglucose (5 mmol/L glucose in maintenance media) or glucose deprived media (0 mmol/L glucose in maintenance media). Plates were then placed in normoxic or hypoxic conditions for 48 h.
Hypoxic conditions were achieved by placing plates in a Billups-Rothenberg chamber flushed with 95% N2/5% CO2 until the partial pressure of O2 in the chamber was below 0.7% of atmospheric pressure then sealed. For normoxic conditions the chamber was left open for free gas exchange and placed in an incubator at 37 °C and 95% air (21% O2)/5% CO2. Humidity was maintained by placing water-soaked gauze on the bottom of the chamber. Following the hypoxic/normoxic period the plates were removed from their respective chambers and placed in an incubator at 37 °C and 95% O2/5% CO2 for 24 to 72 h. The media was never changed following the hypoxic/normoxic period, but glucose was returned to the media, when specified as OGD w/rep. In some experiments a small amount of media was removed for early analysis of lactate dehydrogenase (LDH) levels.
Glucose deprivation without oxygen deprivation (Gluc dep w/return) was achieved by treating plates as described for OGD except that plates were maintained in a normoxic environment throughout the treatment period. Following the return of glucose, oxygen, or oxygen and glucose, viability was assessed from cellular respiration monitored using an alamarBlue® assay and/or LDH levels in the media. After testing for alamarBlue® and LDH, the cultures were fixed and processed for immunocytochemistry. Human NPCs were treated in a similar manner and served as a positive control for NPCs derived from an ischemia vulnerable species.
Measures of cell viability and cell death
Media (100 μL) was assayed for LDH (Roche Diagnostics) as a measure of cell death, according to manufacturer’s instructions. AlamarBlue® (Invitrogen), a redox indicator that yields a fluorescent signal in response to metabolic activity and is proportional to cell number under the conditions described, was assayed as a measure of cell viability. AlamarBlue® solution was added to 200 μL of media in each well of the 96 well plate 2 h prior to assay. Following 2 h at 37 °C and 95% air/5% CO2, respiration was determined in the cells by removing 100 μL of media into an empty 96-well plate to measure a respiratory factor using a fluorescence plate reader.
ATP measurements
Immediately following OGD/normoxia or following the 24 h reperfusion period, media was removed from all wells and then 100 μL of buffer and substrates were added to cells according to manufacturer’s directions (ATPlite Kit, PerkinElmer). The plates were agitated on an orbital shaker for 2 min. The lysate was transferred to a black 96-well plate and luminescence measured on a Wallac Victor2 Multiplate reader.
Immunocytochemistry
Cells in 96-well plates were fixed for 20 min with 4% paraformaldehyde and incubated with antibodies for Ki67, MAP2ab or TUJ1. The cells were rinsed numerous times with PBS (Ca2+, Mg2+ free), the cells were blocked with Triton X-100 containing blocking buffer and then; β-tubulin polyclonal antibody TUJ1 (Covance Cat# PRB-435P), 1/2000 dilution; MAP2 (a and b) (Abcam Cat#ab3096), 1/300 dilution; GFAP (Abcam Cat#ab16997), 1/100; Nestin (Abcam Cat #ab7659), 1/200 and Ki67 (Abcam Cat #ab695093), 1/1000 dilution. The cells were again rinsed several times with PBS then secondary antibody (Alexa fluor 546 goat anti-rabbit IgG (H + L) (Life Technologies - Molecular Probes, Cat #A11010) was added along with Hoechst dye 33342 (Life Technologies - Molecular Probes Cat. # H3570). The abundance of immunoreactive cells was measured using the neuronal profiling software of the Cellomics Arrayscan instrument to assess total number of cells within each field (Hoechst positive cells) and number of neurons within each field with a neuronal process (TUJ1-positive and Hoechst-positive cells). Hoechst dye 33342 labeled nuclei of all cells. Ki67 antibody was used when identifying dividing cells of any type.
Statistical analysis
Data are shown as mean ± SD. Unless otherwise indicated, data were analyzed by one-way ANOVA followed by Holm-Sidak multiple comparisons or t-test for pairwise comparisons when data were normally distributed, and by Mann-Whitney rank sum test or Kruskal-Wallis one way ANOVA on ranks followed by Dunn’s multiple comparisons when data were not normally distributed. Software for analysis included Excel 2010 and SigmaPlot (v. 11.0). Sample size (n) refers to the number of wells per treatment condition unless indicated otherwise. P < 0.05 was interpreted as statistically significant.
RESULTS
NPCs are identified in cultures of human cells by expression of TUJ1 and neurons by MAP2ab at 14 DIV (Figure 1). By contrast, AGS cultures begin as NSCs, identified by nestin-ir and an absence of GFAP-ir at 7 DIV (Figure 2). By 14 DIV, AGS cultures express TUJ1 and GFAP characteristic of proliferating progenitors committed to neuronal or astrocytic fates[8] and nestin is no longer expressed. When cultured in NeuraLife™ AGS cells remain viable out to 21 DIV, continue to express TUJ1 and begin to express MAP2ab (Figure 2).
Figure 1.
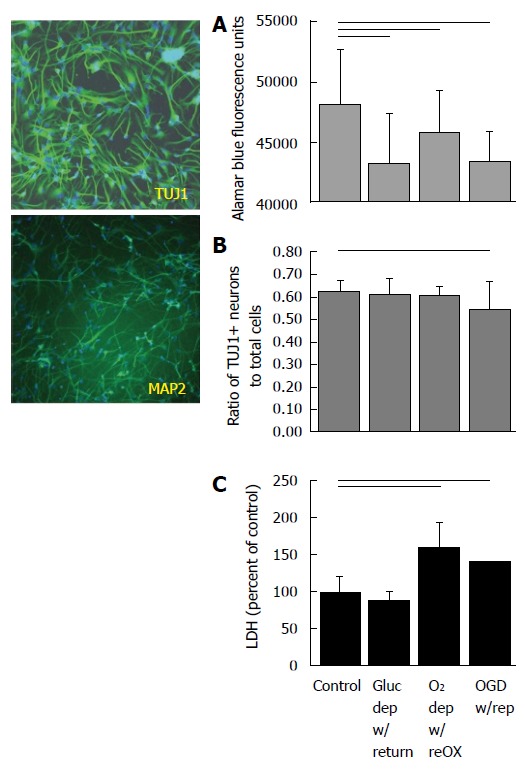
Cultures of human neuronal progenitor cells express TUJ1 and MAP2ab at 14 d in vitro (left) and die within 24 h of re-oxygenation (right). Cultures were deprived of oxygen and/or glucose for 48 h and resources were replaced for 24 h before assaying for viability (A), proportion NPC to total cells (B) or cell death (C). Horizontal lines indicate P < 0.05; ANOVA followed by a Holm-Sidak test (A, B) or Kruskal-Wallis ANOVA on ranks followed by Dunn’s multiple comparisons (C). Mean ± SD. Only differences relative to control are shown. NPC: Neuronal progenitor cell.
Figure 2.
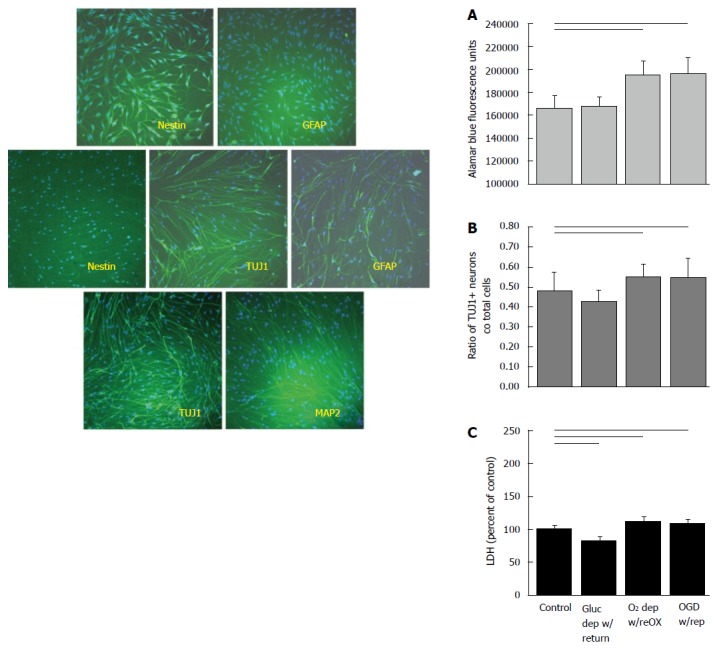
Cultures of arctic ground squirrel neuronal progenitor cells do not begin to express MAP2ab, characteristic of mature neurons, until 21 d in vitro. Cultures of AGS NPC express nestin, a marker of NSC, but not GFAP, a marker for astrocytes at 7 d in vitro (DIV) (top left). By 14 DIV cultures express TUJ1 a marker of neuronal progenitor cells that are committed to a neuronal fate, and GFAP a marker of progenitor cells commitment to an astrocytic fate. Nestin is no longer expressed at 14 DIV (middle left). At 21 DIV cultures express TUJ1 and begin to express MAP2ab (bottom left). AGS NPCs at 14 DIV increase in viability and appear to proliferate when deprived of oxygen or oxygen and glucose (right). When AGS NPC were deprived of oxygen or oxygen and glucose for 48 h followed by 24 h of resource replacement, cultures showed an increase in viability (A) and an increase in the proportion of neuronal progenitors (B) despite signs of cell death (C). Horizontal lines P < 0.05; ANOVA followed by a Holm-Sidak test. Mean ± SD. Only differences relative to control are shown. AGS: Arctic ground squirrel; NPC: Neuronal progenitor cell; OGD: Oxygen glucose deprivation.
Viability of human NPCs and neurons at 14 DIV depended on both glucose and oxygen availability, although combined deprivation of both substrates produced the most consistent decrease in viability and neuron numbers. Depriving human cultures of glucose, oxygen or oxygen and glucose produced a decrease in alamar Blue® fluorescence (P < 0.001; ANOVA, n = 72-96, Figure 1A). All treatments combined showed a significant decrease in the ratio of TUJ1+ NPCs to total cells (P < 0.05, ANOVA, n = 15-20, Figure 1B) although post-hoc analysis showed that only cultures exposed to OGD with reperfusion had significantly fewer NPCs to total cells compared to controls. Oxygen deprivation with reoxygenation and OGD with reperfusion produced a significant increase in LDH released into the media (P < 0.05, Kruskal-Wallis, ANOVA on ranks, n = 18-24) (Figure 1C).
By contrast, when AGS NPCs were exposed to oxygen deprivation with reoxygenation or OGD with reperfusion at 14 DIV, cell viability increased (P < 0.001, ANOVA, n = 5-6, Figure 2A), and the ratio of NPCs to total cells increased (P < 0.01, ANOVA, n = 9-12, Figure 2B) despite a small, but significant increase in LDH (P < 0.001, ANOVA, n = 6-9, Figure 2C). When deprived of glucose alone, LDH decreased relative to control and viability and neuron numbers remained unchanged. Viability of the cultured cells after a period of recovery was also evident from measures of ATP. Although AGS NPCs lost ATP within 40 h after oxygen or oxygen and glucose deprivation (P < 0.001, ANOVA, n = 6, Figure 3A), ATP returned to or exceeded control levels within 24 h of reintroducing resources (P < 0.001, ANOVA, n = 5-6, Figure 3B). Results are expressed as total ATP luminescence units because the ATP assay superseded cell counts.
Figure 3.
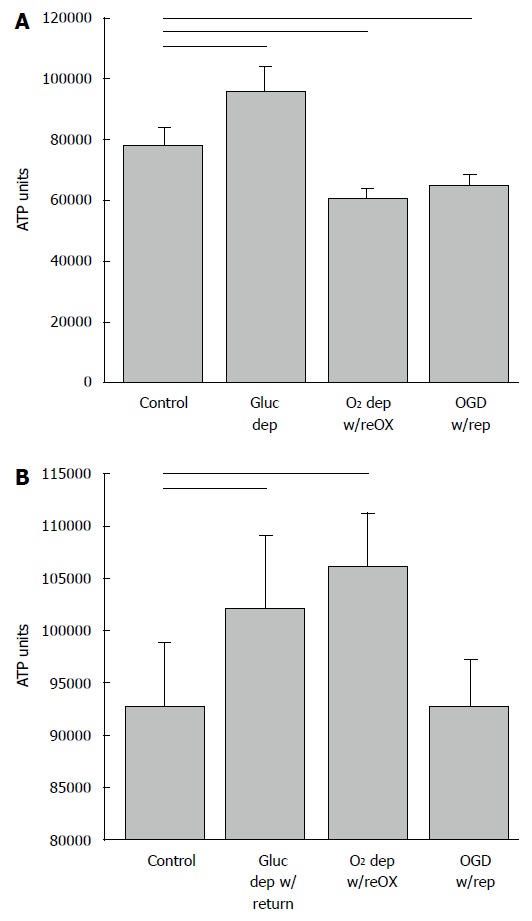
ATP (shown as relative luminescence units) declines in arctic ground squirrel neuronal progenitor cells during oxygen and glucose deprivation. NPCs at 14 d in vitro deprived for 48 h followed by 24 h resource replacement lost ATP when deprived of oxygen or oxygen and glucose (A). ATP recovered within 24 h of reoxygenation or reperfusion (modeled by return of oxygen and glucose) (B). Horizontal lines P < 0.05; ANOVA followed by a Holm-Sidak test. Mean + SD. Only differences relative to control are shown. OGD: Oxygen and glucose deprivation; NPCs: Neuronal progenitor cells.
The increase in viability and number of TUJ1 positive NPCs to total cells in the presence of cell death suggested that TUJ1 positive NPCs were proliferating in response to injury at a rate faster than other cell lineages. To test this hypothesis we identified dividing cells as those immunoreactive for Ki67, a cell cycle protein expressed during the G1 phase of the cell cycle. As expected, the ratio of dividing (NPCs) (Ki67 and TUJ1 positive) to the total number of dividing cells (Ki67 positive) increased following oxygen deprivation and oxygen and glucose deprivation (P < 0.01, Kruskal-Wallis, n = 5-8, Figure 4A). The number of dividing neuronal progenitors remained constant while the number of total dividing cells decreased (P < 0.001, ANOVA, n = 6-8, Figure 4B).
Figure 4.
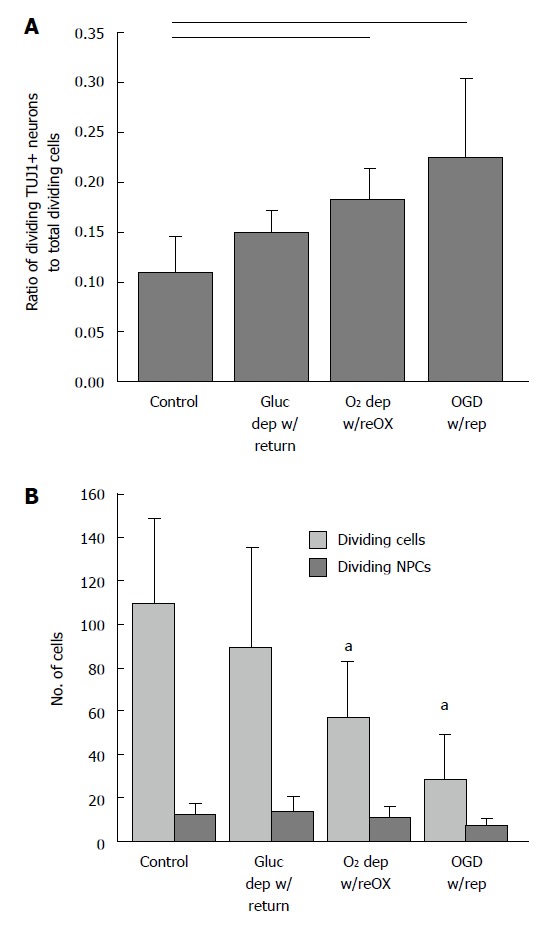
Arctic ground squirrel neuronal progenitor cells proliferate in response to injury faster than other cell lineages. AGS NPCs at 14 d in vitro deprived of oxygen or oxygen and glucose for 48 h followed by 24 h of reoxygenation or reperfusion (modeled by return of oxygen and glucose). The proportion of dividing NPCs (TUJ1+ and Ki67+/Ki67+) (A) increased because the number of dividing NPCs (TUJ1+ and Ki67+) did not change while the number of dividing cells (Ki67+) decreased (B). Horizontal lines P < 0.05; aP < 0.05 compared to control; ANOVA followed by a Holm-Sidak test. Mean ± SD. Only differences relative to control are shown. OGD: Oxygen glucose deprivation; NPCs: Neuronal progenitor cells; AGS: Arctic ground squirrel.
We next asked if encouraging AGS NPCs to terminally differentiate by extending the number of DIV beyond 21 d would abolish ischemia-induced neurogenesis and tolerance to oxygen deprivation. Using a novel culture media (NeuraLife™, Lifeline Cell Technology, Frederick, MD) cultures remained viable beyond 3 wk and began to express MAP-2ab (Figure 2). After this additional week in culture, 48 h of hypoxia followed by 24 h of re-oxygenation produced an increase in LDH in the media (P < 0.001, t-test, n = 83-84, Figure 5A). However, the number of NPCs relative to total cells did not change (Figure 5B), and the extended culture period did not abolish neurogenesis. The number of dividing NPCs (cells positive for Ki67 and TUJ1) 24 h after re-oxygenation increased when compared to normoxia treated control cultures (Figure 5C). NPCs counts and evidence of neurogenesis persisted up to 72 h after re-oxygenation. By 48 and 72 h after re-oxygenation the ratio of NPCs to total cells increased slightly [48 h, Figure 6A, P < 0.01, t-test, n = 4 (averages of 6 replicates)] or remained the same (72 h, Figure 6C). In addition, the number of dividing NPCs increased (48 h, Figure 6B) or the decline in dividing NPCs was less than the decline in proliferation of other cell lineages (72 h, Figure 6D) (P < 0.05, t-test, n = 4, averages of 6 replicates).
Figure 5.
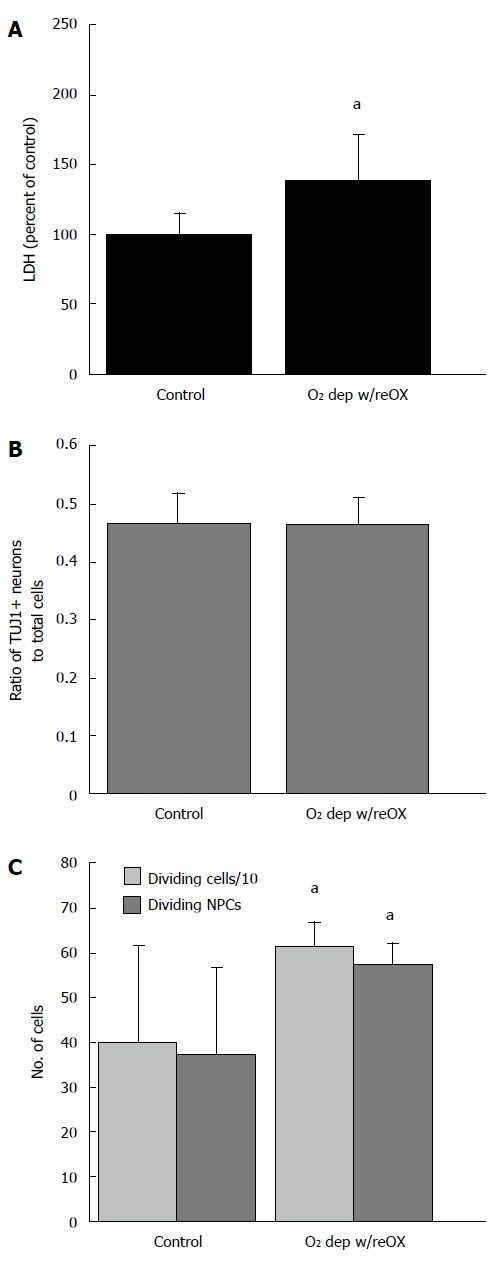
Arctic ground squirrel neuronal progenitor cell cultured for 21 d in vitro are more vulnerable to oxygen deprivation than cells cultured for 14 d in vitro. Longer time in culture lowers resistance to oxygen deprivation /reoxygenation indicated by an increase in LDH (A) not seen after 14 d in vitro (DIV). However, after 24 h of O2 deprivation, followed by 24 h of reoxygenation the proportion of neuronal to nonneuronal progenitors did not change (B) while proliferation of NPCs increased (C). Data are from AGS NPCs cultured for 21 DIV that were deprived of oxygen followed by 24 h of reoxygenation. aP < 0.05 compared to control, t-test. LDH: Lactate dehydrogenase; NPCs: Neuronal progenitor cells; AGS: Arctic ground squirrel.
Figure 6.
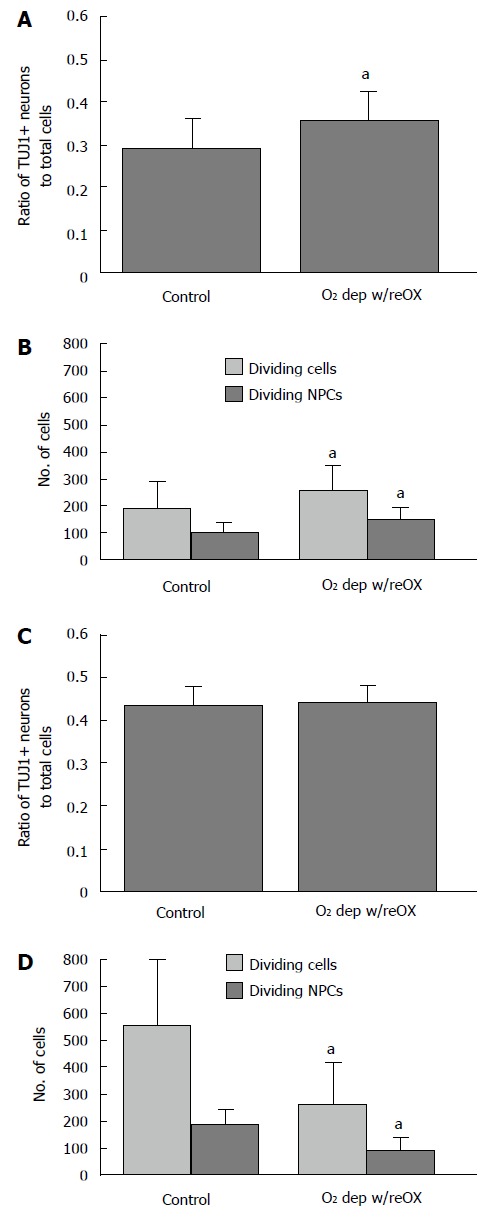
By 48 and 72 h after re-oxygenation the ratio of neuronal progenitor cells to total cells increases slightly or remains the same. AGS NPCs cultured for 21 d in vitro were deprived of oxygen followed by 48 h (A, B) or 72 h (C, D) of re-oxygenation. After 48 h of reoxygenation, the proportion of NPCs increased relative to total cells because the number of dividing NPCs increased (B). After 72 h the proportion of NPCs was sustained (C) because division of NPCs declined less than other cell lineages (D). aP < 0.05 compared to control, t-test. Mean ± SD. AGS: Arctic ground squirrel; NPCs: Neuronal progenitor cells.
Increased antioxidant capacity may protect AGS during periods of reperfusion during arousal from torpor[16-18]. We therefore asked if AGS cultures would resist injury induced by the pro-oxidant H2O2. AGS NPCs were in fact more sensitive to H2O2 than cultures of human NPCs and neurons (Figure 7). While the viability of human cultures, indicated from alamarBlue® fluorescence, decreased after 6 h of exposure to 50 μmol/L H2O2 (P < 0.05, Kruskal-Wallis, ANOVA on ranks, n = 20), the viability of AGS cultures decreased after just 2 h of exposure (P < 0.05, Kruskal-Wallis, ANOVA on ranks, n = 25). Differences in percent glia in cultures can directly affect neuroprotective capacity. In looking at the percent of GFAP+ glial cells in each culture, differentiated AGS NPCs and differentiated human NPCs cultures, the number of GFAP positive glial cells in the AGS cultures (DIV12) was considerably lower than the percentage of GFAP+ glial cells in the differentiated human cultures (6.2% ± 3.7% vs 37.0% ± 1.9%, respectively, n = 6-12 wells).
Figure 7.
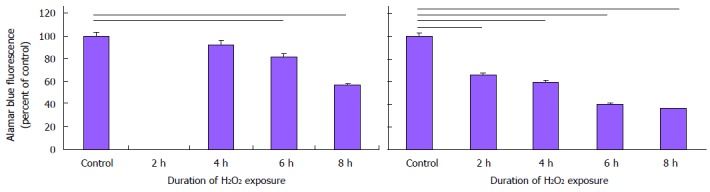
Arctic ground squirrel neuronal progenitor cells are vulnerable to oxidative stress. Cells, 14 d in vitro, were exposed to 50 μmol/L H2O2. After 2 to 8 h of exposure cellular respiration, a measure of cellular viability, was measured using alamarBlue®. The viability of human cultures (A) decreased after 6 h exposure while the viability of AGS NPCs (B) decreased after 2 h of exposure to H2O2. AGS: Arctic ground squirrel; NPCs: Neuronal progenitor cells.
DISCUSSION
AGS offer a unique and robust model of resistance to brain injury following global cerebral ischemia in vivo and OGD in vitro[3,4,6,7]. This resistance may be in part, intrinsic to AGS neurons and thus persist in culture. To study AGS neurons in culture we isolated AGS NSCs that were then differentiated into NPCs prior to insult with oxygen and/or glucose deprivation. Here we show that proliferation and differentiation of NSCs obtained from adult AGS hippocampus follows a characteristic progression of lineage-specific markers described for NSCs from other species[8]. We show that at 7 DIV AGS cultures express nestin but not GFAP, a characteristic of NSCs. These cells commit to a neuronal (TUJ1 positive) or astrocytic (GFAP positive) fate by 14 DIV. AGS NPCs continue to mature if cultured under conditions that favor survival and by 3 wk begin to express MAP2ab a marker of mature, terminally differentiated neurons. Interestingly, the human cultures at only 14 DIV already contain a large fraction of MAP2ab-ir cells showing that AGS NPCs mature more slowly than hNPCs in culture.
Glucose deprivation had little influence on viability or cell death and did not stimulate proliferation of NPCs. Similarly the effects of OGD were similar to the effects of hypoxia without glucose deprivation. We have shown previously that cultures of AGS NPCs passaged and differentiated in much the same manner as described here are vulnerable to iodoacetate, a chemical inhibitor of glycolysis, and less vulnerable to inhibition of oxidative metabolism[19]. Lack of effect of glucose deprivation, in the present study may be due to astrocytic stores of glycogen and the capacity for these cell cultures to survive on anaerobic glycolysis. These results are consistent with other studies showing that NSCs/NPCs cultured under low oxygen consume more glucose, produce energy by glycolysis, and upregulate genes involved in glycolysis[20]. Because glycogen is stored primarily in astrocytes, higher proportions of astrocytes to neurons in AGS NPCs cultures could contribute to lower levels of cell death compared to human NPCs. However, astrocytes do not appear to contribute to neuroprotection in AGS NPCs since the percentage of GFAP+ astrocytes are considerably lower in AGS NPCs cultures than in human NPCs cultures. Although the degree of hypoxia experienced in culture is difficult to compare directly to hypoxia sensed by neurons in vivo the partial pressure of O2 in our hypoxic chambers was 0.7% of atmospheric pressure or lower. This low level of O2 is well below normal physiological levels[21].
Interpretation of a direct comparison with human NPCs is limited for several reasons. The percentage of MAP2ab-ir cells may influence tolerance to OGD since neurons are not anaerobic[22]. The human cultures at 14 DIV contained MAP2ab positive neurons, whereas the AGS cultures did not. Perhaps the increase in LDH with hypoxic conditions of AGS cultures at 21 DIV reflects the greater number of mature neurons lost. In order to increase the number of mature MAP2ab-ir cells we extended the culture period, but only in the presence of AGS neuron beneficial media, NeuraLife™. There was no discernible difference in TUJ1+ cell numbers early in the culture period, but as with MAP2ab-ir cells, the number of TUJ1+ cells (neurons and progenitors) was also greater at longer culture period, specifically 21 DIV. While the influence of relative proportions of astrocytes, NPCs and mature neurons to the response to oxygen and glucose deprivation may be inferred from these results it is important to not over-interpret the comparison between human and AGS cultures. hNPCs and AGS NSCs were derived from 2 different anatomical sources, the adult hippocampus for AGS and the first trimester fetal brains for the human line. Second, the stage of development differed between the two lines and time points studied. Humans are, however, a species known to be vulnerable to cerebral ischemia/reperfusion injury and for this reason hNPCs were included as a positive control.
Neurogenesis following cerebral ischemia in vivo has not been monitored in AGS or in any other hibernating species, to our knowledge. However, immature cells have been captured in a mitotic state in the adult dentate gyrus of a closely related species of ground squirrel where cooling during hibernation slowed and facilitated observations of mitosis[23]. AGS and other species of ground squirrels regenerate synapses and AGS show enhanced cognitive function after arousal from hibernation[24,25] when hemoglobin-oxygen saturation levels fall to as low as 57%[16]. Whether the tendency for AGS NPCs to resist terminal differentiation and to proliferate in response to a low oxygen environment contributes to this species’ ability to tolerate or recover from global cerebral ischemia/reperfusion in vivo remains to be determined.
Cerebral ischemia associated with stroke, cardiac arrest, and traumatic brain injury leads to death or disability due in large part to progressive pathology for which effective therapeutic interventions are lacking. Neuroprotective therapeutics, have consistently failed in clinical trials to improve outcome following stroke, cardiac arrest and TBI despite positive preclinical results[26,27]. The reasons for clinical failure of neuroprotectants could be many fold[28], yet the possibility remains that without an actual reversal of ischemic injury the outcome will always be negative or not sufficient. Neurorestorative therapies for stroke[29] may provide improved outcomes over neuroprotectants. Recent evidence suggests that injury-induced proliferation of NSCs may provide a means to enhance recovery and decrease morbidity after stroke[9,30,31], although aberrant neurogenesis following stroke may contribute to aberrant functional outcomes[32]. Better understanding of mechanisms sufficient to promote survival and proliferation of AGS NPCs has the potential to unveil mechanisms that will also promote proliferation of human NPCs following brain injury. AGS NPCs offer a platform to investigate mechanisms of ischemia tolerance to inform development of novel therapies that target signal transduction pathways that contribute to ischemia tolerance and neurogenesis. Methods to promote neurogenesis after brain injury are expected to complement traditional neuroprotective therapies[33,34].
COMMENTS
Background
Many people have lost loved ones to cardiac arrest or stroke. Death is most typically due to brain injury caused by a disruption in blood supply. Patients who survive are often burdened with severe neurological disabilities. Hibernating species resist brain injury following disruption of blood flow that would be lethal in other mammalian species, including humans. Unique physiology of these animals contributes to this resistance, but, it was unknown if cellular processes independent of environmental influences played a role in this tolerance. Here for the first time, we show aspects of cellular physiology in arctic ground squirrel (AGS) neurons that could contribute to ischemia tolerance. These observations now establish a foundation to study mechanisms unique to the AGS that may lead to the development of novel and efficacious treatments to reduce brain injury following cardiac arrest and stroke in humans and reduce effects of further neurodegenerative events.
Research frontiers
Opportunities to promote neurogenesis and neuron regeneration are a new area, ripe for drug development. While much is now known about how hypoxia influences proliferation and differentiation of neuronal stem cells, little work has been directed at understanding the longevity and survival of neuronal progenitor cells (NPCs), i.e., young neurons, largely committed to a neuronal fate that retain the capacity to divide. Using the AGS as a unique model of ischemia tolerance we found that AGS NPCs persist longer in culture than human NPCs and divide in response to hypoxia. The mechanisms behind this phenomenon can now be studied and drugs developed to mimic these mechanisms for the treatment of cardiac arrest and stroke.
Innovations and breakthroughs
Characterization of unique properties of NPCs derived from the ischemia-resistant AGS is an innovative approach to understanding mechanisms of cellular response to hypoxia that will inform future drug development.
Applications
Better understanding of mechanisms sufficient to promote survival and proliferation of AGS NPCs has the potential to unveil mechanisms that will also promote proliferation of human NPCs following brain injury. AGS NPCs offer a platform to investigate mechanisms of ischemia tolerance to inform development of novel therapies that target signal transduction pathways that contribute to ischemia tolerance and neurogenesis. Methods to promote neurogenesis after brain injury are expected to complement traditional neuroprotective-targeted therapeutic approaches.
Terminology
NPCs: A NPCs is like a neural stem cell in that both have the ability to divide. A NPCs, however, has already begun to differentiate into a neuron and is more committed to a neuronal fate than a neural stem cell. NPCs are found in the adult, mammalian central nervous system, in two distinct brain regions, where they are thought to function in repair.
Peer-review
This study characterized an interesting oxygen and glucose deprivation resistance in a model of AGS derived NPCs. This study demonstrated an acute response of AGS NPCs to oxygen and glucose deprivation in a number of parameters, including proliferation, ATP, survival etc. The key to this study is the use of human NPCs under similar conditions as a comparison. The current data did clearly demonstrate a resistance of AGS NPCs cells, but also showed that the AGS NPCs are more vulnerable to H2O2, as compared to human NPCs. The study presented an interesting possibility that the AGS may carriy a different genetic background or be primed with differential epigenetic coding that bestows the differential resistance or response to environmental factors, e.g., oxygen or glucose. This interpretation of the differential response was interpreted that AGS offer a unique and robust model of resistance to brain injury following global cerebral ischemia in vivo.
Footnotes
Supported by The US Army Medical Research and Materiel Command, No. 05178001; the National Institute of Neurological Disorders and Stroke, Nos. NS041069-06 and R15NS070779.
Institutional review board statement: Not applicable.
Institutional animal care and use committee statement: Not applicable.
Conflict-of-interest statement: Two of the authors have financial interests in companies with commercial interests in neural stem cells isolated from the arctic ground squirrel. Kelleher-Andersson J works for Neuronascent, Inc. a Maryland Corporation. McGee R works for Lifeline Cell Technology, a subsidiary of International Stem Cell Corporation. Both companies shared commercial interest in AGS NSC. Kelleher-Andersson J owns stock in Neuronascent, Inc. McGee R holds stock options in International Stem Cell Corporation. Drew KL receives no financial support from Neuronascent, Inc. or Lifeline Cell Technology and has no other financial interests in AGS NSCs.
Data sharing statement: No other data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 16, 2015
First decision: September 17, 2015
Article in press: January 11, 2016
P- Reviewer: Amornyotin S, Zhou FC S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
References
- 1.Ornato JP, Peberdy MA. Prehospital and emergency department care to preserve neurologic function during and following cardiopulmonary resuscitation. Neurol Clin. 2006;24:23–39. doi: 10.1016/j.ncl.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 3.Dave KR, Prado R, Raval AP, Drew KL, Perez-Pinzon MA. The arctic ground squirrel brain is resistant to injury from cardiac arrest during euthermia. Stroke. 2006;37:1261–1265. doi: 10.1161/01.STR.0000217409.60731.38. [DOI] [PubMed] [Google Scholar]
- 4.Dave KR, Anthony Defazio R, Raval AP, Dashkin O, Saul I, Iceman KE, Perez-Pinzon MA, Drew KL. Protein kinase C epsilon activation delays neuronal depolarization during cardiac arrest in the euthermic arctic ground squirrel. J Neurochem. 2009;110:1170–1179. doi: 10.1111/j.1471-4159.2009.06196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frerichs KU, Hallenbeck JM. Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J Cereb Blood Flow Metab. 1998;18:168–175. doi: 10.1097/00004647-199802000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Christian SL, Ross AP, Zhao HW, Kristenson HJ, Zhan X, Rasley BT, Bickler PE, Drew KL. Arctic ground squirrel (Spermophilus parryii) hippocampal neurons tolerate prolonged oxygen-glucose deprivation and maintain baseline ERK1/2 and JNK activation despite drastic ATP loss. J Cereb Blood Flow Metab. 2008;28:1307–1319. doi: 10.1038/jcbfm.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross AP, Christian SL, Zhao HW, Drew KL. Persistent tolerance to oxygen and nutrient deprivation and N-methyl-D-aspartate in cultured hippocampal slices from hibernating Arctic ground squirrel. J Cereb Blood Flow Metab. 2006;26:1148–1156. doi: 10.1038/sj.jcbfm.9600271. [DOI] [PubMed] [Google Scholar]
- 8.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 9.Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci USA. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jessberger S, Zhao C, Toni N, Clemenson GD, Li Y, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci. 2007;27:9400–9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emery DL, Fulp CT, Saatman KE, Schütz C, Neugebauer E, McIntosh TK. Newly born granule cells in the dentate gyrus rapidly extend axons into the hippocampal CA3 region following experimental brain injury. J Neurotrauma. 2005;22:978–988. doi: 10.1089/neu.2005.22.978. [DOI] [PubMed] [Google Scholar]
- 12.Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drew KL, McGee RC, Wells MS, Kelleher-Andersson JA. Growth and differentiation of adult hippocampal arctic ground squirrel neural stem cells. J Vis Exp. 2011:e2199. doi: 10.3791/2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rex A, Bert B, Fink H, Voigt JP. Stimulus-dependent changes of extracellular glucose in the rat hippocampus determined by in vivo microdialysis. Physiol Behav. 2009;98:467–473. doi: 10.1016/j.physbeh.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Ma YL, Zhu X, Rivera PM, Tøien Ø, Barnes BM, LaManna JC, Smith MA, Drew KL. Absence of cellular stress in brain after hypoxia induced by arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1297–R1306. doi: 10.1152/ajpregu.00260.2005. [DOI] [PubMed] [Google Scholar]
- 17.Tøien Ø KL, Chao ML, Rice ME. Ascorbate dynamics and oxygen consumption during arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2001;281:R572–R583. doi: 10.1152/ajpregu.2001.281.2.R572. [DOI] [PubMed] [Google Scholar]
- 18.Drew KL, Tøien Ø, Rivera PM, Smith MA, Perry G, Rice ME. Role of the antioxidant ascorbate in hibernation and warming from hibernation. Comp Biochem Physiol C Toxicol Pharmacol. 2002;133:483–492. doi: 10.1016/s1532-0456(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 19.McGee RC, Drew KL, Wells MS, Kelleher-Andersson JA. Neurons derived from arctic ground squirrel neural stem cells tolerate oxygen-glucose deprivation by enhanced capacity for anaerobic metabolism and neurogenesis 151.13. Society for Neuroscience, Washington, DC; 2008. [Google Scholar]
- 20.Zhu LL, Zhao T, huang X, Liu ZH, Wu LY, Wu KW, Fan M. Gene expression profiles and metabolic changes in embryonic neural progenitor cells under low oxygen. Cell Reprogram. 2011;13:113–120. doi: 10.1089/cell.2010.0043. [DOI] [PubMed] [Google Scholar]
- 21.Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220:562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 22.Genc S, Kurnaz IA, Ozilgen M. Astrocyte-neuron lactate shuttle may boost more ATP supply to the neuron under hypoxic conditions--in silico study supported by in vitro expression data. BMC Syst Biol. 2011;5:162. doi: 10.1186/1752-0509-5-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popov VI, Kraev IV, Ignat’ev DA, Stewart MG. Suspension of mitotic activity in dentate gyrus of the hibernating ground squirrel. Neural Plast. 2011;2011:867525. doi: 10.1155/2011/867525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von der Ohe CG, Darian-Smith C, Garner CC, Heller HC. Ubiquitous and temperature-dependent neural plasticity in hibernators. J Neurosci. 2006;26:10590–10598. doi: 10.1523/JNEUROSCI.2874-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weltzin MM, Zhao HW, Drew KL, Bucci DJ. Arousal from hibernation alters contextual learning and memory. Behav Brain Res. 2006;167:128–133. doi: 10.1016/j.bbr.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Savitz SI, Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann Neurol. 2007;61:396–402. doi: 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]
- 27.Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx. 2004;1:36–45. doi: 10.1602/neurorx.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cook DJ, Tymianski M. Translating promising preclinical neuroprotective therapies to human stroke trials. Expert Rev Cardiovasc Ther. 2011;9:433–449. doi: 10.1586/erc.11.34. [DOI] [PubMed] [Google Scholar]
- 29.Jäkälä P, Jolkkonen J. Time for a neurorestorative therapy in stroke. Expert Opin Biol Ther. 2012;12:267–270. doi: 10.1517/14712598.2012.656086. [DOI] [PubMed] [Google Scholar]
- 30.Lagace DC. Does the endogenous neurogenic response alter behavioral recovery following stroke? Behav Brain Res. 2012;227:426–432. doi: 10.1016/j.bbr.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 31.Yagita Y, Kitagawa K, Ohtsuki T, Takasawa Ki T, Okano H, Hori M, Matsumoto M. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke. 2001;32:1890–1896. doi: 10.1161/01.str.32.8.1890. [DOI] [PubMed] [Google Scholar]
- 32.Niv F, Keiner S, Krishna -, Witte OW, Lie DC, Redecker C. Aberrant neurogenesis after stroke: a retroviral cell labeling study. Stroke. 2012;43:2468–2475. doi: 10.1161/STROKEAHA.112.660977. [DOI] [PubMed] [Google Scholar]
- 33.Liu XY, Zhou XY, Hou JC, Zhu H, Wang Z, Liu JX, Zheng YQ. Ginsenoside Rd promotes neurogenesis in rat brain after transient focal cerebral ischemia via activation of PI3K/Akt pathway. Acta Pharmacol Sin. 2015;36:421–428. doi: 10.1038/aps.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan XB, Hou HL, Wu LM, Liu J, Zhou JN. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology. 2007;53:487–495. doi: 10.1016/j.neuropharm.2007.06.020. [DOI] [PubMed] [Google Scholar]


