Abstract
Three important physical properties which may affect the performance of glycoconjugate vaccines against serious disease are molar mass (molecular weight), heterogeneity (polydispersity), and conformational flexibility in solution. The dilute solution behaviour of native and activated capsular polyribosylribitol (PRP) polysaccharides extracted from Haemophilus influenzae type b (Hib), and the corresponding glycoconjugate made by conjugating this with the tetanus toxoid (TT) protein have been characterized and compared using a combination of sedimentation equilibrium and sedimentation velocity in the analytical ultracentrifuge with viscometry. The weight average molar mass of the activated material was considerably reduced (Mw ~ 0.24 × 106 g.mol−1) compared to the native (Mw ~ 1.2 × 106 g.mol−1). Conjugation with the TT protein yielded large polydisperse structures (of Mw ~ 7.4 × 106 g.mol−1), but which retained the high degree of flexibility of the native and activated polysaccharide, with frictional ratio, intrinsic viscosity, sedimentation conformation zoning behaviour and persistence length all commensurate with highly flexible coil behaviour and unlike the previously characterised tetanus toxoid protein (slightly extended and hydrodynamically compact structure with an aspect ratio of ~3). This non-protein like behaviour clearly indicates that it is the carbohydrate component which mainly influences the physical behaviour of the glycoconjugate in solution.
Haemophilus influenzae is a small non-motile Gram negative bacterium1,2 present in the nasopharynx of approximately 75 % of healthy children and adults and is regarded as normal flora3. A minority (~3–7%) of healthy individuals intermittently harbour the carbohydrate encapsulated H. influenzae strains (types a, b, c, d, e, and f ) in the upper respiratory tract4. It was mistakenly thought to be the cause of influenza disease: as a consequence it was named accordingly5,6. However the considered opinion now is that H. influenzae is most likely to have been an important secondary invader to the influenza virus in the 1890 pandemic and many subsequent influenza epidemics. Nonetheless it is still considered responsible for a wide range of clinical diseases such as meningitis and pneumonia3,7.
Invasive diseases caused by H. influenzae seem to occur in humans only and are primarily due to type b Haemophilus influenzae or “Hib” (>95% of cases) where it remains a major cause of acute bacterial meningitis. Until the availability of the Hib vaccine, the type b H. influenzae was the main cause of meningitis in children between 6 months and 5 years old, although older children, adolescents and adults can also be infected8.
Since 1931 it has been known that some strains of Haemophilus influenzae possess a polysaccharide capsule and that there are 6 capsular serotypes (a–f )9. In 1953, Zamenhof and co-workers10 postulated that the type-specific substance of Haemophilus influenzae type b, was composed of polyribose-ribitol chains with (3→5) phosphate diester linkages between the ribose moieties. Further work11,12,13,14 established the double ribose unit as β-D-Ribf-/β-D-Ribf. Anderson and colleagues15 and Schneerson and coworkers16 have also reported equimolar ratios of phosphorus and ribose. Thus, the polyribosyl ribitol phosphate (PRP) capsule of Hib is a linear copolymer composed of repeated units of→3)-β-D-Ribf-(1→1)-D-ribitol-(5-OPO3→[(C10H19O12P)n] with a defined molecular size2,17. The size of the polysaccharide used for conjugation should be defined and controlled.
Unlike other bacteria (e.g Streptococcus pneumoniae and Neisseria meningitidis) the type b polysaccharide capsule of H. influenzae is attractive as a vaccine antigen since invasive disease is almost exclusively restricted to only one serotype, which in turn has rendered its capsular polysaccharide a prime candidate for vaccine studies. The first generation of Hib vaccine was based on the purified type b capsular polysaccharide and its ribose-ribitol phosphate repeating units2,18,19,20. Conjugation of the polysaccharide with a suitable protein to stimulate a stronger and longer lasting T-cell based immunity21 has been the target of research. Connaught Laboratories (now Sanofi Pasteur) produced the first licensed Hib conjugate vaccine in 1987 after the pioneering work by Schneerson and co-workers22, through random conjugation of PRP to diphtheria toxoid. Following on the heels of this vaccine three other vaccines from the biopharmaceutical companies, namely Wyeth (now Pfizer), Sanofi-Pasteur and Merck, all differing in their carrier protein and method of conjugation, received approval from the Food and Drug Administration (FDA)2. Recently approved glycoconjugate vaccines include a Hib-Tetanus toxoid (TT) conjugate Hiberix® and a novel Hib-MenCY-TT (MenHibrix®) by GSK Vaccines. MenHibrix® vaccine administered in accordance to the current Hib vaccine schedule (USA) would have the potential to induce protective antibodies against Hib and meningococcal-CY disease in children23.
Important to their function as a vaccine is the structure and stability of the polysaccharide and conjugates thereof. Molecular weight (molar mass) and molar mass distribution have been documented as being the most important physical parameters affecting the immunogenicity of capsular polysaccharides24,25. While many other aspects of polysaccharide characterisation have been relatively thoroughly explored26,27,28, the physical characterisation of capsular polysaccharides (molecular size and mass distribution and conformational flexibility) has been less extensively pursued. Such physical studies for capsular polysaccharides have been generally limited to low pressure chromatographic analyses calibrated with “standards” and more recently to high performance size exclusion chromatography (SEC) coupled to on-line refractive index detector (RI), multi-angle light scattering (MALS). In this study we used the analytical ultracentrifuge due to its larger dynamic range (molar masses from 103 to >108 g.mol−1), particularly appropriate for large glycoconjugates, many of which are beyond the exclusion limit of SEC-MALS29. This study is designed principally to characterise the purified native and activated capsular polyribosylribitol polysaccharides (PRP) from Haemophilus influenzae type b (referred to as PRP native and PRP-ADH respectively) as well as the final PRP- TT conjugate (with a polysaccharide: protein ratio of ca. 0.4) and to establish whether it is the protein component or carbohydrate component which principally influences the physical or hydrodynamic properties in solution This study has also been designed to demonstrate the usefulness of analytical ultracentrifuge based procedures – all not requiring a separation column or matrix - in the characterisation of large glycoconjugate vaccines.
Results
Sedimentation velocity in the analytical ultracentrifuge was first applied as the primary method for assessing the size heterogeneity. Bimodal plots of apparent sedimentation coefficient distributions g*(s) versus s were seen in the case of both native Hib polysaccharide (Fig. 1a) and the ADH activated Hib (Fig. 1b) whereas a unimodal profile for the much larger conjugate structure was observed (Fig. 1c). This does not mean that the conjugate was monodisperse as the broadness of the peak is commensurate with a broad distribution of sizes of a (quasi-) continuous type arising from polydispersity of the carbohydrate chains. Under these conditions Hib polysaccharides and conjugate have apparent weight average sedimentation coefficients ranging from 5.9S to 30S as reported in Table 1. All three showed classical dependencies of s20,w on c, (decrease of s20,w with increase of c) indicative of non-ideality and the absence of significant reversible associative effects (Fig. 2).
Figure 1.
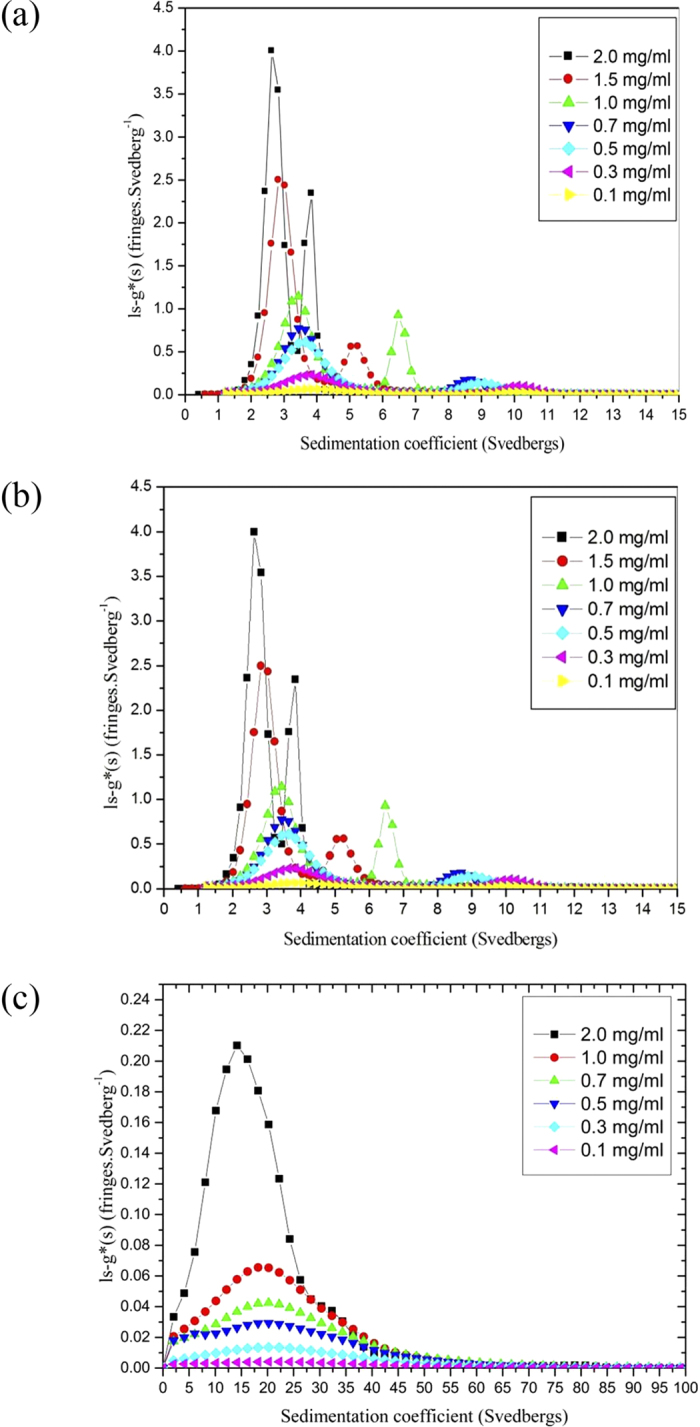
Sedimentation coefficient distributions, g*(s) vs s profiles, at different concentrations for (a) Hib PRP-native capsular polysaccharide (b) Hib PRP-ADG (c) Hib PRP-TT conjugate. The apparent sharpening of the peaks as the concentration increases is due to “hypersharpening” through the combined effects of polydispersity and non-ideality: the faster moving species in a distribution are slowed down by having to sediment through a solution of the slower ones. These effects diminish as the concentration is reduced.
Table 1. Hydrodynamic properties for Hib PRP-native, Hib PRP-ADH and Hib PRP-TT derivatives.
| Sample | so20,w (S) | ks mL.g−1 | 10−3 × Mwa g.mol−1 | 10−3 × Mzb g.mol−1 | [η]c mL.g−1 | [η]d mL.g−1 | [η]e mL.g−1 | ks/[η] | f/fo |
|---|---|---|---|---|---|---|---|---|---|
| PRP-native | 5.9 ± 0.2 | 400 ± 40 | 1200 ± 50 | 1250 ± 60 | 447 ± 14 | 445 ± 8 | 445 ± 6 | 0.9 ± 1 | 9.8 |
| PRP-ADH | 6.0 ± 0.2 | 260 ± 40 | 240 ± 10 | 325 ± 20 | 275 ± 4 | 273 ± 3 | 275 ± 3 | 0.9 ± 2 | 3.3 |
| PRP-TT | 30.0 ± 0.5 | 190 ± 10 | 7300 ± 420 | 7700 ± 390 | 225 ± 2 | 224 ± 6 | 224 ± 4 | 0.9 ± 1 | 6.4 |
| TTf monomer | 7.6 ± 0.1 | 150 ± 5 | 5.7 ± 0.1 | 5.7 ± 0.1 | 1.3 | ||||
| TTf dimer | 11.6 ± 0.2 | 270 ± 15 |
In phosphate-chloride buffer (pH = 6.8, I = 0.10).
aSedimentation equilibrium SEDFIT-MSTAR analysis.
bSedimentation equilibrium MFIT analysis.
cHuggins extrapolation procedure.
dKraemer extrapolation procedure.
eSolomon-Ciuta procedure.
ffrom ref. 33.
Figure 2. Concentration dependence (reciprocal) sedimentation coefficient plot for Hib PRP-native, Hib PRP-ADH and Hib PRP-TT, to remove the effects on non-ideality.
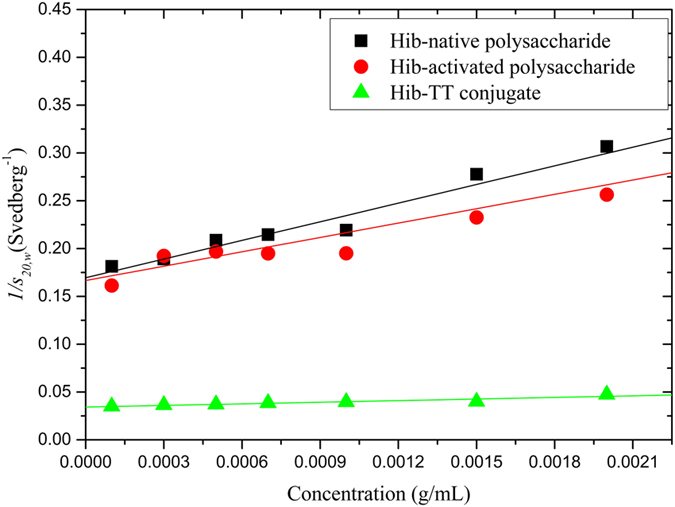
Sedimentation coefficients measured in the phosphate chloride buffer (pH = 6.8, I = 0.10) had been normalized to standard conditions (the viscosity and density of water at a temperature of 20.0 °C).
Molar mass values estimated from sedimentation equilibrium using the SEDFIT-MSTAR and MFIT algorithms for weight-average molar mass and z-average molar mass, respectively are shown in Table 1 with an example of a determination for the PRP-TT conjugate in Fig. 3. The weight average molar mass values for the glycoconjugate obtained using the matrix-free technique of sedimentation in the analytical ultracentrifuge29 appear to be commensurate with a recent study by Lockyer and coworkers30 using size exclusion chromatography (SEC) coupled to multi-angle light scattering, but without the problem of large molar mass species (>2 × 106) eluting in the void volume of the SEC.
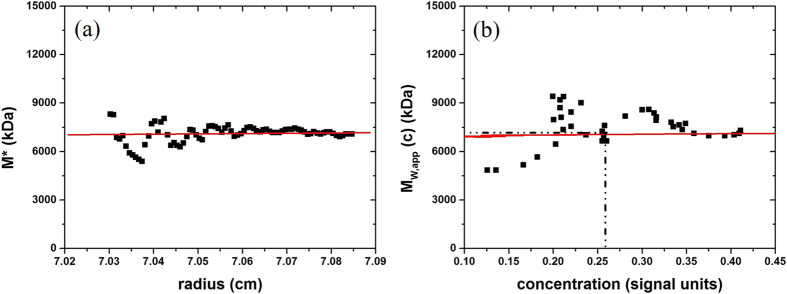
Figure 3. SEDFIT-MSTAR output for analysis of Hib PRP-TT conjugate at a loading concentration of 0.3 mg.mL−1 to find the (apparent) weight average molar mass Mw,app over the whole distribution.
(a) The operational point average molar mass M*(r) plotted as a function of radial position from the centre of rotation r. Mw,app, the (apparent) weight average molar mass for the whole distribution being measured = M* extrapolated to the radial position at the cell base. Retrieved Mw,app from this extrapolation = (7.3 ± 0.4) × 106 g.mol−1. (b) Plot of the “point” or “local” apparent average molar mass Mw,app(r) at radial positions r, as a function of local concentration c(r) in the ultracentrifuge cell. The “hinge point” corresponds to the radial position where the c(r) = the initial loading concentration. At this hinge point Mw,app(r) = (7.3 ± 0.5) × 106 g.mol−1. Although not as precise a way of estimating Mw,app from the sedimentation equilibrium records it does provide an internal check for consistency.
The sedimentation coefficient distribution – which itself gives a good measure of sample heterogeneity - for Hib PRP-TT conjugate was then transformed into a corresponding distribution of molar mass using the Extended Fujita Approach of Harding et al.31 (Fig. 4).
Figure 4. Molar mass distribution f(M) profile from sedimentation velocity for Hib PRP-TT conjugate.
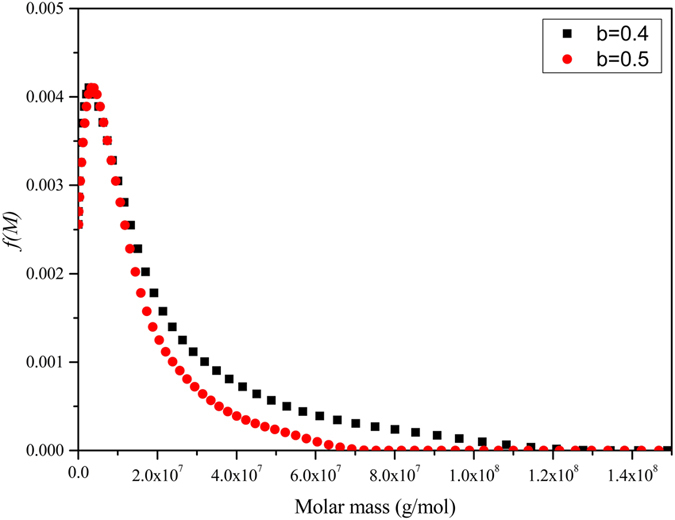
Obtained by transforming the sedimentation coefficient distribution of Fig. 1c by the Extended Fujita method, using the weight average sedimentation coefficient with the weight average Mw,app (from Fig. 3) molar mass and two different plausible values of the conformation parameter b. The broad distribution is completely different for the sharp monomer-dimer distribution we observed earlier for TT by itself 33.
The transformation is as follows:
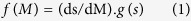 |
with
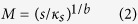 |
and
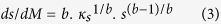 |
b is a conformation parameter that has already been estimated for a number of polysaccharides32 and κs can be found from equation (3) provided that at least one value of M (e.g. Mw from sedimentation equilibrium) is known for one value of s (e.g. the weight average s value). The distributions so obtained for two plausible values of b are shown in Fig. 4. The broad distribution is completely different for the sharp monomer-dimer distribution we observed earlier for TT by itself 33.
For macromolecules of known molar mass the intrinsic viscosity [η] can provide an important measure of the conformational flexibility (especially when used in conjunction with the sedimentation coefficient, so20,w, and the concentration dependence coefficient ks). Three different and complementary extrapolation methods values to zero concentration (to eliminate complications through non-ideality effects) were used for all 3 samples – all gave good agreement and are shown in Table 1.
Discussion
The hydrodynamic data collectively enable us to establish what is the conformational flexibility of the Hib glycoconjugate and to establish whether it is either the protein component or the carbohydrate component which is more strongly influencing the hydrodynamic properties. There are four approaches, one using the sedimentation data alone, the others using various combinations.
Conformational analysis: translational frictional ratio
The translational frictional ratio, f/fo is a parameter which depends on conformation and molecular expansion through hydration effects34. It can be measured experimentally from the sedimentation coefficient and molar mass:
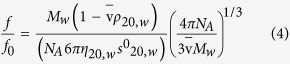 |
where NA is Avogadro’s number. f is the friction coefficient of the molecule and fo the corresponding value for a spherical particle of the same mass and (anhydrous) volume31. Departures from f/fo = 1 are due to either asymmetry (for example the modest asymmetries seen in the tetanus toxoid protein – Table 1 and ref. 33) or an increase in volume due to swelling through particle solvation effects – the high values in Table 1 could be due to either (or both) and without other information it is impossible to distinguish between the two. Combination with viscosity data and how viscosity and the sedimentation coefficient change with molar mas, however, help us to be more specific.
Conformational analysis: the Wales-van Holde ratio
The Wales-van Holde ratio35, R = ks/[η] - where ks is the concentration dependence of the sedimentation coefficient or “Gralen” coefficient - is perhaps the simplest indicator of a macromolecules conformational flexibility in solution. The limits are ~1.6 for a compact sphere or a non-draining random coil, and ~0.1 for a stiff rod36. From Table 1 it seems that the values for the polysaccharide and glycoconjugate are identical (R ~ 0.9) and consistent with a flexible coil structure.
Conformational analysis: Estimation of the Persistence Length L p
For a more quantitative estimate of chain flexibility we can use the persistence length Lp, which has theoretical limits of 0 for a random coil and ∞ for a stiff rod. Practically the limits are ∼1–2 nm for a random coil (such as the polysaccharide pullulan) and ∼200–300 nm for a very stiff rod shaped macromolecule (such as xanthan or schizophyllan)29. Several methods are available for the estimation of Lp using either intrinsic viscosity37,38,39 or sedimentation coefficient40 measurements. For example the Bohdanecky-Bushin relation
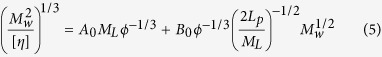 |
where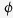 is the Flory-Fox coefficient (2.86 × 1023 mol−1) and A0 and B0 are tabulated coefficients, and the Yamakawa–Fujii equation40
is the Flory-Fox coefficient (2.86 × 1023 mol−1) and A0 and B0 are tabulated coefficients, and the Yamakawa–Fujii equation40
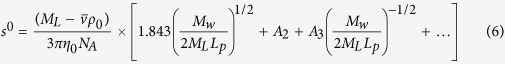 |
Yamakawa and Fujii39 showed that A2 can be considered as –ln(d/2Lp) and A3 = 0.1382 if the Lp is much higher than the chain diameter, d. Difficulties arise if the mass per unit length is not known, although both relations have recently been built into an algorithm Multi-HYDFIT40 which estimates the best estimates or best range of values of Lp and ML based on minimization of a target function Δ. An estimate for the chain diameter d is also required but extensive simulations have shown that the results returned for Lp are relatively insensitive to the value chosen for d which was fixed at an average of ∼0.8 nm41,42. ML and Lp were treated as variables and the minimum value of the target function Δ was estimated on a 2D contour plot for each sample (see Fig. 5) and the values estimated given in Table 2. All the values are consistent with flexible random coil structures with persistence lengths between 4.5 and 7 nm.
Figure 5. Plot of mass per unit length ML versus persistence length Lp evaluation using the multi-HYDFIT procedure of Ortega and Garcia de la Torre41.
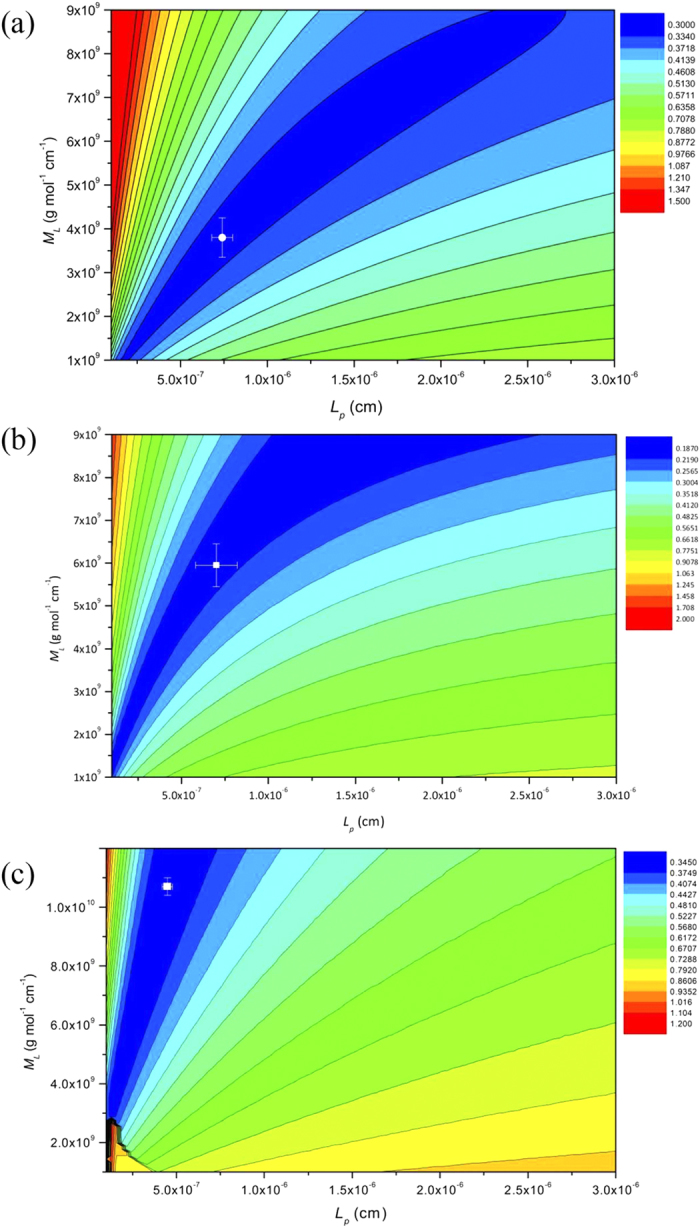
(a) the Hib PRP-native polysaccharide. The plot yields Lp ∼ 7.0 × 10−7 (cm) and ML ∼ 3.8 × 109 (g.mol−1.cm−1) at the minimum target (error) function (indicated by the white cross). (b) Hib PRP-ADH, Lp ∼ 7.0 × 10−7 (cm) and ML ∼ 6.0 × 109 (g. mol−1. cm−1); (c) Hib PRP-TT Lp ∼ 4.5 × 10−7 (cm) and ML ∼ 10.7 × 109 (g. mol−1. cm−1).
Table 2. Values for the mass per unit length ML and the persistence length Lp from global hydrodynamic analysis for PRP-native, PRP-ADH and PRP-TT.
| Sample | 10−7 × ML (g.mol−1.cm−1) | 107 × Lp (cm) |
|---|---|---|
| PRP-native | 380 ± 50 | 7.0 ± 1.0 |
| PRP-ADH | 600 ± 50 | 7.0 ± 1.2 |
| PRP-TT | 1070 ± 50 | 4.5 ± 0.3 |
Sedimentation Conformation Zoning
This high flexibility is confirmed by “Sedimentation Conformation Zoning”, introduced by Pavlov et al.43,44. This involves plotting ksML versus [s]/ML,onto a zonal template established from macromolecules of known conformation type. ks is the Gralen coefficient, ML the mass per unit length and the “intrinsic” sedimentation coefficient [s] is given by:
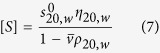 |
with η20,w and ρ20,w the viscosity and density of water at 20.0 °C, and 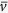 the partial specific volume. Figure 6 – confirms that both the native and activated polysaccharides, and the glycoconjugate are all highly flexible structures (falling just within the Zone D area – randomly coiled structures), and not too different with capsular polysaccharides from Streptococcus pneumoniae45 (Zone C – semi-flexible). The tetanus toxoid protein by itself - slightly extended and hydrodynamically compact34 – is by contrast a “Zone E” particle.
the partial specific volume. Figure 6 – confirms that both the native and activated polysaccharides, and the glycoconjugate are all highly flexible structures (falling just within the Zone D area – randomly coiled structures), and not too different with capsular polysaccharides from Streptococcus pneumoniae45 (Zone C – semi-flexible). The tetanus toxoid protein by itself - slightly extended and hydrodynamically compact34 – is by contrast a “Zone E” particle.
Figure 6. Conformation Zoning plot, Hib PRP-native, PRP-ADH and PRP-TT conjugate (with spacer) all have very flexible structures in the “Zone D” region close to Zone C Zone A: Rigid rod with no flexibility; Zone B: Rigid rod with some flexibility; Zone C: Semi-flexible coil; Zone D: Random coil; Zone E: Globular or heavily branched structures. See ref. 56.
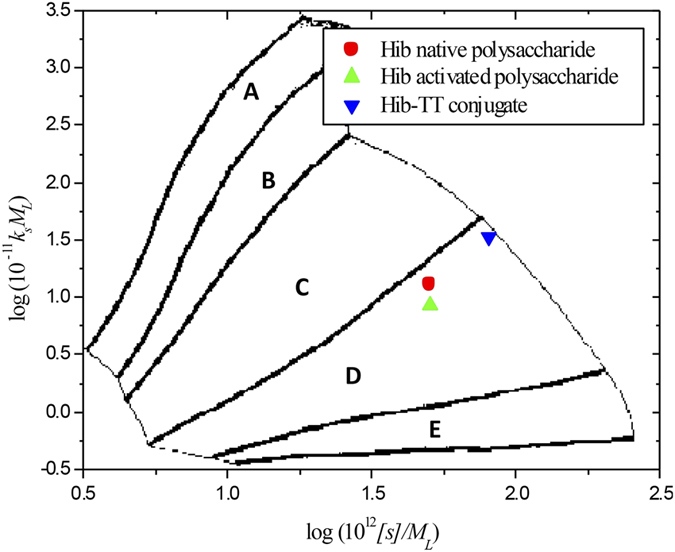
All four conformation approaches (frictional ratio, Wales-van Holde ratio, persistence length and conformation zoning) show that conjugation with the tetanus protein yielded large polydisperse structures of (Mw ~ 7.3 × 106 g/mol), but which retained the high flexibility of the native and activated polysaccharide, similar to what we found earlier for capsular polysaccharides from Streptococcus pneumoniae45 – and very different for the previously characterised tetanus toxoid protein34, as summarized in Table 1. These findings supplement other biophysical studies on other glycoconjugate vaccines. The high flexibility arising from carbohydrate chains is consistent for example with the 1H-NMR relaxation studies of Berti and coworkers46 on Hib conjugated to the non-toxic mutant of diphtheria toxin CRM197: this work also provided evidence of reduced hydration of the protein as a result of the conjugation. Using circular dichroism and fluorescence spectroscopy and 1H-NMR, the protein component of Hib and MenC glycoconjugate vaccines was nonetheless found to be important for pH and thermal stability47,48. In more recent work Pecetta49 and coworkers have provided evidence based on differential scanning calorimetry and circular dichroism of conjugation causing some structural changes of the CRM197 protein.
Concluding Remarks
The flexible chain-like hydrodynamic properties of the PRP-TT glycoconjugates are commensurate with those of a polysaccharide rather than a protein. Tetanus toxoid protein is known to be a globular protein of aspect ratio ~3:1 with a tendancy to form small amounts of dimer (~14%)34. Intrinsic viscosities of the protein are small and typical of globular proteins and very different from the Hib polysaccharide and glycoconjugate. This clearly indicates that it is the carbohydrate component which most strongly influences the physical behaviour of these substances in solution. This study has also shown the usefulness of the analytical ultracentrifuge as a matrix free method for ‘quality control’ assessment of the heterogeneity and conformational flexibility of the Hib vaccine preparations.
Methods
Sample preparation
The bulk Hib manufacturing process consists of the following steps: after initial solid preculture steps, the bacteria are incubated in shake flasks. Once the appropriate cell density is reached, the inoculum is transferred to a fermentor for further amplification. The virulent strain of Hib is grown in a medium supplemented with hematin and nicotinamide adenine dinucleotide (NAD). During the growth phase, the pH is regulated with concentrated NaOH solution and the dissolved oxygen is regulated by the stirring speed and air flow rate. At the onset of the drift phase (7–8 hours after inoculation), the pH and dissolved oxygen regulations are switched off and the parameters are left to drift. The fermentation is terminated 10 hours after the inoculation. The broth containing the polyribosyl (PRP) polysaccharide of interest is inactivated by heat and centrifuged to remove cell debris. Purification is assured by Hib precipitation with Cetavlon in the presence of Celite. The complex PRP-CTAB is an insoluble complex retained on the Celite. The PRP polysaccharide is detached from the Cetavlon by using a high salt concentration (0.5 M NaCl). The product is ultrafiltered to eliminate residual nucleic acids, then precipitated with ethanol, dried and kept at −20 °C until conjugation. The residuals (DNA (<0.2%), proteins (<0.1%), LPS(<0.03%)) were very low in the final bulk of polysaccharide and met all the required specifications of the pharmacopeia (WHO/EMEA).
Hib PRP-polysaccharide activation (using adipic acid dihydrazide as linker) prior to its conjugation was using the cyanogen bromide method50,51,52. By-products were removed by further ultrafiltration (30 kDa cut-off). The conjugation process did not involve oxidization of PRP with periodic acid: the PRP is directly activated. After activation (6 minutes), the ADH is added to form a covalent polysaccharide-ADH product which is then able to be conjugated with the –COOH groups of the tetanus toxoid (TT) protein by using carbodimide conjugation. The conjugation between the activated (PRP-ADH) and the TT protein was performed using EDC (1-ethyl-3-(3-dimethylaminopropyl)-carbodimide hydrochloride)53,54. The Hib PRPTT conjugate was then purified on a Sephacryl S500HR column using 0.2 M NaCl as the elution solution. The conjugate was then sterile filtered before a final ultrafiltration step, which led to the product meeting the required specifications of the pharmacopeia, with <15% of unconjugated polysaccharide and <5% unconjugated protein. All samples were dissolved in phosphate buffered saline pH ~6.8, I = 0.1 M55, at 20.0 °C and mixed by magnetic stirring at room temperature for 24 hours. All solutions were then diluted to the appropriate (total macromolecular) concentrations required.
Sedimentation velocity in the analytical ultracentrifuge
A general description of the ultracentrifugal methods and how they can be used for glycoconjugate and polysaccharide characterisation is given in two recent references29,56. Specifically for this study sedimentation velocity experiments were performed using a Beckman (Palo Alto, CA, USA) Optima XL-I analytical ultracentrifuge equipped with Rayleigh interference optics and an automatic on-line data capture system. Conventional 12 mm double-sector epoxy cells with sapphire windows were loaded with 400 μL of different (total macromolecular) concentrations (0.1–2.0 mg mL−1) of each sample and a matching amount of the corresponding reference buffer (phosphate buffered saline) in appropriate channels. Samples were run at a rotor speed of 45000 rpm (~150,000 g) at a temperature of 20.0 °C. Concentration profiles and the movement of the sedimenting boundary in the analytical ultracentrifuge cell were recorded using the Rayleigh interference optical system and converted to concentration (in units of fringe displacement relative to the meniscus, j) versus radial position, r26. Data was analysed using the least squares boundary ls-g*(s) model incorporated into the SEDFIT analytical algorithm of Dam & Schuck57. SEDFIT generates an apparent distribution of sedimentation coefficients in the form of g*(s) versus s, where s is the sedimentation coefficient (in Svedberg units S = 10−13 sec). The * indicates the profiles are not corrected for diffusion broadening (likely to be small for slow-diffusing polysaccharides and glycoconjugates). This data analysis was followed by the correction to standard solvent conditions - namely the density and viscosity of water at 20.0 °C - to yield s20,W using the algorithm SEDNTERP58, which also incorporates the partial specific volume (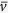 ) of the samples
) of the samples 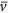 ~ 0.63 mL.g−1. To account for hydrodynamic non-ideality (co-exclusion and backflow effects), the apparent sedimentation coefficients (s20,w) were calculated at a series of different cell loading concentration and extrapolated to infinite dilution using the Gralén relation59:
~ 0.63 mL.g−1. To account for hydrodynamic non-ideality (co-exclusion and backflow effects), the apparent sedimentation coefficients (s20,w) were calculated at a series of different cell loading concentration and extrapolated to infinite dilution using the Gralén relation59:
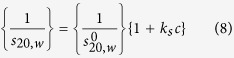 |
where ks is the Gralén or concentration dependence coefficient.
Sedimentation equilibrium in the analytical ultracentrifuge
Sedimentation equilibrium experiments were also performed using the Beckman (Palo Alto, CA, USA) Optima XL-I analytical ultracentrifuge again using the Rayleigh interference optics and an automatic on-line data capture system to record equilibrium concentration distribution profiles. The modified long (20 mm) optical path length double-sector titanium cells with sapphire windows were selected and loaded with 0.070 mL of solution (dialysed at room temperature against the phosphate buffered saline for 48 hours) and a matching amount of reference buffer dialysate in the appropriate channels. Samples were centrifuged at rotor speeds selected to give a sufficient fringe increment from meniscus to base60 i.e. 4000 rpm (~1200 g), 9000 rpm (~5900 g) and 2000 rpm (~300 g) for PRP native, PRP-ADH and the PRP-TT solution, respectively, at a temperature of 20.0 °C. Scans were taken every one hour and equilibrium was reached after approximately 48–72 hours. Optical records (Rayleigh interference profiles) of the relative concentration distribution of the solute at equilibrium were analysed to give the weight (mass) average apparent molar mass Mw,app using the SEDFIT-MSTAR algorithm61. This uses the M* function of Creeth and Harding62, together with the hinge point method (evaluation of the point or weight average molar mass at the radial position in the distribution where the local (total macromolecule) concentration c(r) = the initial loading concentration, c61). The use of long path length cells meant that low loading concentrations could be used to give a sufficient signal (~0.3 mg.mL−1). At such low concentrations, non-ideality effects (which tend to lead to underestimates of the molar mass) may be relatively small and we make the approximation that the apparent weight average molar mass Mw,app is equal to the true weight average molar mass Mw60. We also estimate the apparent z-average molar mass Mz,app using the MFIT algorithm of Ang and Rowe63.
Viscometry
Dynamic viscosity measurements for Hib native, Hib-ADH and Hib-TT, were carried out using the automated micro-viscometer Anton Parr AMVn (Anton Parr, Graz, Austria) at a concentration series (total macromolecular concentration) from 0.1–2.0 mg mL−1 based on the rolling ball viscosity method in which the apparatus measures the time of a (silanized) steel ball needed to roll in a 1.6 mm diameter silanized glass capillary containing the sample. The experiment was performed at different reclining angles of 70° n = 4 times), 60° (n = 4 times) and 50° (n = 6 times) under precise temperature control (20.00 ± 0.01 °C). Huggins64 and Kraemer65 extrapolations forms (see, also ref. 38) were performed to obtain the intrinsic viscosity (Equations 9, 10). Intrinsic viscosities were also estimated using the Solomon – Ciutâ relation (Equation 11)66.
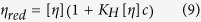 |
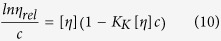 |
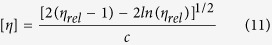 |
where c is the concentration, 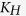 and
and 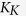 are the Huggins and Kraemer coefficients, repectively.
are the Huggins and Kraemer coefficients, repectively.
Additional Information
How to cite this article: Abdelhameed, A. S. et al. A glycoconjugate of Haemophilus influenzae Type b capsular polysaccharide with tetanus toxoid protein: hydrodynamic properties mainly influenced by the carbohydrate. Sci. Rep. 6, 22208; doi: 10.1038/srep22208 (2016).
Acknowledgments
The authors thank Olivier Laloux and Ghislain Delpierre for helpful discussions. Hiberix and MenHibrix are trademarks of the GSK group of companies. This work was supported by GlaxoSmithKline Biologicals SA. FMA is grateful to the support of the University of Tabuk, Saudi Arabia for a studentship.
Footnotes
PD and KC are, or were at the time of the study, employees of the GSK group of companies.
Author Contributions A.S.A. completed the experimental work and, with F.A. and G.G.A. was responsible for Figs 1,2,3–4. G.A.M. was responsible for Figs 5 and 6. P.D. and K.C. were responsible for the production of the materials. S.E.H. was responsible for directing the research and writing the paper. All authors drafted and reviewed the manuscript.
References
- Gram C. Über die isolierte Färbung der Schizomyceten in Schnitt- und Trockenpräparaten. Fortschr Med 2, 185–189 (1884). [Google Scholar]
- Pon R. A. & Jennings H. J. In Carbohydrate-based Vaccines and Immunotherapies (eds Guo Z. & Boons G.-J.) Ch. 2, 117–166 (Wiley, 2009). [Google Scholar]
- Todar K. On-line Textbook of Bacteriology. (Date of access: 19/05/2015) www.textbookofbacteriology.net/ (2008).
- Ryan K. J. & Ray C. G. In Sherris Medical Microbiology: An Introduction to Infectious Diseases 4th edn (eds Ryan K. J. & Ray C. G.), Ch. 15, 525–528 (McGraw Hill, 2004). [Google Scholar]
- Pfeiffer R. F. J. Vorläufige Mittheilungen über den Erreger der Influenza. Deut Med Wochenschr 18, 28 (1892). [Google Scholar]
- Pfeiffer R. F. J. Die aetiologie der Influenza. Z Hyg Infektionskr 13, 357–386 (1893). [Google Scholar]
- Kuhnert P. & Christensen H. Pasteurellaceae: Biology, Genomics and MolecularAspects (Caister Academic Press, 2008). [Google Scholar]
- Murray P. R. et al. Medical Microbiology. (Elsevier, 1998). [Google Scholar]
- Kelly D. F., Moxon E. R. & Pollard A. J. Haemophilus influenzae type b conjugate vaccines. Immunology 113, 163–174 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamenhof S., Leidy G., Fitzgerald P. L., Alexander H. E. & Chargaff E. Polyribophosphate, the type-specific substance of Hemophilus influenzae, type b. J Biol Chem 203, 695–704 (1953). [PubMed] [Google Scholar]
- Zamenhof S. & Leidy G. Further studies on poly-ribophosphate and other poly-sugarphosphates. Fed Proc 13, 327 (1954). [Google Scholar]
- Rosenberg E. & Zamenhof S. Action of ribonuclease on polyribophosphate and studies on a new polysugarphosphate. Fed Proc 19, 315 (1960). [Google Scholar]
- Rosenberg E. & Zamenhof S. Further studies on polyribophosphate. J Biol Chem 236, 2845–2849 (1961). [PubMed] [Google Scholar]
- Rosenberg E. & Zamenhof S. A new naturally occurring disaccharide, β-D-ribofuranosyl β–D-ribofuranoside. J Biol Chem 237, 1040–1042 (1962). [PubMed] [Google Scholar]
- Anderson P., Peter G., Johnston R. B. J., Wetterlow L. H. & Smith D. H. Immunization of humans with polyribophosphate, the capsular antigen of Hemophilus influenzae, Type b. J Clin Invest 51, 39–44 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R. et al. An Escherichia coli antigen cross-reactive with the capsular polysaccharide of Haemophilus influenzae Type b: Occurrence among known serotypes, and immunochemical and biological properties of E. coli antisera toward H. influenzae Type b. J Immunol 108, 1551–1562 (1972). [PubMed] [Google Scholar]
- European Pharmacopoeia 5.0. Haemophilus type b conjugate vaccine. (The Council of Europe, 2004). [Google Scholar]
- Park J.-C. J., Schneerson R., Robbins J. B. & Schlesselman J. J. Interim report of controlled field trial of immunization with capsular polysaccharides of Haemophilus influenzae type b and group C Neisseria meningitidis in Mercklenburg county, North Carolina (March 1974-March 1976). J Infect Dis 136, S51–S56 (1977). [DOI] [PubMed] [Google Scholar]
- Smith D. H., Peter G., Ingram D. L., Harding A. L. & Anderson P. Responses of children immunized with the capsular polysaccharide of Haemophilus influenzae type b. Pediatrics 5, 637–644 (1973). [PubMed] [Google Scholar]
- Kayhty H., Karanko V., Peltola H. & Makela P. H. Serum antibodies after vaccination with Haemophilus influenzae type b capsular polysaccharide and responses to reimmunization: No evidence of immunologic tolerance or memory. Pediatrics 74, 857–865 (1984). [PubMed] [Google Scholar]
- Astronomo R. D. & Burton D. R. Carbohydrate vaccines: Developing sweet solutions to sticky situations? Nat Rev Drug Discov 9, 308–324 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A. & Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med 152, 361–376 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan T. et al. A novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-Tetanus-toxoid conjugate vaccine is immunogenic and induces immune memory when co-administered with DTPa-HBV-IPV and conjugate pneumococcal vaccines in infants. Vaccine 25, 8487–8499 (2007). [DOI] [PubMed] [Google Scholar]
- Jennings H. J. Capsular polysaccharides as human vaccines. Adv Carbohyd Chem Bi 41, 155–208 (1983). [DOI] [PubMed] [Google Scholar]
- Kabat E. A. & Bezer A. E. The effect of variation in molar mass on the antigenicity of dextran in man. Arch Biochem Biophys 78, 306–318 (1958). [DOI] [PubMed] [Google Scholar]
- Harding S. E. In Analytical Ultracentrifugation: Techniques and Methods (eds Scott D. J., Harding S. E. & Rowe A. J.) Ch. 12, 231–252 (The Royal Society of Chemistry, 2005). [Google Scholar]
- Kök M. S., Abdelhameed A. S., Ang S., Morris G. A. & Harding S. E. A novel global hydrodynamic analysis of the molecular flexibility of the dietary fibre polysaccharide konjac glucomannan. Food Hydrocolloid 23, 1910–1917 (2009). [Google Scholar]
- Harding S. E., Abdelhameed A. S. & Morris G. A. On the hydrodynamic analysis of conformation in mixed biopolymer systems. Polym Int 60, 2–8 (2011). [Google Scholar]
- Harding S. E. et al. Ultracentrifuge methods for the analysis of polysaccharides, glycoconjugates and ligands. Method Enzymol 562, 391–439 (2015). [DOI] [PubMed] [Google Scholar]
- Lockyer A., Gao F., Derrick J. P. & Bolgiano B. Structural correlates of carrier protein recognition in tetanus toxoid-conjugated bacterial polysaccharide vaccines. Vaccine 33, 1345–1352 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S. E. et al. Extended Fujita approach to the molar mass distribution of polysaccharides and other polymeric systems. Methods 54, 136–144 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. A., Adams G. G. & Harding S. E. On hydrodynamic methods for the analysis of the sizes and shapes of polysaccharides in dilute solution: A short review. Food Hydrocolloid 42, 318–334 (2014). [Google Scholar]
- Abdelhameed A. S. et al. An asymmetric and slightly dimerized structure for the tetanus toxoid protein used in glycoconjugate vaccines. Carbohyd Polym. 90, 1831–1835 (2012). [DOI] [PubMed] [Google Scholar]
- Tanford C. Physical Chemistry of Macromolecules. (John Wiley & Sons, 1961). [Google Scholar]
- Wales M. & van Holde K. E. The concentration dependence of the sedimentation constants of flexible macromolecules. J Polym Sci 14, 81–86 (1954). [Google Scholar]
- Creeth J. M. & Knight C. G. J. On the estimation of the shape of macromolecules from sedimentation and viscosity measurements. Biochim Biophys Acta 102, 549–558 (1965). [DOI] [PubMed] [Google Scholar]
- Bushin S., Tsvetkov V., Lysenko E. & Emelianov V. The sedimentation diffusion and viscometric analysis of the conformation properties and molecular rigidity of ladder-like polyphenyl siloxane in solution. Vysokomol Soedin A 23, 2494–2503 (1981). [Google Scholar]
- Bohdanecky M. New method for estimating the parameters of the wormlike chain model from the intrinsic viscosity of stiff-chain polymers. Macromolecules 16, 1483–1492 (1983). [Google Scholar]
- Harding S. E. The intrinsic viscosity of biological macromolecules. Progress in measurement, interpretation and application to structure in dilute solution. Prog Biophys Mol Bio 68, 207–262 (1997). [DOI] [PubMed] [Google Scholar]
- Yamakawa H. & Fujii M. Translational friction coefficient of wormlike chains. Macromolecules 6, 407–415 (1973). [Google Scholar]
- Ortega A. & Garcia de la Torre J. Equivalent radii and ratios of radii from solution properties as indicators of macromolecular conformation, shape, and flexibility. Biomacromolecules 8, 2464–2475 (2007). [DOI] [PubMed] [Google Scholar]
- Morris G. A. et al. Molecular flexibility of citrus pectins by combined sedimentation and viscosity analysis. Food Hydrocolloid 22, 1435–1442 (2008). [DOI] [PubMed] [Google Scholar]
- Pavlov G. M., Harding S. E. & Rowe A. J. Normalized scaling relations as a natural classification of linear macromolecules according to size. Prog Coll Pol Sci S 113, 76–80 (1999). [Google Scholar]
- Pavlov G. M., Rowe A. J. & Harding S. E. Conformation zoning of large molecules using the analytical ultracentrifuge. TRAC-Trend Anal Chem 16, 401–405 (1997). [Google Scholar]
- Harding S. E. et al. Solution properties of capsular polysaccharides from Streptococcus pneumoniae. Carbohyd Polym 90, 237–242 (2012). [DOI] [PubMed] [Google Scholar]
- Berti F., Costantino P., Fragai M. & Luchinat C. Water accessibility, aggregation, and motional features of polysaccharide-protein conjugate vaccines. Biophys J 86, 3–9 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. M., Lemercinier X., Bolgiano B., Crane D. & Corbel M. J. Solution stability studies of the subunit components of meningococcal C oligosaccharide-CRM197 conjugate vaccines. Biotechnol Appl Bioc 33, 91–98 (2001). [DOI] [PubMed] [Google Scholar]
- Crane D. T., Bolgiano B. & Jones C. Comparison of the diphtheria mutant toxin, CRM197, with a Haemophilus influenzae type-b polysaccharide-CRM197 conjugate by optical spectroscopy. Eur J Biochem 246, 320–327 (1997). [DOI] [PubMed] [Google Scholar]
- Pecetta S. et al. Carrier priming with CRM197 or diptheria toxoid has a different impact on the immunogenicity of the respective glycoconjugates: Biophysical and immunochemical interpretation. Vaccine 33, 314–320 (2015). [DOI] [PubMed] [Google Scholar]
- Kohn J. & Wilchek M. A new approach (cyano-transfer) for cyanogen bromide activation of Sepharose at neutral pH, which yields activated resins, free of interfering nitrogen derivatives Biochem Bioph Res Co 107, 878–884 (1982). [DOI] [PubMed] [Google Scholar]
- Kohn J. & Wilchek M. Mechanism of activation of Sepharose and Sephadex by cyanogen bromide. Enzyme Microb Tech 4, 161–163 (1982). [Google Scholar]
- Kohn J. & Wilchek M. The use of cyanogen bromide and other novel cyanylating agents for the activation of polysaccharide resins. Appl Biochem Biotech 9, 285–305 (1984). [Google Scholar]
- Sheehan J., Cruickshank P. & Boshart G. Notes- A convenient synthesis of water soluble carbodiimides. J Org Chem 26, 2525–2528 (1961). [Google Scholar]
- Hermanson G. T. Bioconjugate Techniques 1st edn (Academic Press, 1996). [Google Scholar]
- Green A. A. The preparation of acetate and phosphate buffer solutions of known pH and ionic strength. J Am Chem Soc 55, 2331–2336 (1933). [Google Scholar]
- Harding S. E., Abdelhameed A. S., Gillis R. B., Morris G. A. & Adams G. G. Characterization of capsular polysaccharides and their glycoconjugates by hydrodynamic methods. Methods Mol Biol 1331, 211–227 (2015) [DOI] [PubMed] [Google Scholar]
- Dam J. & Schuck P. Calculating sedimentation coefficient distributions by direct modeling of sedimentation velocity concentration profiles. Method Enzymol 384, 185–212 (2004). [DOI] [PubMed] [Google Scholar]
- Laue T. M., Shah B. D., Ridgeway T. M. & Pelletier S. L. In Analytical Ultracentrifugation in Biochemistry and Polymer Science (eds Harding S. E., Rowe A. J. & Horton J. C.) Ch. 6, 90–125 (The Royal Society of Chemistry, 1992). [Google Scholar]
- Gralén N. Sedimentation and diffusion measurements on cellulose and cellulose derivatives, PhD Dissertation, University of Uppsala, (1944). [Google Scholar]
- Harding S. E. Challenges for the modern analytical ultracentrifuge analysis of polysaccharides. Carbohyd Res 340, 811–826 (2005). [DOI] [PubMed] [Google Scholar]
- Schuck P. et al. SEDFIT–MSTAR: molar mass and molar mass distribution analysis of polymers by sedimentation equilibrium in the ultracentrifuge. Analyst 139, 79–92 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeth J. M. & Harding S. E. Some observations on a new type of point average molecular weight. J Biochem Bioph Meth 7, 25–34 (1982). [DOI] [PubMed] [Google Scholar]
- Ang S. & Rowe A. J. Evaluation of the information content of sedimentation equilibrium data in self-interacting systems. Macromol Biosci 10, 798–807 (2010). [DOI] [PubMed] [Google Scholar]
- Huggins M. L. The viscosity of long chain molecules IV: Dependance on concentration. J Am Chem Soc 64, 2716–2718 (1942). [Google Scholar]
- Kraemer E. O. Molecular weights of cellulose and cellulose derviatives. Ind Eng Chem 30, 1200–1203 (1983). [Google Scholar]
- Solomon O. F. & Ciuta I. Z. Détermination de la viscosité intrinsèque de solutions de polymères par une simple détermination de la viscosité. J Appl Polym Sci 6, 683–686 (1962). [Google Scholar]