Abstract
AIM: To investigate the relationship of serum levels of polyunsaturated fatty acid (PUFA) with kinds of cytokines in colorectal cancer (CRC).
METHODS: Serum samples of 100 CRC patients were collected. The concentration of total n-3 PUFA which included C18:3 n-3, C20:5 n-3, C22:5 n-3, C22:6 n-3 and the total n-6 PUFA included C18:2 n-6, C18:3 n-6, C20:3 n-6, C20:4 n-6, and C22:5 n-6 were detected on GC-2010 Plus Gas Chromatograph with a OmegawaxTM 250 column. Cytokines were detected by MagPlexTM-C microspheres. P values for the trend were estimated by creating a continuous variable using the median value within quartiles.
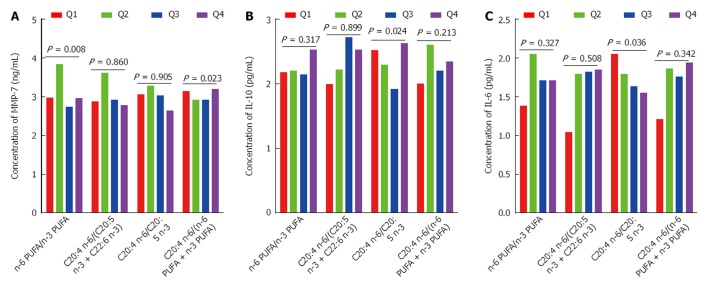
RESULTS: Interleukin-6 (IL-6) showed significantly positive association with the C20:4 n-6 (P for trend = 0.004). Interferon gamma (IFN-γ) showed significant positive association with the C22:5 n-3 (P for trend = 0.035). IL-8 and matrix metalloproteinase-9 (MMP-9) showed significant inverse association with the C22:6 n-3 (P for trend = 0.049, and 0.021). MMP-2 showed significant inverse association with the C20:5 n-3 (P for trend = 0.008). MMP-7 showed significantly positive association with the ratio of n-6 PUFA and n-3 PUFA (P for trend = 0.008). MMP-7 also showed significantly inverse association with the ratio of C20:4 n-6 and (n-6 PUFA + n-3 PUFA) (P for trend = 0.024). IL-10 (P for trend = 0.023) and IL-6 (P for trend = 0.036) showed significantly positive association with the ratio of C20:4 n-6 and C20:5 n-3.
CONCLUSION: Our data suggested that serum levels of PUFA is related to the inflammation of CRC, and also play different role in regulation of immune response.
Keywords: Polyunsaturated fatty acids, Cytokine, Colorectal cancer, Odds ratio, Nutrition
Core tip: Serum levels of polyunsaturated fatty acid (PUFA) with interferon gamma (IFN-γ), interleukin-10 (IL-10), IL-6, IL-8, tumor necrosis factor-α, matrix metalloproteinase-2 (MMP-2), MMP-7 and MMP-9 in colorectal cancer (CRC) were analyzed. IL-6 showed significantly positive association with the C20:4 n-6. IFN-γ showed significant positive association with the C22:5 n-3. IL-8 and MMP-9 showed significant inverse association with the C22:6 n-3. MMP-2 showed significant inverse association with the C20:5 n-3. Our data suggested that nutritional intervention may be related to the inflammation of CRC, and also found that their different role in the regulation of immune response.
INTRODUCTION
Cancer has been demonstrated to be closely related to inflammation, and inflammatory processes may result in tumor progression and a poorer prognosis[1-3]. Many previous studies have shown that n-3 polyunsaturated fatty acids (PUFA) have the potential to inhibit inflammation and support cancer treatment[4,5]. n-3 and n-6 PUFA have multiple mechanisms of action, including regulation of anti-inflammatory and inflammatory responses[6,7].
n-3 PUFA can inhibit the production of interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α)[7] and has been shown to be independently associated with low levels of pro-inflammatory markers (IL-6 and TNF-α) and higher levels of anti-inflammatory markers (soluble IL-6r, IL-10 and TGF-β)[8]. In American men and women, the dietary intake of n-3 and n-6 PUFA was inversely associated with plasma levels of soluble TNF-receptors 1 and 2 but not with the plasma levels of other cytokines[9]. n-6 PUFA, such as arachidonic acid, a molecule that is esterified to membrane phospholipids, are precursors of pro-inflammatory mediators, and the metabolites of arachidonic acid play important roles in regulating the immune response[10,11]. PUFAs played important role in promoting inflammation by increasing vascular permeability and vasodilatation and directing the synthesis of pro-inflammatory cytokines and their migration to the site of inflammation[12,13].
In our study, we investigated the relationships between the serum levels of PUFAs and the serum levels of cytokines in colorectal cancer (CRC). Our study may be useful for determining new nutritional adjuvant treatments for colorectal cancer in clinical practice.
MATERIALS AND METHODS
Study population
Our study was reviewed and approved by the Ethics Committee of the Chinese PLA General Hospital. All individuals provided informed written consent. The serum samples were collected before the administration of any CRC treatment, such as surgery, chemotherapy and radiation therapy. CRC was diagnosed according to combined clinical criteria, including imaging data and serum tumor markers and was further confirmed by histopathological analysis. All study subjects were Han Chinese in origin, lived in northern inland cities, and did not have an increased dietary intake of PUFAs. Body mass index (BMI) was calculated as weight (kilograms)/height (square meters). Smoking and alcohol consumption statuses were reported as current (C), former (F), or never (N)[14]. The average daily intakes of energy, protein, fat and carbohydrates were estimated using the Chinese PLA General Hospital Nutrition Analyzer System. The clinical characteristics of all CRC samples used in this study were described in our previous study[15].
Measurement of serum PUFA
200 μL serum sample was transferred to the glass methylation tube. 5 μg intern control C23:0 The, 1 mL hexane and 1 mL 14% BF3/MeOH reagent were added and mixed into the methylation tube. After blanketing by nitrogen, and heated at 100 °C for 45 min. Then the tube was cooled to room temperature, added 1 mL H2O into the tube. After centrifugation at 1200 r/min for 5 min, the upper hexane layer was transferred to a new tube, and then concentrated by nitrogen. The total fatty acid methyl esters were detected by GC-2010 Plus Gas Chromatograph (Chiyoda-ku, Tokyo, Japan) with a OmegawaxTM 250 column (Supelco, Belletonte, PA, United States) 30 m × 0.25 mm × 0.25 μm film thickness. The parameter of the Column temperature Program was 210 °C and 45 min. The levels of polyunsaturated fatty acids were showed as a percentage. The indicators detected in our study included C18:3 n-3, C20:5 n-3, C22:5 n-3, C22:6 n-3, C18:2 n-6, C18:3 n-6, C20:3 n-6, C20:4 n-6, and C22:5 n-6.
Multiplex microbead immunoassay and clinical serum marker detection
A multiplex bead-based sandwich immunoassay kits was used to detect the serum concentration of interferon gamma (IFN-γ), IL-10, IL-6, IL-8, TNF-α, matrix metalloproteinase-2 (MMP-2), MMP-7 and MMP-9 according to the instruction of manufacturer (HCYTOMAG-60K, Millipore, Billerica, MA, United States). Briefly, 25 μL serum sample and 25 μL beads were incubated at 4 °C overnight in a 96 well solid plate. Then the plates were washed twice by automated plated washer, and 25 μL biotinylated detecting antibodies cocktail was added, after shaking at room temperature for 1 h. 25 μL streptavidin-phycoerythrin solution was then added and shakedat room temperature for 30 min. After washing the plate twice, 150 μL sheath fluid was added. After the procedures metioned above, the fluorescent signal of the beads was detected by a Luminex 200 (Luminex, Austin, TX, United States). The levels were calculated according to the standard curves which were established by 5 different concentration ranged from 0-10000 pg/mL (IFN-γ, IL-10, IL-6, IL-8, TNF-α, and MMP-9), 0-50000 pg/mL (MMP-2) and 0-40000 pg/mL (MMP-7). The levels were reported as median fluorescent intensity. Total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides were detected by Cobas 8000 modular analyzer series (Roche Diagnostic, Mannheim, Germany).
Statistical analysis
All statistical analyses were performed on SAS 9.2 statistical package (SAS Institute, Inc. Cary, United States). Serum levels of n-3 and n-6 PUFA were divided into quartiles. The relationship of n-3 and n-6 PUFA with cytokines were analyzed by computing age, sex, smoke, alcohol drinking, body mass index (BMI), total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, total energy, protein, fat and carbohydrate intake adjusted partial Person correlation. P values for the trend were estimated by creating a continuous variable using the median value within quartiles, and P < 0.05 showed significant difference.
RESULTS
Association of n-6 PUFA and indicators with cytokines
In this study, we analyzed the associations of n-6 PUFAs with IFN-γ, IL-10, IL-6, IL-8, TNF-α, MMP-2, MMP-7 and MMP-9. As shown in Figure 1, IL-6 had a significant positive association with C20:4 n-6; when the percentage of C20:4 n-6 increased from Q1 to Q4, the concentration of IL-6 increased from 1.55 to 1.92 pg/mL (P for trend = 0.004). However, IL-6 concentration was not significantly associated with C18:2 n-6, C18:3 n-6, C22:5 n-6 or n-6 PUFA. As shown in Table 1, IFN-γ, IL-10, IL-8, TNF-α, MMP-2, MMP-7 and MMP-9 were not significantly associated with C18:2 n-6, C18:3 n-6, C20:4 n-6, C22:5 n-6 or n-6 PUFA.
Figure 1.
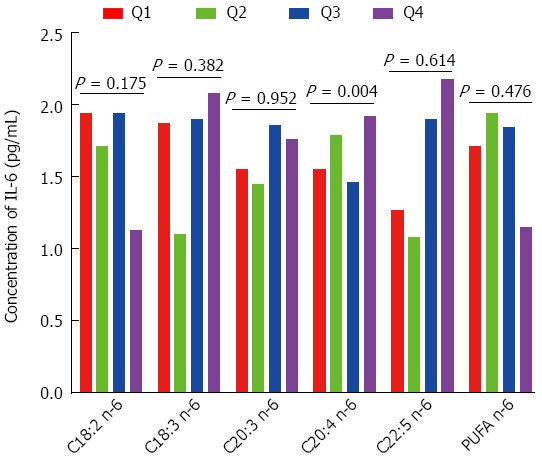
Analysis of the association of n-6 polyunsaturated fatty acid with the concentration of interleukin-6 in colorectal cancer. Q means quartiles. PUFA: Polyunsaturated fatty acids; IL-6: Interleukin-6.
Table 1.
Multivariate analysis of the relationship between n-6 polyunsaturated fatty acid and cytokines
| C18:2 n-6 (quartiles),%1 | C18:3 n-6 (quartiles),%1 | C20:3 n-6 (quartiles),%1 | |||||||||||||
| Quartiles limits | < 22.74 | 22.74-24.46 | 24.46-26.50 | > 26.50 | P value for trend | < 0.21 | 0.21-0.28 | 0.28-0.34 | > 0.34 | P value for trend | < 1.28 | 1.28-1.59 | 1.59-1.92 | > 1.92 | P value for trend |
| Median | 21.55 | 23.75 | 25.47 | 28.54 | 0.17 | 0.24 | 0.31 | 0.40 | 1.05 | 1.38 | 1.72 | 2.13 | |||
| IFN-γ (pg/mL) | 0.55 | 0.22 | 0.81 | 0.33 | 0.834 | 0.41 | 0.27 | 0.40 | 0.46 | 0.943 | 0.30 | 0.46 | 0.34 | 0.41 | 0.760 |
| IL-10 (pg/mL) | 2.63 | 2.06 | 2.70 | 1.98 | 0.959 | 2.19 | 2.27 | 1.99 | 2.68 | 0.828 | 2.04 | 2.10 | 2.50 | 2.63 | 0.552 |
| IL-8 (pg/mL) | 66.00 | 48.43 | 51.01 | 30.76 | 0.259 | 57.55 | 21.77 | 45.19 | 73.44 | 0.436 | 62.18 | 34.07 | 47.60 | 64.69 | 0.266 |
| TNF-α (pg/mL) | 7.36 | 7.34 | 9.23 | 6.68 | 0.443 | 9.74 | 6.69 | 7.32 | 8.30 | 0.450 | 7.76 | 6.97 | 8.29 | 8.43 | 0.743 |
| MMP-2 (ng/mL) | 12.26 | 12.86 | 10.89 | 8.80 | 0.485 | 13.54 | 9.68 | 7.54 | 12.24 | 0.488 | 10.79 | 11.57 | 12.86 | 11.62 | 0.863 |
| MMP-7 (ng/mL) | 2.96 | 3.10 | 2.94 | 3.06 | 0.735 | 3.39 | 2.55 | 3.22 | 3.00 | 0.426 | 3.01 | 3.10 | 2.62 | 3.14 | 0.444 |
| MMP-9 (ng/mL) | 12.03 | 12.16 | 10.75 | 10.71 | 0.058 | 10.86 | 9.89 | 12.14 | 12.57 | 0.255 | 10.61 | 9.81 | 14.03 | 11.83 | 0.187 |
| C20:4 n-6 (quartiles),%1 | C22:5 n-6 (quartiles),%1 | PUFA n-6 (quartiles),%1 | |||||||||||||
| Quartiles limits | < 6.72 | 6.72-7.75 | 7.75-9.01 | > 9.01 | P value for trend | < 0.13 | 0.13-0.18 | 0.18-0.25 | > 0.25 | P value for trend | < 32.52 | 32.52-34.53 | 34.53-36.58 | 36.58 | P value for trend |
| edian | 5.99 | 7.29 | 8.19 | 9.74 | 0.06 | 0.15 | 0.21 | 0.31 | 31.07 | 33.65 | 35.56 | 37.92 | |||
| IFN-γ (pg/mL) | 0.41 | 0.46 | 0.22 | 0.41 | 0.298 | 0.52 | 0.22 | 0.27 | 0.41 | 0.709 | 0.30 | 0.67 | 0.41 | 0.27 | 0.610 |
| IL-10 (pg/mL) | 2.14 | 2.97 | 1.85 | 2.63 | 0.681 | 2.20 | 1.74 | 2.68 | 2.57 | 0.453 | 2.23 | 2.77 | 2.00 | 2.01 | 0.287 |
| IL-8 (pg/mL) | 41.89 | 44.92 | 41.14 | 65.83 | 0.129 | 46.68 | 30.06 | 66.74 | 62.79 | 0.348 | 47.90 | 41.00 | 78.72 | 43.19 | 0.983 |
| TNF-α (pg/mL) | 6.45 | 7.78 | 7.39 | 9.38 | 0.182 | 7.32 | 6.67 | 8.41 | 7.43 | 0.457 | 7.17 | 6.83 | 9.38 | 8.61 | 0.480 |
| MMP-2 (ng/mL) | 12.39 | 11.16 | 8.45 | 13.54 | 0.741 | 9.75 | 6.28 | 14.83 | 13.41 | 0.223 | 10.60 | 12.19 | 13.41 | 8.88 | 0.065 |
| MMP-7 (ng/mL) | 3.02 | 3.04 | 2.94 | 3.52 | 0.055 | 3.10 | 2.80 | 2.87 | 3.55 | 0.981 | 3.04 | 2.91 | 3.20 | 3.03 | 0.870 |
| MMP-9 (ng/mL) | 10.94 | 10.93 | 11.96 | 11.81 | 0.200 | 10.91 | 9.97 | 13.00 | 11.01 | 0.493 | 10.32 | 12.40 | 12.56 | 10.89 | 0.868 |
The association of n-6 PUFA and cytokines were adjusted for potential confounding factors, including age, sex, smoke, alcohol drinking, body mass index, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, total energy, protein, fat and carbohydrate intake. PUFA: Polyunsaturated fatty acids; IFN-γ: Interferon gamma; IL-10: Interleukin-10; TNF-α: Tumor necrosis factor-α; MMP-2: Matrix metalloproteinase-2.
Association of n-3 PUFA and indicators with cytokines
As shown in Figure 2A, IFN-γ had a significant positive association with C22:5 n-3; when the percentage of C22:5 n-3 increased from Q1 to Q4, the concentration of IFN-γ increased from 0.38 to 0.57 pg/mL (P for trend = 0.035). As shown in Figure 2B, IL-8 had a significant inverse association with C22:6 n-3; when the percentage of C22:6 n-3 increased from Q1 to Q4, the concentration of IL-8 decreased from 53.01 to 38.10 pg/mL (P for trend = 0.049). As shown in Figure 2C, MMP-2 had a significant inverse association with C20:5 n-3; when the percentage of C20:5 n-3 increased from Q1 to Q4, the concentration of MMP-2 decreased from 12.39 to 6.30 ng/mL (P for trend = 0.008). As shown in Figure 2D, MMP-9 had a significant inverse association with C22:6 n-3; when the percentage of C22:6 n-3 increased from Q1 to Q4, the concentration of MMP-9 decreased from 11.76 to 8.86 ng/mL (P for trend = 0.021). As shown in Table 2, C18:3 n-3, C20:5 n-3, C22:5 n-3, C22:6 n-3, and n-3 PUFA were not significantly associated with IL-10, IL-6, TNF-α, or MMP-7.
Figure 2.
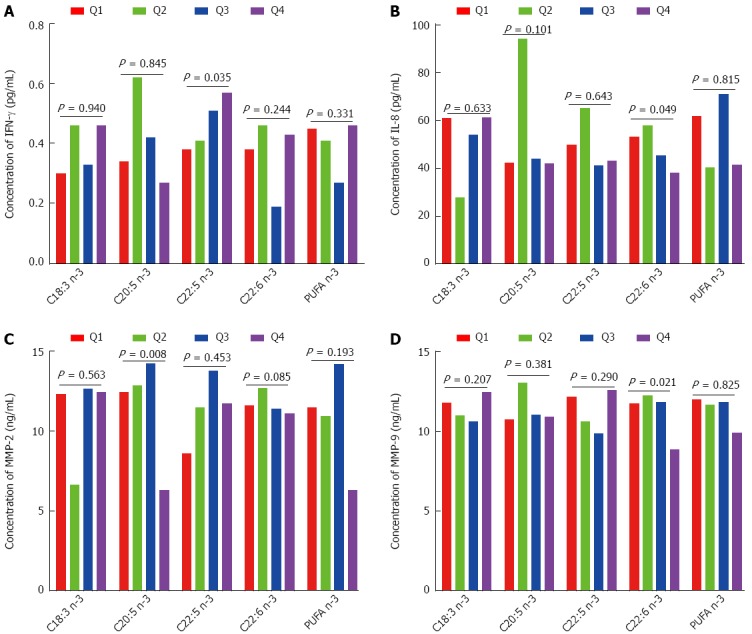
Analysis of the association of n-3 polyunsaturated fatty acid with the concentration of interferon gamma, interleukin-8, matrix metalloproteinase-2 and matrix metalloproteinase-9 in colorectal cancer. Q means quartiles. PUFA: Polyunsaturated fatty acids; IFN-γ: Interferon gamma; IL-8: Interleukin-8; MMP-2: Matrix metalloproteinase-2.
Table 2.
Multivariate analysis of the relationship between n-3 polyunsaturated fatty acid and cytokines
| C18:3 n-3 (quartiles),%1 | C20:5 n-3 (quartiles),%1 | C22:5 n-3 (quartiles),%1 | ||||||||||||||
| Quartiles limits | < 0.33 | 0.33-0.47 | 0.47-0.61 | > 0.61 | P value for trend | < 0.20 | 0.20-0.31 | 0.31-0.46 | > 0.46 | P value for trend | Quartiles limits | < 0.32 | 0.32-0.40 | 0.40-0.49 | > 0.49 | P value for trend |
| Median | 0.26 | 0.41 | 0.53 | 0.74 | 0.15 | 0.25 | 0.37 | 0.60 | Median | 0.27 | 0.36 | 0.44 | 0.59 | |||
| IL-10 (pg/mL) | 2.58 | 2.34 | 1.93 | 2.42 | 0.465 | 2.38 | 1.96 | 2.54 | 2.46 | 0.959 | IL-10 | 2.47 | 2.66 | 2.06 | 2.00 | 0.478 |
| IL-6 (pg/mL) | 1.75 | 1.43 | 1.34 | 1.90 | 0.184 | 1.45 | 1.89 | 1.74 | 2.09 | 0.810 | IL-6 | 1.55 | 1.71 | 1.79 | 1.94 | 0.088 |
| TNF-α (pg/mL) | 7.35 | 7.78 | 8.36 | 7.43 | 0.644 | 7.45 | 8.59 | 7.43 | 6.92 | 0.821 | TNF-α | 7.27 | 8.71 | 6.73 | 7.43 | 0.373 |
| MMP-7 (ng/mL) | 3.02 | 2.97 | 2.87 | 3.27 | 0.520 | 2.62 | 3.10 | 3.10 | 3.14 | 0.412 | MMP-7 | 2.66 | 3.08 | 3.00 | 3.14 | 0.323 |
| C22:6 n-3 (quartiles),%1 | PUFA n-31 | |||||||||||||||
| Quartiles limits | < 1.56 | 1.56-2.13 | 2.13-2.62 | > 2.62 | P value for trend | < 2.79 | 2.79-3.30 | 3.30-4.08 | > 4.08 | P value for trend | ||||||
| Median | 1.31 | 1.79 | 2.36 | 3.01 | 2.45 | 3.04 | 3.73 | 4.45 | ||||||||
| IL-10 (pg/mL) | 2.63 | 2.64 | 1.93 | 2.30 | 0.838 | 2.63 | 2.25 | 1.98 | 2.34 | 0.074 | ||||||
| IL-6 (pg/mL) | 1.79 | 1.87 | 1.43 | 1.34 | 0.679 | 1.85 | 1.51 | 1.25 | 1.83 | 0.279 | ||||||
| TNF-α (pg/mL) | 7.03 | 7.66 | 7.07 | 8.40 | 0.636 | 7.27 | 7.08 | 9.49 | 7.21 | 0.728 | ||||||
| MMP-7 (ng/mL) | 2.94 | 3.13 | 3.26 | 2.97 | 0.827 | 2.92 | 2.69 | 3.61 | 3.20 | 0.160 | ||||||
The association of n-6 PUFA and cytokines were adjusted for potential confounding factors, including age, sex, smoke, alcohol drinking, body mass index, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, total energy, protein, fat and carbohydrate intake. PUFA: Polyunsaturated fatty acids; IFN-γ: Interferon gamma; IL-10: Interleukin-10; TNF-α: Tumor necrosis factor-α; MMP-7: Matrix metalloproteinase-7.
Association of n-6 PUFA and n-3 PUFA indicators with cytokines
As shown in Figure 3A, MMP-7 had a significant positive association with the ratio of n-6 PUFA to n-3 PUFA (P for trend = 0.008). MMP-7 also had a significant inverse association with the ratio of C20:4 n-6 to (n-6 PUFA + n-3 PUFA) (P for trend = 0.024). As shown in Figure 3B and C, IL-10 (P for trend = 0.023) and IL-6 (P for trend = 0.036) had significant positive associations with the ratio of C20:4 n-6 to C20:5 n-3. As shown in Table 3, IFN-γ, IL-8, TNF-α, MMP-2, MMP-9 were not significantly associated with n-6 PUFA/n-3 PUFA, C20:4 n-6/(C20:5 n-3 + C22:6 n-3), C20:4 n-6/C20:5 n-3, and C20:4 n-6/(n-6 PUFA + n-3 PUFA).
Figure 3.
Analysis of the association of n-6 polyunsaturated fatty acids and n-3 polyunsaturated fatty acids indicators with the concentration of matrix metalloproteinase-7, interleukin-10 and interleukin-6 in colorectal cancer. Q means quartiles. PUFA: Polyunsaturated fatty acids. PUFA: Polyunsaturated fatty acids; IL-10: Interleukin-10; MMP-7: Matrix metalloproteinase-7.
Table 3.
Multivariate analysis of the relationship between n-6, n-3 polyunsaturated fatty acid indicators and cytokines
| n-6 PUFA/n-3 PUFA1 | C20:4 n-6/(C20:5 n-3 + C22:6 n-3)1 | |||||||||
| Quartiles limits | < 8.47 | 8.47-10.25 | 10.25-12.66 | > 12.66 | P value for trend | < 2.64 | 2.64-3.21 | 3.21-3.94 | > 3.94 | P value for trend |
| Median | 7.49 | 9.41 | 11.64 | 14.08 | 2.16 | 2.95 | 3.56 | 4.79 | ||
| IFN-γ (pg/mL) | 0.45 | 0.46 | 0.30 | 0.41 | 0.240 | 0.27 | 0.55 | 0.55 | 0.30 | 0.157 |
| IL-8 (pg/mL) | 23.82 | 70.90 | 48.43 | 50.67 | 0.495 | 18.58 | 53.84 | 63.53 | 60.30 | 0.413 |
| TNF-α (pg/mL) | 7.01 | 10.53 | 6.62 | 7.45 | 0.600 | 6.81 | 9.20 | 7.98 | 7.36 | 0.094 |
| MMP-2 (ng/mL) | 5.80 | 14.95 | 11.55 | 11.37 | 0.549 | 4.12 | 11.48 | 14.13 | 11.37 | 0.980 |
| MMP-9 (ng/mL) | 9.88 | 11.81 | 11.27 | 12.24 | 0.734 | 9.73 | 10.30 | 12.56 | 12.26 | 0.495 |
| C20:4 n-6/C20:5 n-31 | C20:4 n-6/(n-6 PUFA + n-3 PUFA)1 | |||||||||
| Quartiles limits | < 16.65 | 16.65-25.18 | 25.18-39.20 | > 39.20 | P value for trend | < 0.18 | 0.18-0.21 | 0.21-0.23 | > 0.23 | P value for trend |
| edian | 12.66 | 19.67 | 30.67 | 48.51 | 0.16 | 0.20 | 0.22 | 0.25 | ||
| IFN-γ(pg/mL) | 0.29 | 0.46 | 0.46 | 0.30 | 0.906 | 0.41 | 0.27 | 0.55 | 0.46 | 0.769 |
| IL-8 (pg/mL) | 26.78 | 41.71 | 80.35 | 50.88 | 0.978 | 23.13 | 43.41 | 71.56 | 65.01 | 0.108 |
| TNF-α (pg/mL) | 6.63 | 8.01 | 8.47 | 8.30 | 0.334 | 6.29 | 8.15 | 8.70 | 8.40 | 0.432 |
| MMP-2 (ng/mL) | 8.99 | 10.84 | 12.81 | 13.77 | 0.977 | 9.10 | 11.35 | 12.24 | 12.81 | 0.200 |
| MMP-9 (ng/mL) | 9.73 | 11.32 | 12.59 | 12.14 | 0.058 | 10.85 | 10.52 | 12.57 | 11.90 | 0.350 |
The association of n-6 PUFA and cytokines were adjusted for potential confounding factors, including age, sex, smoke, alcohol drinking, body mass index, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, total energy, protein, fat and carbohydrate intake. PUFA: Polyunsaturated fatty acids; IFN-γ: Interferon gamma; IL-8: Interleukin-8; TNF-α: Tumor necrosis factor-α; MMP-2: Matrix metalloproteinase-2.
DISCUSSION
Previsous studies have demonstrated that inflammation is closely related to cancer development[16-18]. The PUFAs may involve in the inflammation of CRC, and may be a potential biomarker for prognosis of CRC[19]. When the PUFAs were loaded with magnetoliposomes, they may serve as a novel theranostic anti-inflammatory formulations[20]. Some authors found that n-3 PUFA increases B-cell CD69 surface expression, IL-6 and IFN-γ secretion. It can promote pro-inflammatory responses[21]. In addition, they may also related to the risk of colorectal cancer according to microsatellite instability[22]. In our study, IFN-γ has a significant positive association with C22:5 n-3. Our results were consistent with those of previous studies. Previous studies also shown that PGE2 can affect the Th1/Th2 balance. It not only decreases the production of Th1-type cytokines, such as IFN-γ and IL-2, but also enhances the production of Th2-type cytokines, such as IL-4 and IL-5. n-3 PUFA can decrease the PGE2 concentration to increase the IFN-γ levels. Previous studies found that n-3 PUFA bind to peroxisome proliferator-activated receptor-γ (PPAR-γ)[23]. PPARγ decreases the mRNA stability of IL-8[24], and the concentration of n-3 PUFA has been shown to be inversely associated with IL-8[25,26]. We observed an inverse association between n-3 PUFA and IL-8, which is consistent with the results of previous studies. An increase in theconcentration of PPAR-γ results in decrease in the concentration of MMP-2[27,28], and PPARγ agonists can activate pro-MMP-2[29]. In our study, MMP-2 was inversely associated with n-3 PUFA. n-3 PUFA also reduces the release of inflammatory promoters and promote the release of IL-10, improve the nutritional state of patients[30]. The n-3 PUFA sensitivity of colon cancer cells is closely related to autophagy, and may have potential therapeutic effects against cancer cells with low autophagy[31]. n-3 epoxides may also serve as regulators of inflammation and autophagy in insulin-sensitive tissues and postulate sEH as a druggable target in metabolic diseases[32]. Some authors have demonstrated a positive association between endogenous PGE2, and eicosanoids derived from n-6 PUFA and release of the inflammatory cytokine IL-6[33]. In a recent study of chronic obstructive pulmonary disease patients, higher n-6 PUFA intake was associated with higher IL-6 concentrations[34].Increased consumption of n-6 PUFA altered the production of important mediators and regulators of inflammation and immune responses towards a pro-inflammatory profile, with increased production of PGE2, LTB4, TXA2, IL-1β and IL-6[35]. In our study, n-6 PUFA had a significant positive association with IL-6. Previous studies support our results, and IL-6 may be important for the regulation of immune responses by n-6 PUFA.
In conclusion, our results demonstrated a significant positive association between IL-6 and C20:4 n-6. MMP-2 had a significant inverse association with C20:5 n-3. IFN-γ had a significant positive association with C22:5 n-3. IL-8 and MMP-9 had significant inverse associations with C22:6 n-3. MMP-7 had a significant positive association with the ratio of n-6 PUFA to n-3 PUFA (P for trend = 0.008) and a significant inverse association with the ratio of C20:4 n-6 to (n-6 PUFA + n-3 PUFA). IL-10 and IL-6 both had a significant positive association with the ratio of C20:4 n-6 to C20:5 n-3. Our data suggest that nutritional intervention may impact inflammation in colorectal cancer and that different PUFA play different roles in regulating immune responses.
COMMENTS
Background
Lots of previous studies have demonstrated that the n-3 polyunsaturated fatty acid (PUFA) may have potential action for inhibiting inflammation, and further to support cancer treatment. n-3 PUFA and n-6 PUFA involve multiple mechanisms, including regulation the anti-inflammation and inflammation response.Little study was performed to investigate the relationship of serum levels of PUFAs with cytokines in colorectal cancer (CRC).
Research frontiers
To investigate the relationship of serum levels of PUFA with interferon gamma (IFN-γ), interleukin-10 (IL-10), IL-6, IL-8, tumor necrosis factor-α (TNF-α), matrix metalloproteinase-2 (MMP-2), MMP-7 and MMP-9 in CRC.
Innovations and breakthroughs
IL-6 showed significantly positive association with the C20:4 n-6. IFN-γ showed significant positive association with the C22:5 n-3. IL-8 and MMP-9 showed significant inverse association with the C22:6 n-3. MMP-2 showed significant inverse association with the C20:5 n-3. MMP-7 showed significantly positive association with the ratio of n-6 PUFA and n-3 PUFA. MMP-7 also showed significantly inverse association with the ratio of C20:4 n-6 and (n-6 PUFA + n-3 PUFA). IL-10 and IL-6 showed significantly positive association with the ratio of C20:4 n-6 and C20:5 n-3.
Applications
The data suggested that that nutritional intervention may be related to the inflammation of CRC, and also found that their different role in the regulation of immune response.
Terminology
n-3 PUFA and n-6 PUFA involve in regulation the anti-inflammation and inflammation response which played important role in the development of kinds of cancers.
Peer-review
This study investigates the relationship between the serum levels of polyunsaturated fatty acids and cytokines in CRC patients. The study is very interesting and well-conducted.
Footnotes
Supported by The National High Technology Research and Development Pro-gram 863, NO. 2011AA02A111; The Capital Health Development Special Scientific Research Projects, NO. 2014-2-2154; China Postdoctoral Science Special Foundation Funded Project, NO. 2014T70963; and China Postdoctoral Science Foundation Funded Project, NO. 2013M532110.
Institutional review board statement: The study was reviewed and approved by the Chinese PLA General Hospital Review Board.
Informed consent statement: All study participants or their legal guardians provided written informed consent prior to study enrollment.
Conflict-of-interest statement: We declare that we have no financial or personal relationships with other individuals or organizations that can inappropriately influence our work and that there is no professional or other personal interest of any nature in any product, service and/or company that could be construed as influencing the position presented in or the review of the manuscript.
Data sharing statement: The technical appendix, statistical code, and dataset are available from the corresponding author at tianyp61@gmail.com and drzhuxu@163.com. The study participants provided informed consent for data sharing. No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 12, 2016
First decision: January 22, 2016
Article in press: February 20, 2016
P- Reviewer: Leto SM, Printz C S- Editor: Gong ZM L- Editor: A E- Editor: Zhang DN
References
- 1.Crawford S. Anti-inflammatory/antioxidant use in long-term maintenance cancer therapy: a new therapeutic approach to disease progression and recurrence. Ther Adv Med Oncol. 2014;6:52–68. doi: 10.1177/1758834014521111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pengjun Z, Xinyu W, Feng G, Xinxin D, Yulan L, Juan L, Xingwang J, Zhennan D, Yaping T. Multiplexed cytokine profiling of serum for detection of colorectal cancer. Future Oncol. 2013;9:1017–1027. doi: 10.2217/fon.13.71. [DOI] [PubMed] [Google Scholar]
- 4.Vaughan VC, Hassing MR, Lewandowski PA. Marine polyunsaturated fatty acids and cancer therapy. Br J Cancer. 2013;108:486–492. doi: 10.1038/bjc.2012.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy RA, Mourtzakis M, Mazurak VC. n-3 polyunsaturated fatty acids: the potential role for supplementation in cancer. Curr Opin Clin Nutr Metab Care. 2012;15:246–251. doi: 10.1097/MCO.0b013e328351c32f. [DOI] [PubMed] [Google Scholar]
- 6.Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002;61:345–358. doi: 10.1079/pns2002166. [DOI] [PubMed] [Google Scholar]
- 7.Khan SA, Vanden Heuvel JP. Role of nuclear receptors in the regulation of gene expression by dietary fatty acids (review) J Nutr Biochem. 2003;14:554–567. doi: 10.1016/s0955-2863(03)00098-6. [DOI] [PubMed] [Google Scholar]
- 8.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 9.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 10.Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2012;112:1029–1041. doi: 10.1016/j.jand.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Calder PC. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res. 2008;52:885–897. doi: 10.1002/mnfr.200700289. [DOI] [PubMed] [Google Scholar]
- 12.Dupertuis YM, Meguid MM, Pichard C. Colon cancer therapy: new perspectives of nutritional manipulations using polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2007;10:427–432. doi: 10.1097/MCO.0b013e3281e2c9d4. [DOI] [PubMed] [Google Scholar]
- 13.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Lee IM, Manson JE, Buring JE, Sesso HD. Alcohol consumption, weight gain, and risk of becoming overweight in middle-aged and older women. Arch Intern Med. 2010;170:453–461. doi: 10.1001/archinternmed.2009.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang P, Wen X, Gu F, Zhang X, Li J, Liu Y, Dong J, Deng X, Zhu X, Tian Y. Role of serum polyunsaturated fatty acids in the development of colorectal cancer. Int J Clin Exp Med. 2015;8:15900–15909. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P, Zou M, Wen X, Gu F, Li J, Liu G, Dong J, Deng X, Gao J, Li X, et al. Development of serum parameters panels for the early detection of pancreatic cancer. Int J Cancer. 2014;134:2646–2655. doi: 10.1002/ijc.28584. [DOI] [PubMed] [Google Scholar]
- 17.Stark T, Livas L, Kyprianou N. Inflammation in prostate cancer progression and therapeutic targeting. Transl Androl Urol. 2015;4:455–463. doi: 10.3978/j.issn.2223-4683.2015.04.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang K, Li H, Dong J, Dong Y, Wang CZ. Expression profile of polyunsaturated fatty acids in colorectal cancer. World J Gastroenterol. 2015;21:2405–2412. doi: 10.3748/wjg.v21.i8.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calle D, Negri V, Ballesteros P, Cerdán S. Magnetoliposomes loaded with poly-unsaturated fatty acids as novel theranostic anti-inflammatory formulations. Theranostics. 2015;5:489–503. doi: 10.7150/thno.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockett BD, Salameh M, Carraway K, Morrison K, Shaikh SR. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. J Lipid Res. 2010;51:1284–1297. doi: 10.1194/jlr.M000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song M, Nishihara R, Wu K, Qian ZR, Kim SA, Sukawa Y, Mima K, Inamura K, Masuda A, Yang J, et al. Marine ω-3 polyunsaturated fatty acids and risk of colorectal cancer according to microsatellite instability. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allred CD, Talbert DR, Southard RC, Wang X, Kilgore MW. PPARgamma1 as a molecular target of eicosapentaenoic acid in human colon cancer (HT-29) cells. J Nutr. 2008;138:250–256. doi: 10.1093/jn/138.2.250. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Pascual G, Glass CK. Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol Cell Biol. 2000;20:4699–4707. doi: 10.1128/mcb.20.13.4699-4707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra A, Chaudhary A, Sethi S. Oxidized omega-3 fatty acids inhibit NF-kappaB activation via a PPARalpha-dependent pathway. Arterioscler Thromb Vasc Biol. 2004;24:1621–1627. doi: 10.1161/01.ATV.0000137191.02577.86. [DOI] [PubMed] [Google Scholar]
- 26.Marion-Letellier R, Butler M, Déchelotte P, Playford RJ, Ghosh S. Comparison of cytokine modulation by natural peroxisome proliferator-activated receptor gamma ligands with synthetic ligands in intestinal-like Caco-2 cells and human dendritic cells--potential for dietary modulation of peroxisome proliferator-activated receptor gamma in intestinal inflammation. Am J Clin Nutr. 2008;87:939–948. doi: 10.1093/ajcn/87.4.939. [DOI] [PubMed] [Google Scholar]
- 27.Yiqin Y, Meilin X, Jie X, Keping Z. Aspirin inhibits MMP-2 and MMP-9 expression and activity through PPARalpha/gamma and TIMP-1-mediated mechanisms in cultured mouse celiac macrophages. Inflammation. 2009;32:233–241. doi: 10.1007/s10753-009-9125-3. [DOI] [PubMed] [Google Scholar]
- 28.He Q, Chen J, Lin HL, Hu PJ, Chen MH. Expression of peroxisome proliferator-activated receptor gamma, E-cadherin and matrix metalloproteinases-2 in gastric carcinoma and lymph node metastases. Chin Med J (Engl) 2007;120:1498–1504. [PubMed] [Google Scholar]
- 29.Kim KH, Cho YS, Park JM, Yoon SO, Kim KW, Chung AS. Pro-MMP-2 activation by the PPARgamma agonist, ciglitazone, induces cell invasion through the generation of ROS and the activation of ERK. FEBS Lett. 2007;581:3303–3310. doi: 10.1016/j.febslet.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Zhang H, Zhang Y, Li W, Sun X, Xing Y, Suo J. [Effects of omega-3 polyunsaturated fatty acids on postoperative inflammatory reaction and clinical efficacy] Zhonghua Wei Chang Wai Ke Zazhi. 2015;18:651–655. [PubMed] [Google Scholar]
- 31.Pettersen K, Monsen VT, Hakvåg Pettersen CH, Overland HB, Pettersen G, Samdal H, Tesfahun AN, Lundemo AG, Bjørkøy G, Schønberg SA. DHA-induced stress response in human colon cancer cells - Focus on oxidative stress and autophagy. Free Radic Biol Med. 2016;90:158–172. doi: 10.1016/j.freeradbiomed.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 32.López-Vicario C, Alcaraz-Quiles J, García-Alonso V, Rius B, Hwang SH, Titos E, Lopategi A, Hammock BD, Arroyo V, Clària J. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides. Proc Natl Acad Sci USA. 2015;112:536–541. doi: 10.1073/pnas.1422590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Batlle J, Sauleda J, Balcells E, Gómez FP, Méndez M, Rodriguez E, Barreiro E, Ferrer JJ, Romieu I, Gea J, et al. Association between Ω3 and Ω6 fatty acid intakes and serum inflammatory markers in COPD. J Nutr Biochem. 2012;23:817–821. doi: 10.1016/j.jnutbio.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J Nutr Metab. 2012;2012:539426. doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]