Abstract
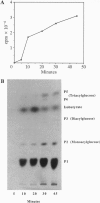
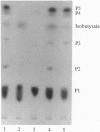
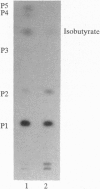
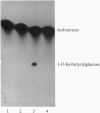
Glandular trichomes of the wild tomato Lycopersicon pennellii Corr. (D'Arcy) secrete large amounts of 2,3,4-tri-O-acylglucoses possessing straight- and branched-chain fatty acids of short to medium chain length (C4-C12). Although previous biosynthetic studies suggested that glucose acylation proceeded via acyl CoA intermediates, repeated attempts to demonstrate isobutyryl-CoA-dependent glucose acylation were unsuccessful. When [14C]isobutyrate is administered to detached L. pennellii leaves, the label is readily converted to 1-O-isobutyryl-beta-D-glucose. This is immediately followed by the appearance of di- and triacylated glucose esters. L. pennellii extracts catalyzed the formation of 1-O-isobutyryl-beta-D-glucose from isobutyrate and UDPglucose, and detached L. pennellii trichomes catalyzed transfer of the isobutyryl moiety from synthetic 1-O-isobutyryl-beta-D-glucose to D-glucose. Detached L. pennellii trichomes also catalyzed the formation of diacylglucose and triacylglucose via transfer of the isobutyryl moiety from 1-O-[14C]isobutyryl-beta-D-glucose to mono- or diacylglucoses, respectively. These studies suggest a multistep mechanism in which activation of fatty acids to their respective high-energy 1-O-acyl-beta-D-glucopyranose derivatives is followed by transfer of the 1-O-acyl moiety to non-anomeric positions of other glucose and/or partially acylated glucose molecules. This appears to be the primary mechanism of activation and fatty acid esterification to glucose in L. pennellii trichomes. Cultivated tomato, L. esculentum Mill., also activates free fatty acids to their 1-O-acyl-beta-D-glucose derivatives but lacks the acyl transfer mechanism for synthesizing polyacylated sugars.
Full text
PDF

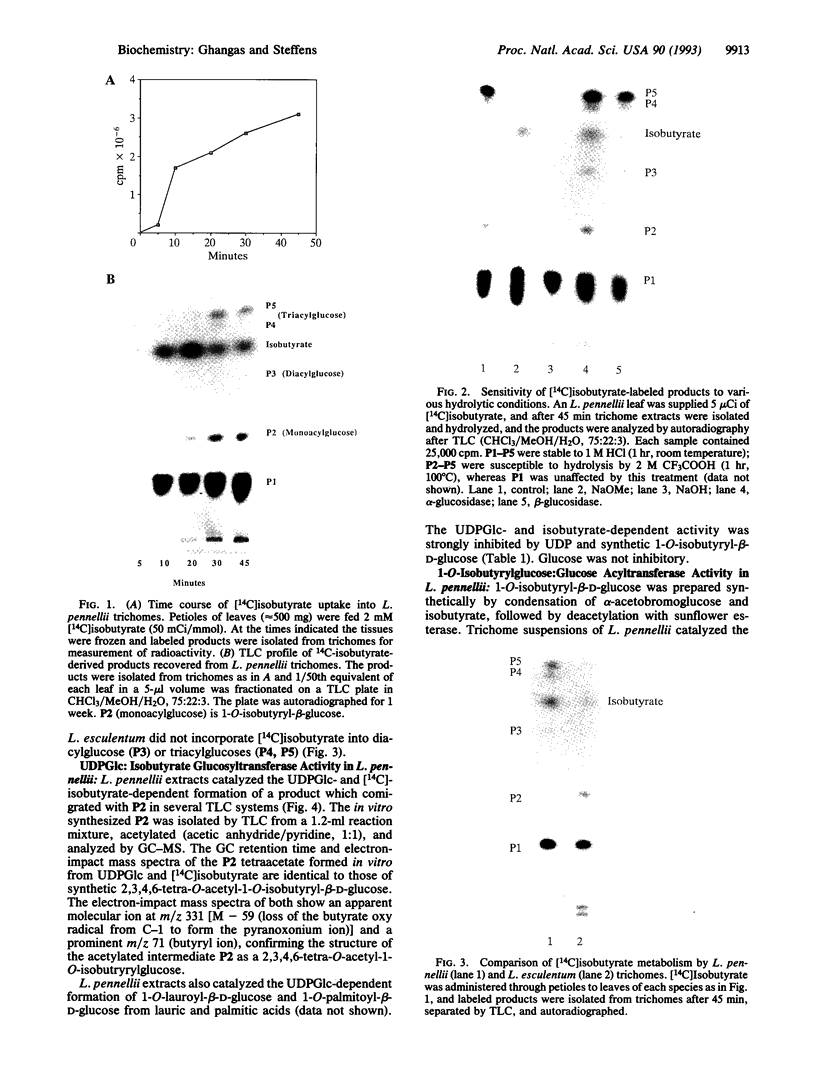
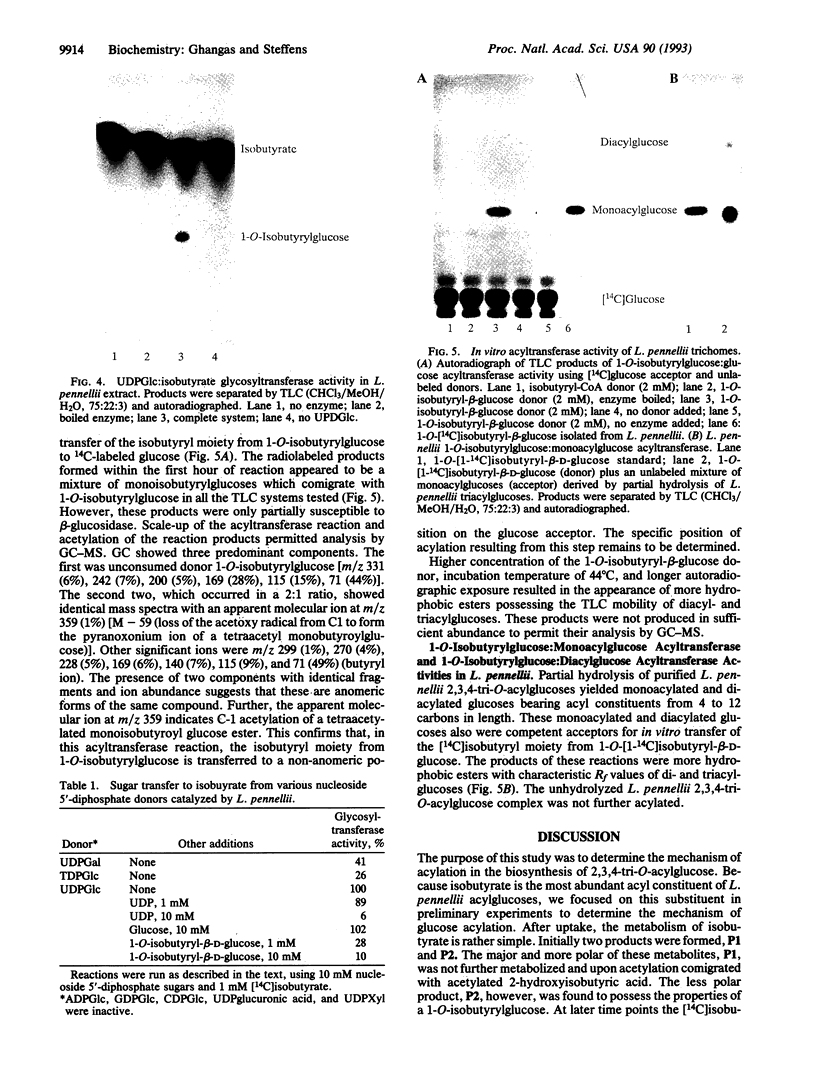

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Fobes J. F., Mudd J. B., Marsden M. P. Epicuticular Lipid Accumulation on the Leaves of Lycopersicon pennellii (Corr.) D'Arcy and Lycopersicon esculentum Mill. Plant Physiol. 1985 Mar;77(3):567–570. doi: 10.1104/pp.77.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffreda J. C., Szymkowiak E. J., Sussex I. M., Mutschler M. A. Chimeric tomato plants show that aphid resistance and triacylglucose production are epidermal autonomous characters. Plant Cell. 1990 Jul;2(7):643–649. doi: 10.1105/tpc.2.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandra G., Severson R., Wagner G. J. Modified branched-chain amino acid pathways give rise to acyl acids of sucrose esters exuded from tobacco leaf trichomes. Eur J Biochem. 1990 Mar 10;188(2):385–391. doi: 10.1111/j.1432-1033.1990.tb15415.x. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Kojima M. Partial purification and characterization of UDPG:t-cinnamate glucosyltransferase in the root of sweet potato, Ipomoea batatas Lam. J Biochem. 1984 Jan;95(1):205–212. doi: 10.1093/oxfordjournals.jbchem.a134586. [DOI] [PubMed] [Google Scholar]
- Shinozaki Y., Matsuzaki T., Suhara S., Tobita T., Shigematsu H., Koiwai A. New types of glycolipids from the surface lipids of Nicotiana umbratica. Agric Biol Chem. 1991 Mar;55(3):751–756. [PubMed] [Google Scholar]
- Southwick S. M., Chung A., Davenport T. L., Ryan J. W. A rapid, simple synthesis and purification of abscisic Acid glucose ester. Plant Physiol. 1986 May;81(1):323–325. doi: 10.1104/pp.81.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas R. J., Kojima M. Purification and characterization of hydroxycinnamoyl D-glucose. Quinate hydroxycinnamoyl transferase in the root of sweet potato, Ipomoea batatas Lam. J Biol Chem. 1986 Jul 5;261(19):8729–8733. [PubMed] [Google Scholar]
- Walters D. S., Steffens J. C. Branched Chain Amino Acid Metabolism in the Biosynthesis of Lycopersicon pennellii Glucose Esters. Plant Physiol. 1990 Aug;93(4):1544–1551. doi: 10.1104/pp.93.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerger E. H., Grazzini R. A., Hesk D., Cox-Foster D. L., Craig R., Mumma R. O. A rapid method for isolating glandular trichomes. Plant Physiol. 1992 May;99(1):1–7. doi: 10.1104/pp.99.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]