Abstract
AIM: To examine the expression of SphK1, an oncogenic kinase that produces sphingosine 1-phosphate (S1P), and its correlation with the expression of LPAR2, a major lysophosphatidic acid (LPA) receptor overexpressed in various cancers, in human colorectal cancer.
METHODS: Real-time reverse-transcription polymerase chain reaction was used to measure the mRNA expression of SphK1, LPAR2, and the three major S1P receptors in 27 colorectal cancer samples and corresponding normal tissue samples. We also examined the correlation between the expression of SphK1 and LPAR2.
RESULTS: Colorectal cancer tissue in 22 of 27 patients had higher levels of SphK1 mRNA than in normal tissue. In two-thirds of the samples, SphK1 mRNA expression was more than two-fold higher than in normal tissue. Consistent with previous reports, LPAR2 mRNA expression in 20 of 27 colorectal cancer tissue samples was higher compared to normal tissue samples. Expression profiles of all three major S1P receptors, S1PR1, S1PR2, and S1PR3, varied without any trend, with no significant difference in expression between cancer and normal tissues. A highly significant positive correlation was found between SphK1 and LPAR2 expression [Pearson’s correlation coefficient (r) = 0.784 and P < 0.01]. The mRNA levels of SphK1 and LPAR2 did not correlate with TNM stage.
CONCLUSION: Our findings suggest that S1P and LPA may play important roles in the development of colorectal cancer via the upregulation of SphK1 and LPAR2, both of which could serve as new therapeutic targets in the treatment of colorectal cancer.
Keywords: Sphingosine kinase 1, Lysophosphatidic acid receptor 2, Carcinogenesis, Colorectal cancer, Sphingosine 1-phosphate
Core tip: This is the first study examining the mRNA expression of SphK1, an oncogenic kinase that produces sphingosine 1-phosphate (S1P), at the mRNA level and its correlation with the expression of lysophosphatidic acid receptor 2 (LPAR2), a major lysophosphatidic acid (LPA) receptor overexpressed in various cancers, in human colorectal cancer. Colorectal cancer tissue in 22 of 27 patients had higher levels of SphK1 mRNA than in normal tissue. A highly significant positive correlation was found between SphK1 and LPAR2 expression (r = 0.784 and P < 0.01). Our findings suggest that S1P and LPA may play important roles in the development of colorectal cancer via the upregulation of SphK1 and LPAR2.
INTRODUCTION
Two simple lysophospholipids, sphingosine 1-phosphate (S1P) and lysophosphatidic acid (LPA), are natural bioactive mediators of diverse cellular processes. The biological effects of these serum-borne lipids are mainly mediated by a family of G protein-coupled receptors, six specific for LPA and five specific for S1P, termed LPAR1-6 and S1PR1-5, respectively[1]. Lymphocyte egress from both the thymus and peripheral lymphoid organs depends on S1PR1[2]. FTY720 (known as fingolimod) is a pro-drug which alters lymphocyte trafficking via S1PR1. FTY720 itself is not bioactive, but when phosphorylated (FTY720-P) by sphingosine kinase, it becomes active and modulates the activity of S1P receptors. In more than 80 countries, FTY720 has been approved and clinically used to treat multiple sclerosis, a common autoimmune disorder affecting the central nervous system[3].
Both S1P and LPA are also implicated in the etiology of cancer due to their involvement in tumor growth, angiogenesis, and metastatic potential[4-6]. Several lines of evidence support the importance of these lysolipids in colon cancer, one of the most common causes of cancer-related deaths worldwide[4,7,8]. Colorectal cancer with ulcers is often associated with local bleeding, subjecting cancer cells to high concentrations of platelet-derived mediators. These mediators include S1P and LPA, which induce various important biological responses. For example, both S1P and LPA can transactivate epidermal growth factor receptor (EGFR), c-Met, and ErbB-2, all three of which are prognostic markers of gastrointestinal cancers that correlate with poor clinical outcomes[9,10].
Sphingosine kinase 1 (SphK1) is the key kinase that produces S1P. There is strong evidence from cellular and animal systems that SphK1 is a major player in oncogenesis, contributing to cell survival, proliferation, and transformation, the prevention of apoptosis, and the stimulation of angiogenesis[5,11,12]. Thus, SphK1 is considered an oncogenic kinase. There is also evidence from clinical samples that SphK1 is overexpressed in many human cancers, such as breast cancer, prostate cancer, ovarian cancer, lung cancer, and acute myeloid leukemia[5,12]. Increased SphK1 expression is associated with poor prognosis in patients with certain cancers, such as glioblastoma, breast cancer, and gastric cancer[12-14]. Human colon cancers are positive for SphK1 by immunohistochemistry, whereas normal colon mucosa is negative or stains weakly[15,16]. These previous reports have focused on the expression of SphK1 protein; however, little is known about the expression of SphK1 transcripts in human colorectal cancer.
Malignant transformation in ovarian and thyroid cancers results in the aberrant expression of lysophosphatidic acid receptor 2 (LPAR2) (and LPAR3 in ovarian cancer), suggesting that LPA receptor expression shifts during malignant transformation in these cancer types[17,18]. In human colorectal cancer tissue, we previously found that LPAR2 is overexpressed at both the mRNA and protein levels[19]. Thus, LPA and one of its receptors, LPAR2, appear to play a role in tumor biology.
We recently found that LPA markedly upregulates the expression of SphK1 and the S1P receptor, S1PR3, in MKN1 gastric cancer cells via the LPAR1 and EGFR transactivation[4]. SphK1 and S1PR3 are critical for LPA-induced chemotaxis and invasion in these cells[4]. These findings provide evidence for crosstalk between LPA and S1P signaling, and reveal a key role for SphK1 in integrating events downstream of LPA and EGF receptors. Thus, we considered it informative to examine the expression of not only LPAR2 mRNA, but also SphK1 mRNA, in human colorectal cancer tissue and to examine the correlation between the two in order to better understand how LPA and S1P contribute to the development of colorectal cancer.
MATERIALS AND METHODS
Patients
Twenty-seven patients with colorectal cancer who underwent surgical resection in the Department of Surgery, Tokyo Metropolitan Bokutoh Hospital, between March 2010 and March 2011 were enrolled in this study. Patients who received preoperative radiotherapy and/or chemotherapy were excluded. Histological examination was performed routinely for all tumors, and all were diagnosed as adenocarcinoma. All cases were staged according to the American Joint Commission on Cancer (AJCC) TNM classification (7th edition). This study was approved by the Institutional Review Board (IRB) of Tokyo Metropolitan Bokutoh Hospital (IRB code: 32-Heisei22). Written informed consent was obtained from all patients who participated.
Isolation of total RNA and reverse transcription
Tumor tissue from the resected primary lesion and paired non-tumor tissue (taken 10 cm away from the neoplasm) were immediately frozen in liquid nitrogen and kept at -80 °C until RNA extraction. Total RNA was extracted from each sample as described previously[19]. Total RNA (1 μg) was reverse-transcribed using PrimeScript® RT reagent kit with gDNA Eraser (Takara Bio, Inc., Shiga, Japan). The reverse transcription reaction was carried out in a total volume of 20 μL according to the manufacturer’s instructions. The cDNA was stored at -20 °C until use.
Real-time fluorescence-based quantitative PCR
Gene expression of SphK1, LPAR2, S1PR1, S1PR2, S1PR3, and β-actin were examined. All primers were chosen from TaqMan® Gene Expression Assays (Life Technologies Co.). Real-time PCR was performed with a StepOne™ System (Life Technologies Co.) using TaqMan® probes with the following profile: one step at 50 °C for 2 min, one step at 95 °C for 10 min, and 40 cycles at 95 °C for 30 s and 60 °C for 1 min. Thermocycling was performed in a final volume of 20 μL containing 1 μL of cDNA sample. StepOne™ software was used to construct a calibration curve by plotting the crossing point (Cp) vs the logarithm of the number of copies for each calibrator. The number of copies in unknown samples was calculated by comparing their Cps with the calibration curve. To correct for differences in both RNA quality and quantity between samples, data were normalized using the ratio of the target cDNA concentration to that of β-actin.
Statistical analysis
Pearson’s product-moment correlation coefficient (r) was used to analyze the relationship between SphK1 and LPAR2 expression, using commercially available software (Microsoft Excel, Microsoft Co., WA, United States). Relationships between the expression of SphK1 and each S1P receptor, as well as LPAR2 and each S1P receptor, were also examined using Pearson’s product-moment correlation coefficient. P < 0.01 was considered significant.
RESULTS
Patient characteristics
Patient characteristics are summarized in Table 1. Twenty-seven patients (20 male and 7 female) were enrolled. The median age was 68 years (range, 45-91 years). The expression of SphK1, LPAR2, three S1P receptors, and β-actin mRNA was evaluated in colorectal cancer tissue samples and corresponding normal tissue samples from the 27 patients.
Table 1.
Patient characteristics
| Sex (male/female) | 20/7 |
| Age (yr) | 68 (range: 45-91) |
| Tumor location | |
| Right colon | 7 |
| Left colon | 15 |
| Rectum | 5 |
| Histological type | |
| Well-differentiated adenocarcinoma | 7 |
| Moderately-differentiated adenocarcinoma | 19 |
| Mucinous adenocarcinoma | 1 |
| Tumor size (cm) | 5.2 ± 1.7 |
| Tumor depth (T1/T2/T3/T4a/T4b) | 0/1/15/6/5 |
| Stage (UICC-7th) | |
| I/II/IIIa/IIIb/IIIc/IV | 1/12/0/4/2/8 |
Quantification of SphK1 mRNA in colorectal cancer tissue and normal tissue
Figure 1A shows ratios of SphK1 mRNA (normalized to β-actin mRNA) in cancer tissue to those in normal tissue. Colorectal cancer tissue in 22 of 27 patients expressed a higher level of SphK1 mRNA relative to normal tissue, whereas four patients had a lower level in colorectal cancer tissue and one patient had similar levels between colorectal cancer tissue and normal tissue. In two-thirds of the samples, expression levels of SphK1 mRNA in cancer tissue were more than two-fold that in normal tissue.
Figure 1.
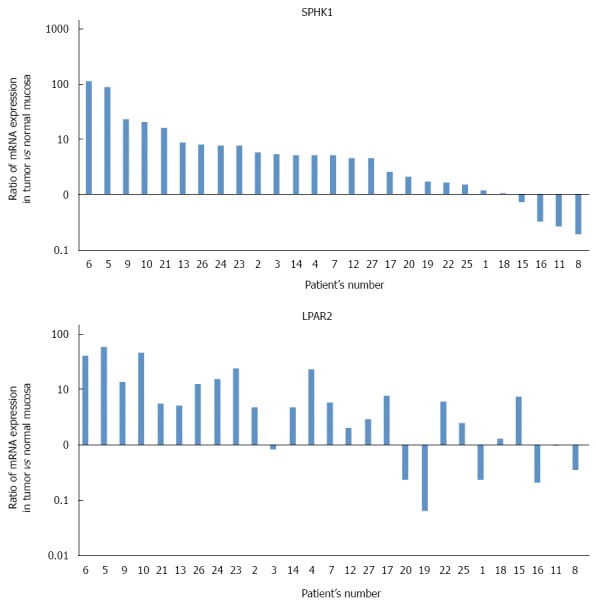
Ratios of SphK1 mRNA (A) and LPAR2 mRNA (B) in cancer tissue to those in normal tissue. Both normalized to β-actin mRNA, as measured by real-time reverse-transcription polymerase chain reaction. In two-thirds of the samples, expression of SphK1 mRNA in cancer tissue was more than two-fold that of normal tissue. Colorectal cancer tissue in 20 of 27 patients expressed a higher level of LPAR2 mRNA than in normal tissue.
Quantification of LPAR2 mRNA in colorectal cancer tissue and normal tissue
Figure 1B shows ratios of LPAR2 mRNA (normalized to β-actin mRNA) in cancer tissue to those in normal tissue. Colorectal cancer tissue in 20 of 27 patients expressed a higher level of LPAR2 mRNA relative to normal tissue, whereas seven patients had a lower level in colorectal cancer tissue. These results are consistent with our previous data using colorectal cancer tissue from a different patient population[19].
Quantification of S1P receptor mRNA in colorectal cancer tissue and normal tissue
Expression profiles of three major S1P receptors, S1PR1, S1PR2, and S1PR3, were also examined. Figure 2 shows ratios of S1P receptor mRNA (normalized to β-actin mRNA) in cancer tissue to those in normal tissue. Expression profiles of all three major S1P receptors varied without any apparent trend, with no significant difference between cancer tissue and normal tissue.
Figure 2.
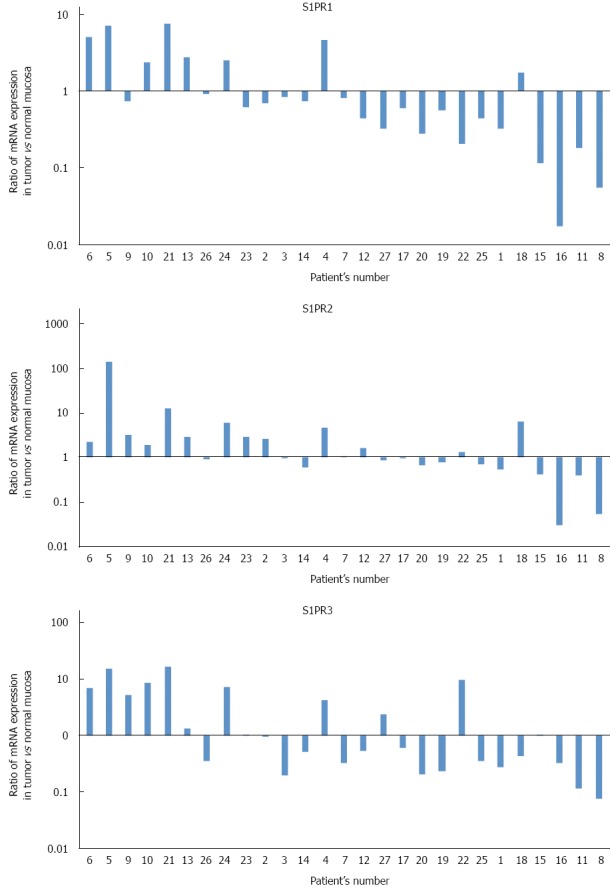
Ratios of sphingosine 1-phosphate receptor mRNA (S1PR1, S1PR2 and S1PR3), normalized to β-actin mRNA, in cancer tissue to those in normal tissue, as measured by real-time reverse-transcription polymerase chain reaction. Expression profiles of all three major sphingosine 1-phosphate (S1P) receptors varied without any clear trend, with no significant difference between cancer tissue and normal tissue.
Correlation of SphK1 expression and LPAR2 expression in human colorectal cancer tissue
We next determined whether there was a correlation between SphK1 and LPAR2 expression. A highly significant positive correlation was observed between SphK1 and LPAR2 expression (Figure 3). Pearson’s correlation coefficient (r) and the corresponding P value for this correlation were r = 0.784 and P < 0.01, respectively (Table 2). Correlation coefficients (r) between SphK1 and each S1P receptor were all less than 0.67, suggesting the lack of a strong correlation. Similarly, correlation coefficients (r) between LPAR2 and each S1P receptor were all less than 0.64, again revealing the lack of a strong correlation (Table 2).
Figure 3.
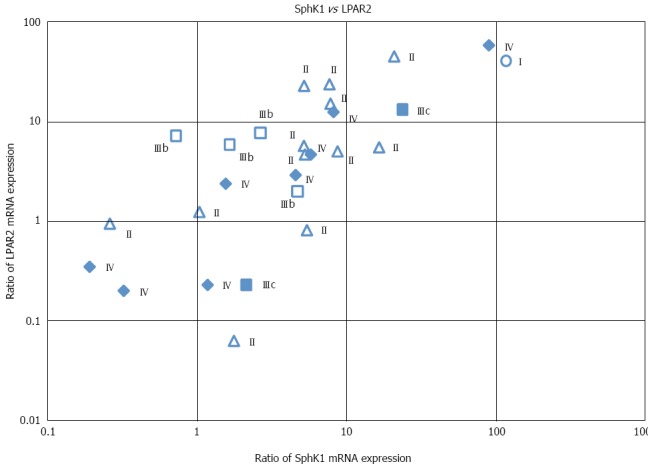
Correlation between SphK1 and LPAR2 mRNA expression in human colorectal cancer tissue. Cancer stages are indicated. Pearson’s correlation coefficient (r) and the corresponding P value for the correlation were r = 0.784 and P < 0.01, respectively.
Table 2.
Correlation coefficients (r) between levels of SphK1, LPAR2, and each S1P receptor
| LPAR2 | SPHK1 | S1PR1 | S1PR2 | S1PR3 | ||
| LPAR2 | Pearson correlation | 1 | 0.7841 | 0.6381 | 0.6391 | 0.620 |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.001 | ||
| SPHK1 | Pearson correlation | 0.7841 | 1 | 0.6611 | 0.577 | 0.553 |
| Sig. (2-tailed) | 0.000 | . | 0.000 | 0.002 | 0.003 | |
| S1PR1 | Pearson correlation | 0.6381 | 0.6611 | 1 | 0.587 | 0.8151 |
| Sig. (2-tailed) | 0.000 | 0.000 | . | 0.001 | 0.000 | |
| S1PR2 | Pearson correlation | 0.6391 | 0.577 | 0.587 | 1 | 0.576 |
| Sig. (2-tailed) | 0.000 | 0.002 | 0.001 | . | 0.002 | |
| S1PR3 | Pearson correlation | 0.620 | 0.553 | 0.8151 | 0.576 | 1 |
| Sig. (2-tailed) | 0.001 | 0.003 | 0.000 | 0.002 | . |
Correlation is significant at the 0.01 level (2-tailed).
Relationship between expression of SphK1 and LPAR2 and clinicopathological factors
We also examined the correlation between SphK1 and LPAR2 expression and various clinicopathological factors. SphK1 mRNA levels did not correlate with tumor location, histological type, tumor depth (data not shown), or TNM stage (Figure 3). Similarly, LPAR2 mRNA levels did not correlate with tumor depth, lymph node metastasis, histological grading (data not shown), or TNM stage (Figure 3). These results are consistent with our previous data[19].
DISCUSSION
In this study, we found that SphK1 mRNA was markedly upregulated in human colorectal cancer tissue, and that SphK1 and LPAR2 mRNA expression was strongly correlated in this cancer type. Quantitative real-time reverse-transcription polymerase chain reaction (RT-PCR) was used to quantify SphK1, LPAR2, and S1P receptor mRNA levels in colorectal cancer and normal mucosa obtained by surgical resection, the reliability of which we previously confirmed with LPAR2 mRNA measurements[19].
Previous studies have shown that SphK1 is overexpressed in many human cancers, including breast cancer, prostate cancer, ovarian cancer, lung cancer, stomach cancer, and acute myeloid leukemia[5,12]. Moreover, increased SphK1 expression is associated with poor prognosis in patients with certain cancers, such as glioblastoma, breast cancer, and gastric cancer[12-14]. Thus, SphK1 may serve as a prognostic biomarker for several cancers. With respect to colon cancer, previous studies reported that most colon cancer tissue samples stained positively for SphK1 protein, whereas normal colon mucosa had negative or weak staining by immunohistochemistry[15,16]. Similarly, in this study, we found that human colorectal cancer expresses high levels of SphK1 mRNA relative to normal mucosa.
S1P produced by upregulation of SphK1 was recently shown to link chronic intestinal inflammation to colitis-associated cancer in mice[20]. Moreover, deletion of the Sphk1 gene in the Min mouse, in which intestinal adenomas develop spontaneously, resulted in reduction of adenoma size[21]. In a mouse model of colitis-driven colon cancer, development of colon cancer was also shown to be suppressed by a small molecule inhibitor of sphingosine kinase[11]. Similarly, in rats, the expression of SphK1 protein is upregulated in colon tumors induced by azoxymethane[22]. These results collectively suggest that upregulation of SphK1 mRNA in human colorectal cancer likely plays a role in tumorigenesis. We also found that the expression of three major S1P receptors, S1PR1, S1PR2, and S1PR3, varied in colorectal cancer tissue without any clear trend whereas SphK1, the key S1P-producing kinase, is upregulated.
The biological effects of LPA are mainly mediated by a family of G protein-coupled receptors, five of which are specific for LPA (LPAR1-6)[23]. These receptors differ with respect to their tissue distribution. For example, LPAR1 is broadly expressed, whereas the expression of LPAR2 and LPAR3 is more restricted, which may account for the various biological effects of LPA[23]. LPAR1 and LPAR2 knockout mice have different phenotypes. Deletion of Lpar1 in mice results in craniofacial dysmorphism, semi-lethality due to defective suckling behavior, and generation of a small fraction of pups with frontal hematoma[24]. In contrast, Lpar2(-/-) mice are born at the expected frequency and display no obvious phenotypic abnormalities[25]. In many colorectal cancer cell lines, at least one LPA receptor is expressed[19]. LPAR1 expression has been linked to cell migration, LPAR2 expression to the production of neovascularizing factors (e.g., IL-8, IL-6, and VEGF), and LPAR3 expression to cell survival. Yet, each of the LPA receptors has the ability to mediate the major functions of LPA, with the relative efficiency determined by the spectrum of receptors activated[8,26]. We previously reported that human colorectal cancers express LPAR1 mRNA at a significantly lower level, and LPAR2 mRNA at a significantly higher level, compared to normal tissue, resulting in a marked increase in the ratio of LPAR2/LPAR1 during malignant transformation (18-fold increase). This supports the characterization of LAPR2 as an oncogene[19]. Indeed, the absence of LPAR2 attenuates tumor formation in an experimental mouse model of colitis-associated cancer[27]. Our present results also revealed high expression of LPAR2 mRNA in human colorectal cancer tissue as compared with normal mucosa, which is consistent with our findings from a different colorectal cancer patient population[19]. These results strongly suggest that LPAR2 expression in colorectal tissue is universally upregulated during malignant transformation of human colorectal cancer. With respect to other tissues, malignant transformation also resulted in aberrant expression of LPAR2 in ovarian and thyroid cancers, suggesting that shifts of LPA receptor expression during malignant transformation are involved in the progression of these cancer types[17,18]. Taken together, these data suggest that overexpression of LPAR2 may be a common finding in a wide range of human cancer tissues and that LPAR2 may be a key receptor involved in the development of various cancers.
In this study, we found a strong positive correlation between SphK1 and LPAR2 mRNA expression in human colorectal cancer (r = 0.784). Although LPAR2 is an oncogenic receptor and SphK1, the key S1P-producing kinase, is associated with cancer, it was somewhat surprising that two leading characters of lysophospholipids and cancer, SphK1 and LPAR2, were strongly correlated in human colorectal cancer. SphK1 also plays a role in linking LPA and S1P, and their cognate G protein-coupled receptors with the EGF tyrosine kinase receptor[4]. These results suggest that SphK1 may control several amplification loops that contribute to cancer progression[5].
The limitation of this study is that there is only one kind of experimentation done to assess the expression of the molecules and the conclusion are based on the results using quantitative real-time RT-PCR, which has become standard method for investigating expression of mRNA because of simplicity and high reproducibility. In our previous paper we have already confirmed mRNA expression of LPA receptors measured by real-time RT-PCR is in consistency with that measured by Northern blot analysis[19]. In addition, in this study, we examined real-time RT-PCR of LPA receptors and SphK1 several times for each sample, and the results were highly reproducible (data not shown). Thus, the data of mRNA expression obtained from RT-PCR method in this study seemed very reliable. Moreover, several groups including us have already confirmed correlation between mRNA expression and protein expression using Western blot analysis and immunohistochemical staining of LPA receptors as well as SphK1[13,19,28]. From these reasons, we consider our mRNA results are reliable and have significant meanings in reality, although further investigation will be needed.
Recently, FTY720 (known as fingolimod) was approved and is now used to treat multiple sclerosis in more than 80 countries[3]. FTY720 is the gold standard of S1P-centric drugs and illustrates the therapeutic value of modulating SphK1 and S1P receptor functions. In the context of cancer, FTY720 was shown to interfere with the SphK1/S1P/S1PR1 axis and suppress the NF-κB/IL-6/Stat3 malicious amplification loop and colitis-associated cancer in mice[20]. This suggests the possibility that FTY720 may also be useful for treating colorectal cancer in humans.
In summary, SphK1 expression was markedly upregulated in human colorectal cancer tissue, and, interestingly, its expression strongly correlated with that of LPAR2. These findings provide evidence for crosstalk between two simple lysophospholipids, LPA and S1P. Given SphK1’s role in linking LPA and S1P, it may serve as an attractive therapeutic target for treating colorectal cancer.
COMMENTS
Background
Two simple lysophospholipids, sphingosine 1-phosphate (S1P) and lysophosphatidic acid (LPA), are natural bioactive mediators of diverse cellular processes. The biological effects of these serum-borne lipids are mainly mediated by a family of G protein-coupled receptors, six specific for LPA and five specific for S1P, termed LPAR1-6 and S1PR1-5, respectively.
Research frontiers
Both S1P and LPA are also implicated in the etiology of cancer due to their involvement in tumor growth, angiogenesis, and metastatic potential. Crosstalk between LPA and S1P signaling and SphK1 which produce S1P as its key role were revealed recently.
Innovations and breakthroughs
This is the first study examining the mRNA expression of SphK1, an oncogenic kinase that produces S1P, at the mRNA level and its correlation with the expression of lysophosphatidic acid receptor 2 (LPAR2), a major LPA receptor overexpressed in various cancers, in human colorectal cancer. Colorectal cancer tissue in 22 of 27 patients had higher levels of SphK1 mRNA than in normal tissue. A highly significant positive correlation was found between SphK1 and LPAR2 expression (r = 0.784 and P < 0.01).
Applications
Current findings suggest that S1P and LPA may play important roles in the development of colorectal cancer via the upregulation of SphK1 and LPAR2.
Terminology
Lymphocyte egress from both the thymus and peripheral lymphoid organs depends on S1PR1. FTY720 (known as fingolimod) is a pro-drug which alters lymphocyte trafficking via S1PR1. FTY720 itself is not bioactive, but when phosphorylated (FTY720-P) by sphingosine kinase, it becomes active and modulates the activity of S1P receptors. In more than 80 countries, FTY720 has been approved and clinically used to treat multiple sclerosis, a common autoimmune disorder affecting the central nervous system.
Peer-review
This is quite an interesting study showing positive correlation between SphK1 and LPAR2 and the development of colorectal. However, the results are solely relied on RT-PCR data without further validation. What would be highly advisable is to screen the replicas of the same tissue samples on a protein level as most of the known functions for the above two genes are performed by encoded proteins.
Footnotes
Supported by Grant 2010 from Tokyo Metropolis, Japan.
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of Tokyo Metropolitan Bokutoh Hospital (IRB code: 32-Heisei22).
Conflict-of-interest statement: The authors declare that they have no competing interests.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 12, 2015
First decision: November 5, 2015
Article in press: December 14, 2015
P- Reviewer: Lyakhovich A, Sameer AS S- Editor: Yu J L- Editor: A E- Editor: Liu XM
References
- 1.Kihara Y, Maceyka M, Spiegel S, Chun J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br J Pharmacol. 2014;171:3575–3594. doi: 10.1111/bph.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 3.Pelletier D, Hafler DA. Fingolimod for multiple sclerosis. N Engl J Med. 2012;366:339–347. doi: 10.1056/NEJMct1101691. [DOI] [PubMed] [Google Scholar]
- 4.Shida D, Fang X, Kordula T, Takabe K, Lépine S, Alvarez SE, Milstien S, Spiegel S. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res. 2008;68:6569–6577. doi: 10.1158/0008-5472.CAN-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S. Targeting SphK1 as a new strategy against cancer. Curr Drug Targets. 2008;9:662–673. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 7.Shida D, Kitayama J, Yamaguchi H, Yamashita H, Mori K, Watanabe T, Nagawa H. Lysophosphatidic acid transactivates both c-Met and epidermal growth factor receptor, and induces cyclooxygenase-2 expression in human colon cancer LoVo cells. World J Gastroenterol. 2005;11:5638–5643. doi: 10.3748/wjg.v11.i36.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, Watanabe T, Takuwa Y, Nagawa H. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res. 2003;63:1706–1711. [PubMed] [Google Scholar]
- 9.Shida D, Kitayama J, Yamaguchi H, Yamashita H, Mori K, Watanabe T, Yatomi Y, Nagawa H. Sphingosine 1-phosphate transactivates c-Met as well as epidermal growth factor receptor (EGFR) in human gastric cancer cells. FEBS Lett. 2004;577:333–338. doi: 10.1016/j.febslet.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Shida D, Kitayama J, Yamaguchi H, Yamashita H, Mori K, Watanabe T, Nagawa H. Lysophospholipids transactivate HER2/neu (erbB-2) in human gastric cancer cells. Biochem Biophys Res Commun. 2005;327:907–914. doi: 10.1016/j.bbrc.2004.12.088. [DOI] [PubMed] [Google Scholar]
- 11.Chumanevich AA, Poudyal D, Cui X, Davis T, Wood PA, Smith CD, Hofseth LJ. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis. 2010;31:1787–1793. doi: 10.1093/carcin/bgq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vadas M, Xia P, McCaughan G, Gamble J. The role of sphingosine kinase 1 in cancer: oncogene or non-oncogene addiction? Biochim Biophys Acta. 2008;1781:442–447. doi: 10.1016/j.bbalip.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Guan HY, Gong LY, Song LB, Zhang N, Wu J, Yuan J, Zheng YJ, Huang ZS, Li M. Clinical significance of sphingosine kinase-1 expression in human astrocytomas progression and overall patient survival. Clin Cancer Res. 2008;14:6996–7003. doi: 10.1158/1078-0432.CCR-08-0754. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Yu CP, Xia JT, Zhang L, Weng GX, Zheng HQ, Kong QL, Hu LJ, Zeng MS, Zeng YX, et al. Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2009;15:1393–1399. doi: 10.1158/1078-0432.CCR-08-1158. [DOI] [PubMed] [Google Scholar]
- 15.Kawamori T, Kaneshiro T, Okumura M, Maalouf S, Uflacker A, Bielawski J, Hannun YA, Obeid LM. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009;23:405–414. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan SS, Khin LW, Wong L, Yan B, Ong CW, Datta A, Salto-Tellez M, Lam Y, Yap CT. Sphingosine kinase 1 promotes malignant progression in colon cancer and independently predicts survival of patients with colon cancer by competing risk approach in South asian population. Clin Transl Gastroenterol. 2014;5:e51. doi: 10.1038/ctg.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, Hasegawa Y, Tanyi JL, LaPushin R, Eder A, et al. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;1582:257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 18.Schulte KM, Beyer A, Köhrer K, Oberhäuser S, Röher HD. Lysophosphatidic acid, a novel lipid growth factor for human thyroid cells: over-expression of the high-affinity receptor edg4 in differentiated thyroid cancer. Int J Cancer. 2001;92:249–256. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1166>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Shida D, Watanabe T, Aoki J, Hama K, Kitayama J, Sonoda H, Kishi Y, Yamaguchi H, Sasaki S, Sako A, et al. Aberrant expression of lysophosphatidic acid (LPA) receptors in human colorectal cancer. Lab Invest. 2004;84:1352–1362. doi: 10.1038/labinvest.3700146. [DOI] [PubMed] [Google Scholar]
- 20.Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, Huang WC, Hait NC, Allegood JC, Price MM, Avni D, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23:107–120. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, Ai Y, Ristimaki AP, Fyrst H, Sano H, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamori T, Osta W, Johnson KR, Pettus BJ, Bielawski J, Tanaka T, Wargovich MJ, Reddy BS, Hannun YA, Obeid LM, et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20:386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- 23.Meyer zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta. 2007;1768:923–940. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci USA. 2000;97:13384–13389. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2) Mol Cell Biol. 2002;22:6921–6929. doi: 10.1128/MCB.22.19.6921-6929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shida D, Kitayama J, Yamaguchi H, Hama K, Aoki J, Arai H, Yamashita H, Mori K, Sako A, Konishi T, et al. Dual mode regulation of migration by lysophosphatidic acid in human gastric cancer cells. Exp Cell Res. 2004;301:168–178. doi: 10.1016/j.yexcr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Lin S, Wang D, Iyer S, Ghaleb AM, Shim H, Yang VW, Chun J, Yun CC. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology. 2009;136:1711–1720. doi: 10.1053/j.gastro.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu G, Zheng H, Zhang Z, Wu Z, Xiong H, Li J, Song L. Overexpression of sphingosine kinase 1 is associated with salivary gland carcinoma progression and might be a novel predictive marker for adjuvant therapy. BMC Cancer. 2010;10:495. doi: 10.1186/1471-2407-10-495. [DOI] [PMC free article] [PubMed] [Google Scholar]


