Abstract
AIM: To analyze the clinical and pathological parameters and expression of the neural cell adhesion molecule (CD56) in patients with biliary atresia (BA).
METHODS: Established clinical laboratory markers of hepatic function, including enzyme activity, protein synthesis, and bilirubin metabolism, were evaluated in patients with BA and compared with those in patients with choledochal cysts and neonatal hepatitis. Pathological changes in tissue morphology and fibrosis were examined by histological and tissue collagen staining. Immunohistochemical staining for the biliary epithelial cell markers CD56 and CK19 together with the Notch signaling related molecules Notch1 and Notch2 was performed in the context of alterations in the structure of intrahepatic biliary ducts.
RESULTS: Differences in some clinical laboratory parameters among the three diseases examined were observed, but they did not correlate with the pathological classification of fibrosis in BA. Immunohistochemical staining showed the presence of CD56-positive immature bile ducts in most patients (74.5%) with BA but not in patients with choledochal cysts or neonatal hepatitis. The number of CD56-expressing cells correlated with disease severity, with more positive cells present in the later stages of liver damage (81.8% vs 18.2%). Furthermore, bile plugs were mainly found in CD56-positive immature biliary ducts. Notch signaling was a key regulatory pathway in biliary duct formation and played a role in tissue fibrosis. Notch1 was co-expressed in CD56-positive cells, whereas Notch2 was found exclusively in blood vessels in the portal area of patients with BA.
CONCLUSION: The maturation of biliary epithelial cells and the expression of Notch may play a role in the pathogenesis of BA.
Keywords: Biliary atresia, CD56, Epithelial cell adhesion molecule, Cytokeratin 7, Biliary epithelial cells, Liver fibrosis
Core tip: Clinical laboratory parameters did not correlate with tissue fibrosis in patients with biliary atresia (BA). Immunohistochemical staining showed that CD56-positive immature bile ducts were present only in patients with BA but not in those with choledochal cyst or neonatal hepatitis. The number of CD56 expressing cells correlated with disease severity, with more positive cells present in the later stages of liver damage. Furthermore, the signaling molecule Notch1, but not Notch2, was co-expressed in CD56 positive cells. Our data provide new insights into the pathogenesis of BA in terms of biliary epithelial cell maturation and Notch expression.
INTRODUCTION
Biliary atresia (BA) is an infantile disorder that affects both intrahepatic and extrahepatic bile ducts, with progressive tissue fibrosis leading to irreversible liver cirrhosis[1]. With advances in clinical care and the availability of Kasai portoenterostomy to re-establish bile drainage, mortality has been significantly reduced; however, patients may still develop cirrhosis without liver transplantation[1,2]. The etiology of BA is still not fully understood, although a variety of pathological processes, such as viral infection[3], dysregulation of the immune system[4], and genetic defects, have been reported[5] in animal models and patients. It has also been proposed that the condition is multifactorial[6]. As transplantation is limited, advancing our knowledge on the pathological processes, such as inflammation and fibrosis, in BA may identify new therapeutic targets for treatment.
In patients with BA, an increase in bile ductules as the disease develops has been demonstrated, a process that is suggested to be associated with liver regeneration[7]. However, the function of these ductules is not fully understood, especially in liver fibrosis. Several markers have been used to differentiate biliary epithelial cells (BECs) from other hepatic cells and blood vessels. The most commonly used marker is cytokeratin 7 (CK7), which stains all BECs but also reacts with some progenitor hepatocytes[8]. Some investigators have used CD56 (neural cell adhesion molecule, NCAM) to identify immature BECs and epithelial cell adhesion molecule (EpCAM) as a marker of mature BECs, and antibodies specific for these molecules have been successfully used to isolate BECs from animal and human samples[9]. The expression of CD56 in patients with BA has been described in some studies[10,11], however, the function and relationship of this marker with the pathological changes that occur in BA have not been well studied.
Notch signaling plays a key role in the development of the biliary system[12]. Alagille syndrome is caused by mutations in Jagged1, which encodes a ligand for Notch1, resulting in a loss of bile duct structure in the portal tracts[13]. However, Notch signaling contributes to the pathology of carbon tetrachloride-induced liver fibrosis in an animal model, and use of the inhibitor γ-secretase reduces fibrosis[14]. In addition, in Alagille syndrome, although cholestasis is severe, progression to biliary cirrhosis is rare[15], further indicating that Notch1-mediated signaling contributes to tissue fibrosis. Notch2 functions in normal peri- and post-natal formation of the intrahepatic bile duct (IHBD), and low expression of this receptor causes malformation of the IHBD. Notch2 can also influence the development of blood vessels[16]. As the pathological processes in BA include the formation of neo-ductules and tissue fibrosis, the function of Notch signaling in BA warrants investigation.
In this study, we report differences in serum levels of markers of bile metabolism, i.e., total bilirubin (TBIL), direct bilirubin (DBIL), and total bile acid levels (TBA), in three cholestatic diseases: BA, neonatal hepatitis (NH), and choledochal cysts (CCs). Significantly higher levels of TBIL, DBIL, and TBA were found in patients with BA and NH compared to those with CC, which suggests that the liver, but not extra-hepatic structures, influences function. In addition, we noted that gamma-glutamyl transferase (γ-GT), which reflects involvement of BECs, was increased in BA, but was less pronounced in NH. Detailed analysis of the phenotype of BECs in the context of CD56, EpCAM, and CK7 expression was performed in order to determine the different stages of maturation. Biliary epithelial cells stained for CD56 were present in higher numbers in patients with BA compared to those with CC or NH, which implies that neo-BECs might contribute directly to liver fibrosis. As Notch signaling is related to tissue fibrosis, increased expression of Notch1 in these cells suggests that CD56-related Notch signaling may also be involved in the pathogenesis of BA.
MATERIALS AND METHODS
Materials
The Trichrome Stain (Masson) Kit, Sirius Red, and other chemical reagents used were purchased from Sigma (Sigma-Aldrich Corporation, St Louis, MO, United States). Antibodies used were mouse anti-human CD56 (Dako Denmark, Glostrup, Denmark), mouse anti-human EpCAM, and mouse anti-human CK7 (Santa Cruz Biotechnology, Dallas, TX, United States), rabbit anti-human Notch1 (Cell Signaling Technology, Beverly, MA, United States), and rabbit anti-human Notch2 (Bethyl Laboratories, Montgomery, TX, United States).
Patients’ medical histories
Patients’ medical histories were obtained from the patient record system of Guangzhou Women and Children’s Medical Center, China. Clinical information relevant to this study is shown in Table 1. The age of patients with BA and NH was often less than 1 year, whereas the age of patients with CC varied from 4 months to 10 years, therefore, the latter group was further categorized by age (i.e., CC-AM < 1 year old) and then compared. The experimental protocols were approved by the Medical Ethics Committee of Guangzhou Women and Children’s Medical Center.
Table 1.
Patient medical histories
| Patient characteristic |
Disease |
||||
| BA-E | BA-ML | NH | CC | CC-AM | |
| Number | 41 | 44 | 32 | 65 | 32 |
| Age1 (mo) | 2.2 ± 1.0 | 2.4 ± 1.7 | 1.9 ± 1.0 | 43.4 ± 31.6 | 2.9 ± 2.5 |
| Sex | M: 19 F: 22 | M: 30 F: 14 | M: 23 F: 9 | M: 17 F: 47 | M: 9 F: 23 |
mean ± SD. BA: Biliary atresia; BA-E: Early stage of BA with less tissue fibrosis; BA-ML: Middle or late stage BA with more or dense tissue fibrosis; CC: Choledochal cyst; CC-AM: Patients aged < 1 year with CC; F: Female; M: Male.
Clinical laboratory investigations
To compare differences in the biochemical parameters for BA, CC, and NH, the serum levels of 10 biochemical parameters were measured and analyzed. The serum samples were obtained from patients before surgery and were analyzed using a Hitachi Pre-Analytical Process Automation System with a 7600 Clinical Analyzer. The 10 parameters analyzed were alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-GT, total protein (TP), albumin (ALB), globulin (GLO), TBIL, DBIL, indirect bilirubin (IBIL), and TBA.
Histology analysis and evaluation of tissue fibrosis
Tissue fibrosis is one of the key pathophysiological changes in patients with BA and is often an indicator of an adverse clinical outcome. The deposition of tissue collagen was assessed as a clinical pathological parameter and classified according to the degree of tissue fibrosis by the pathology department. To confirm the clinical observations, sections were stained with Masson’s Trichrome Stain. In brief, the paraffin sections obtained from the pathology department were dewaxed and hydrated in xylene and descending concentrations of ethanol. The pathological changes were illustrated by Hematoxylin and Eosin staining. For Masson’s Trichrome staining, the sections were re-fixed in Bouin’s solution and then stained with Weigert’s iron hematoxylin solution before Biebrich scarlet-acid fuchsin solution was added. The color was differentiated in phosphomolybdic-phosphotungstic acid solution until the collagen was no longer red. The sections were then transferred to aniline blue solution and finally differentiated in 1% acetic acid solution. To confirm deposition of collagen, the adjacent sections were de-waxed and hydrated, and Sirius Red was applied for 1 h at room temperature; after counterstaining with hematoxylin, the sections were mounted and analyzed.
Immunohistochemical analysis
Tissue sections were dewaxed and hydrated as described above. Depending on the antibody, antigen retrieval was performed on the sections in 0.01 M citrate buffer (pH 6.0) for EpCAM, CK7, and Notch2, or tris-ethylenediaminetetraacetic acid (EDTA) buffer (pH 9.0) for CD56, Notch1, and CD31 in a microwave for 10 min at 95 °C. Endogenous peroxidase was quenched with 3% hydrogen peroxide (H2O2) for 10 min, and non-specific signals were blocked by the addition of 0.1% bovine serum albumin (BSA) and 0.1% goat serum for 30 min at room temperature. The antibodies (1:50-1:200 in dilution buffer, Dako) were added and incubated at 37 °C for 60 min or overnight at 4 °C. The signals were revealed by the Dako EnVision System (Dako Denmark, Glostrup, Denmark) with diaminobenzidine (DAB) or the Polink DS-MR-Hu C1 kit for double staining following the manufacturer’s instructions. The sections were observed using Nikon Eclipse Ci (Nikon Instruments (Shanghai) Co., Ltd., Shanghai, China) and photographed with NIS-Elements software (Nikon Instruments).
Statistical analysis
All clinical data are presented as mean ± standard deviation (SD). The statistical analysis was performed using GraphPad® Prism software (GraphPad Software, Inc., La Jolla, CA, United States). Student’s t test was used when comparing two sets of data and one way analysis of variance (ANOVA) was used when three sets or more were compared. The Tukey multiple comparison test was used for statistical analysis of each individual comparison within a group. P values < 0.05 were considered statistically significant.
RESULTS
Differences in biochemical parameters in patients with BA, CC, and NH
Overall, none of the parameters was significantly different when the CC-AM group was compared with CC group using the Student’s t test (Table 2). However, when CC-AM patients were compared with CC patients excluding the CC-AM group (i.e., CC-AM patients vs CC patients aged > 1 year), the level of γ-GT was significantly different (786.9 ± 759.2 U/L vs 357.2 ± 408.4 U/L, respectively, P < 0.01), as was TBIL (130.7 ± 116.8 μmol/L vs 46.9 ± 58.2 μmol/L, respectively, P < 0.001). The difference in TBIL might have arisen due to increased levels of IBIL in the CC-AM patients (68.4 ± 88.1 μmol/L vs 14.9 ± 16.7 μmol/L, respectively, P < 0.01).
Table 2.
Clinical laboratory analysis of molecules in blood related to liver function
| Population |
Average (SD) value |
||||||||||
| ALT (U/L) | AST (U/L) | γ-GT (U/L) | TP (g/L) | ALB (g/L) | GLO (g/L) | ALB GLO | TBIL (μmol/L) | DBIL (μmol/L) | IBIL (μmol/L) | TBA (μmol/L) | |
| Normal ranges | 9-50 | 5-60 | 10-60 | 65-85 | 40-55 | 20-40 | 1.2-2.4 | 2-17 | 0-7 | 2-13.7 | 0-15 |
| BA (n = 85) | 183.8 (167.9) | 284.8 (279.8) | 898.0 (724.0) | 57.7 (6.7) | 39.0 (5.6) | 21.4 (28.8) | 2.4 (0.9) | 184.8 (60.6) | 136.7 (45.7) | 48.1 (20.7) | 169.7 (79.5) |
| NH (n = 32) | 251.9 (391.2) | 372.7 (425.9) | 271.7d (297.6) | 61.3 (40.5) | 38.1 (6.2) | 39.4 (132.8) | (2.5 (0.8) | 199.0 (96.5) | 134.0 (86.7) | 64.7 (39.2) | 158.0 (96.0) |
| CC | |||||||||||
| All (n = 65) | 105.0b (115.3) | 208.9 (252.1) | 575.4c (644.8) | 62.1 (9.3) | 41.1 (5.6) | 24.3 (27.1) | 3.2 (8.8) | 88.8f (100.8) | 47.2h (61.0) | 41.6 (68.4) | 77.7jl (124.1) |
| Aged < 1 yr (n = 32) | 88.6a (104.3) | 245.6 (326.1) | 786.9e (759.2) | 57.9 (7.7) | 39.6 (5.0) | 18.3 (4.4) | 2.3 (0.5) | 130.7g (116.8) | 62.3i (71.4) | 68.4 (88.1) | 83.9km (91.1) |
| Aged > 1 yr (n = 33) | 117.3 (116.8) | 260.9 (264.2) | 357.2 (408.4) | 66.2 (9.2) | 42.6 (5.8) | 30.4 (37.4) | 4.1 (12.4) | 46.9 (58.2) | 32.0 (44.6) | 14.9 (16.7) | 71.8 (150.7) |
For the statistical analysis, the differences in BA, NH, and CC (total) for each clinical parameter were studied, we first used one-way ANOVA to determine whether the F test was significant and if so, Tukey’s multiple comparisons test was used. A similar procedure was used to analyze age-matched CC. Details of the F test results are shown in the results section.
P < 0.01 and
P < 0.05 vs NH;
P < 0.05 and
P < 0.001 vs BA;
P < 0.01 vs NH;
P < 0.001 and
P < 0.01 vs BA and NH;
P < 0.001 and
P < 0.001 vs BA and NH;
P < 0.001 and
P < 0.001 vs BA;
P < 0.01 and
P < 0.01 vs NH. ALB: Albumin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BA: Biliary atresia; CC: Choledochal cyst; DBIL: Direct bilirubin; γ-GT: Gamma-glutamyl transferase; GLO: Globulin; IBIL: Indirect bilirubin; NH: Neonatal hepatitis; TBA: Total bile acid; TBIL: Total bilirubin; TP: Total protein.
No differences in TP, ALB, GLO, or the ratio of ALB to GLO were observed between the three diseases compared with the normal ranges of expression of these biochemical markers. AST levels were higher than normal (> 3-fold) in all three patient groups, but there was no significant difference. Using one way ANOVA with regard to ALT, there were differences in the means of ALT in BA, NH, and CC patients [F (2, 180) = 5.702, P = 0.0040] and in BA, NH, and CC-AM patients [F (2, 148) = 4.048, P = 0.0194]. There was no statistically significant difference when the patients with BA were compared to those with CC or NH, although there were differences between patients with NH and both the overall CC group and the CC-AM subgroup (P < 0.01 and P < 0.05, respectively). There were differences in the means of γ-GT between BA, NH, and CC patients [F (2, 179) = 11.57, P < 0.0001] and between BA, NH, and CC-AM [F (2, 148) = 9.927, P < 0.0001]. Markedly higher levels of γ-GT were observed in patients with BA (898.0 ± 724.0 U/L), which were 3-fold higher than in the NH group (271.7 ± 297.6 U/L, P < 0.001) and 1.5-fold higher than in the CC group (575.4 ± 644.8 U/L, P < 0.05). There was no significant difference in γ-GT between BA and CC-AM groups, although the level in the CC-AM group was higher (786.9 ± 759.2 U/L). Furthermore, the high level of γ-GT in the CC-AM group reached significance compared with the NH group (P < 0.01).
Bilirubin metabolism is one of the most important indicators of liver function. Levels of all three bilirubin proteins (TBIL, DBIL, and IBIL) were higher than the upper limit of the normal ranges in the three diseases studied. In terms of TBIL, one way ANOVA showed differences in the means of these three proteins in BA, NH, and CC patients [F (2, 180) = 30.48, P < 0.0001], and in BA, NH and CC-AM patients [F (2, 148) = 6.469, P = 0.0020], when compared with the CC group (88.8 ± 100.8 μmol/L). The levels of TBIL were significantly higher in the BA and NH groups (184.8 ± 60.6 μmol/L and 199.0 ± 96.5 μmol/L, respectively; P < 0.001 for both) and the CC-AM group (130.7 ± 116.8 μmol/L, P < 0.01). Similarly, differences in the means of DBIL were observed in the three groups [CC; F (2, 180) = 46.06, P < 0.0001] and CC-AM; F (2, 148) = 17.92, P < 0.0001]. Increased levels of serum DBIL were observed in the BA and NH groups (136.7 ± 45.7 μmol/L and 134.0 ± 86.7 μmol/L, respectively) compared with the CC (47.2 ± 61.0 μmol/L) and CC-AM (62.3 ±7 1.4 μmol/L) subgroups (P < 0.001 for all comparisons). Total bile acid levels reflect liver function in terms of fatty acid metabolism, and the pattern of high levels detected in patients with BA and NH compared to those with CC was similar to that for bilirubin. The same phenomenon was observed for TBA in the three groups, BA, and NH with either CC or CC-AM, and the differences were significant [F (2, 172) = 14.79, P < 0.0001] and [F (2, 140) = 10.50, P < 0.0001]. Increased levels of TBA were observed in the BA and NH groups (169.7 ± 79.5 μmol/L and 158.0 ± 96.0 μmol/L, respectively) compared with the CC and CC-AM groups (71.8 ± 150.7 μmol/L, P < 0.001 and 83.9 ± 91.1 μmol/L, P < 0.01, respectively).
Evaluation of liver tissue fibrosis
In order to determine whether clinical laboratory parameters had any relation to tissue fibrosis, the collagen content in tissue sections was examined with Masson’s Trichrome Stain. We observed that low levels of collagen were deposited in patients with early liver damage [early stage of biliary atresia (BA-E)], in which there was neither fibrosis nor mild portal fibrosis[17,18], and similar results were obtained for patients with NH and CC. However, collagen deposits were markedly increased in patients with moderate or late liver damage [middle or late stage of biliary atresia (BA-ML)], who were reported to have a high degree of fibrosis[17,18] (Figure 1 left panel). These results were confirmed by Sirius Red staining, which allowed more accurate quantification (Figure 1, right panel).
Figure 1.
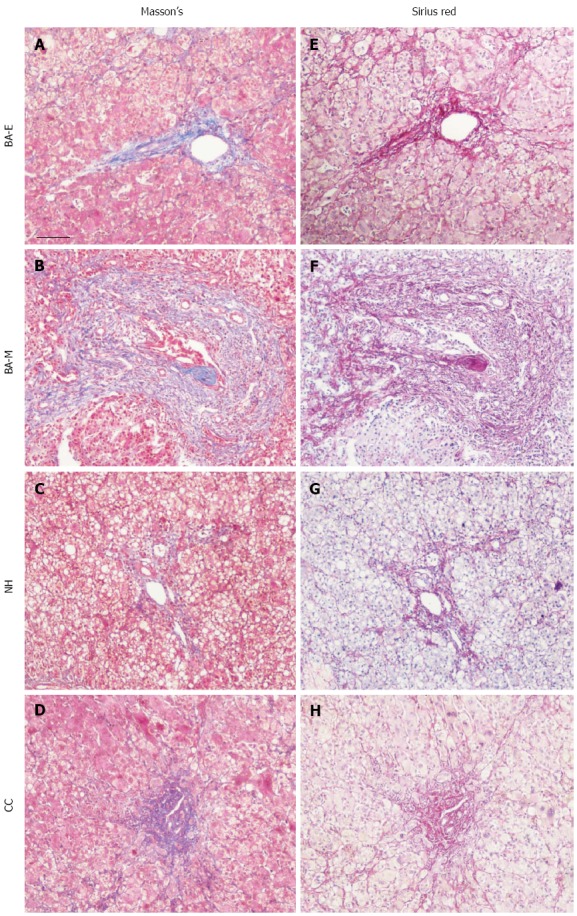
Evaluation of tissue fibrosis in biliary atresia, choledochal cyst, and neonatal hepatitis patients. Liver tissues from patients with BA, CC, and NH were collected and adjacent sections were cut. Tissue fibrosis was evaluated with Masson’s Trichrome Stain, in which the collagen was detected by blue color. Depending on the stage of fibrosis, sections from patients with BA were further separated into two groups: early stage BA (BA-E), with no or mild collagen deposition, and middle or late stage BA (BA-ML), with dense collagen deposition. The adjacent tissue sections were further stained with Sirius Red to validate the results, with collagen detected by pink color. Sections were examined with a Nikon light microscope. Scale bar shown represents 50 μm. NH: Neonatal hepatitis; CC: Choledochal cyst; BA: Biliary atresia.
The levels of biochemical parameters, especially γ-GT, TBIL, and DBIL, were significantly different in the three different cholestatic diseases, and these were reanalyzed in the context of the degree of tissue fibrosis, using BA-E and BA-ML as the stages of disease progression. There were no statistically significant differences, although the trend in the BA-ML group suggested that there might be increases in all three parameters compared with the BA-E group.
Bile plugs in liver samples from patients with BA
No significant differences in serum levels of the biochemical parameters of bilirubin metabolism were found between the BA-E and BA-ML groups, which suggested either a reduction in the expression of bilirubin-related molecules or impairment of the transport of these products. Accumulation of bile plugs is a hallmark of BA, and it can often be found in bile ducts or ductules (Figure 2A, B, E, and F). In contrast, the presence of bile plugs was much less marked in patients with NH and CC (Figure 2C and G; D and H). However, when using BA-E and BA-ML as the different stages of BA, no difference was found in the accumulation of bile plugs, which was extensive in both stages, and their presence in liver tissue was not evenly distributed.
Figure 2.
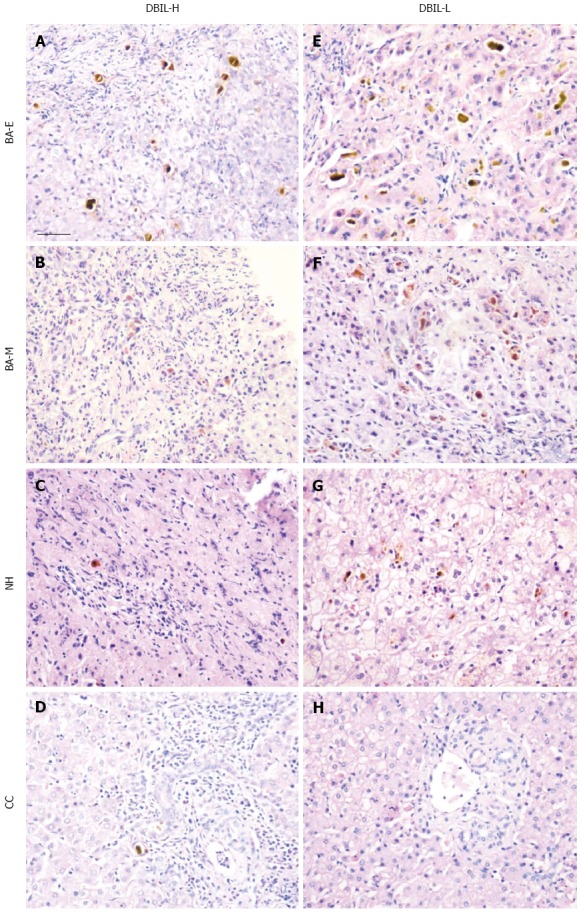
Comparison of bile plugs in biliary atresia, choledochal cyst, and neonatal hepatitis patients. HE staining was performed on tissue sections from patients with three cholestatic diseases: biliary atresia [early (BA-E) and middle and late (BA-ML)], CC, and NH. The sections were separated based on their serum levels of DBIL into two groups: bilirubin high (DBIL-H) and bilirubin low (DBIL-L), with the sections containing the highest number of bile plugs in each group selected and photographed. The sections were examined with a Nikon light microscope. Scale bar represents 50 μm. CC: Choledochal cyst; DBIL: Direct bilirubin; HE: Hematoxylin and eosin; NH: Neonatal hepatitis
Analysis of intrahepatic bile ducts
Changes in morphology of the IHBDs were further examined using different markers for BECs. CK7 has long been used as a marker for BECs and showed positive staining in all tissue sections examined in patients with BA, NH, and CC as well as the disease control (portal vein cavernous transformation, PVCT) (Figure 3A, D, G and J). However, CK7 staining was slightly weaker in BA, which may have been related to its immature status. Compared to the control, the number of CK7-positive cells was greatly increased in patients with BA, but less so in those with NH. In patients with CC, the number of cells was similar to that in the control.
Figure 3.
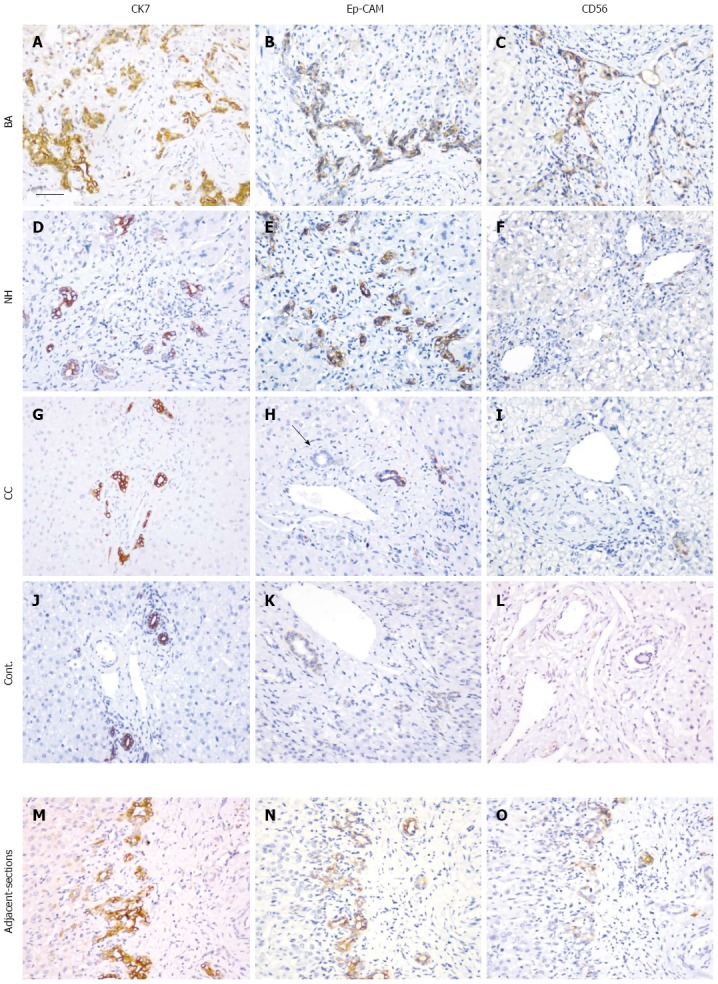
Immunohistochemical analysis of the expression of cytokeratin 7, epithelial cell adhesion molecule, and CD56. Tissue sections from patients with BA (A-C), NH (D-F), CC (G-I), and the disease control (J-L) together with a set of adjacent sections (M-O) were selected. Immunohistochemical staining was performed with antibodies for CK7 (A, D, G, J and M), EpCAM (B, E, H, K and N) and CD56 (C, F, I, L and O). After antigen retrieval, the sections were incubated with antibodies overnight at 37 °C. The black arrow in (H) indicates a bile duct. The sections were examined with a Nikon light microscope. Scale bar represents 50 μm. EpCAM: Epithelial cell adhesion molecule; CK7: Cytokeratin 7; NH: Neonatal hepatitis; CC: Choledochal cyst; BA: Biliary atresia.
EpCAM is a marker of mature BECs and was expressed in BA, NH, CC, and the disease control (Figure 3B, E, H and K). The pattern of EpCAM staining was similar to that of CK7, but the number of cells was less pronounced than that detected for CK7. In some tissue sections, bile ducts in the portal tract did not stain with EpCAM; and, therefore, the staining pattern was different from that of CK7 (Figure 3M and N).
CD56 is a marker of immature BECs. Marked expression of CD56-positive BECs was observed in patients with BA (Figure 3C), but not in those with NH, CC, or in normal control liver (Figure 3F, I and L). Using adjacent tissue sections from patients with BA, a partial co-localization of CD56 and CK7 was observed (Figure 3M and O).
Distribution of CD56-positive cells in patients with BA
Using adjacent tissue sections, expression of CD56 was further analyzed and was observed to co-localize with EpCAM (Figure 4A-H). The CD56-positive immature bile ducts were mostly found in the periportal tract, where bile plugs were often observed (Figure 4I). Inflammatory cell infiltration is an important event in the development of BA, and expression of major histocompatibility complex class II [human leucocyte antigen (HLA)-DR] proteins was detected in tissue sections. The co-localization of CD56 and HLA-DR staining indicated the close relationship between these two sets of cells, and, in some instances, HLA-DR-positive cells were found in CD56-positive immature bile ducts (Figure 4J).
Figure 4.
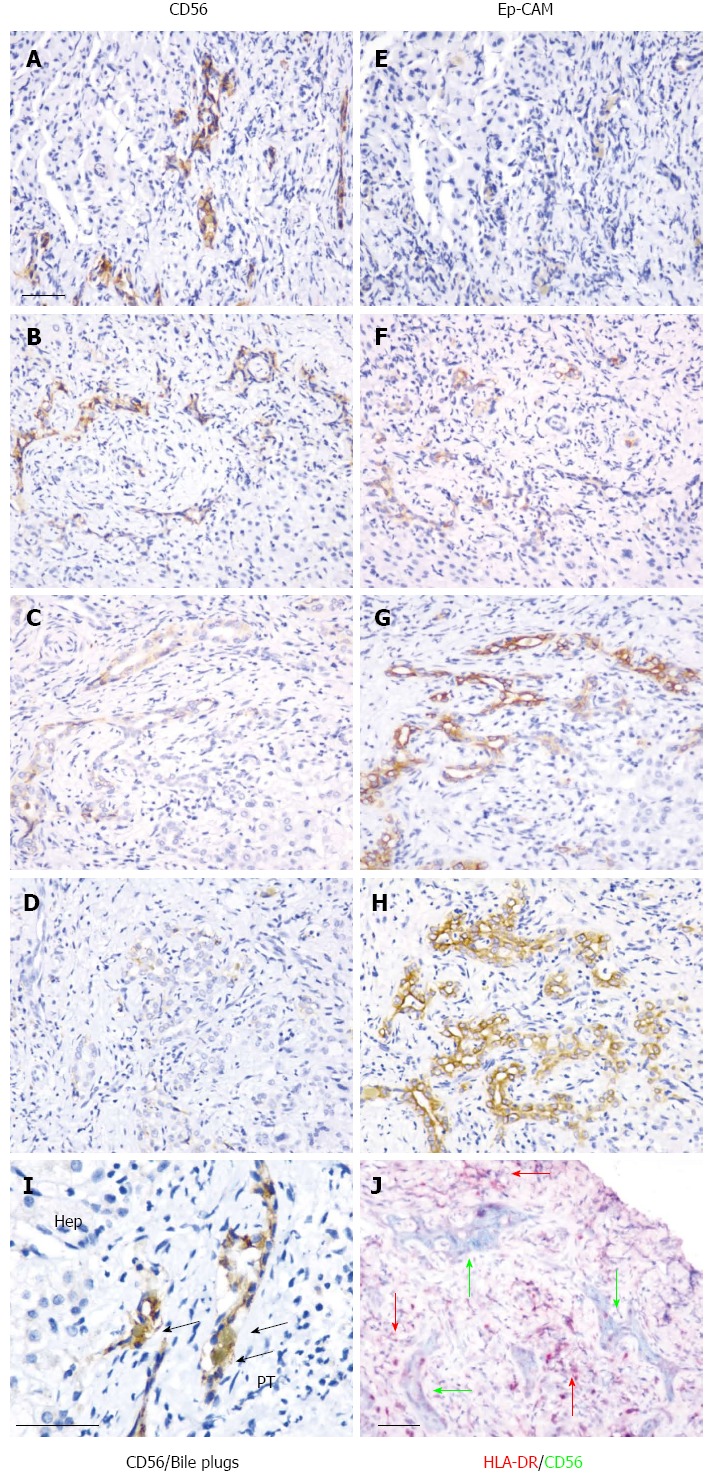
Co-localization of CD56 with epithelial cell adhesion molecule, bile plugs, and human leucocyte antigen-DR. Co-localization of CD56 (A-D) and EpCAM (E-H) is illustrated in adjacent sections using immunohistochemical staining. From the upper (A, E) to the lower (D, H) slides, the full spectrum of the CD56 staining results is shown. Co-localization of CD56 with bile plugs is shown in (I). Localization of CD56 and HLA-DR positive cells is shown in (J). Black arrows indicate bile plugs. Pink arrows indicate HLA-DR-positive cells. Dark green represents CD56-positive cells. The sections were examined and the scale bar represents 50 μm. Hep: Hepatocytes; PT: Portal tract; EpCAM: Epithelial cell adhesion molecule; HLA-DR: Human leucocyte antigen-DR.
Three categories of CD56 expression were identified based on the intensity of staining: strong (++) (Figure 4A and B), weak (+) (Figure 4C), and no signal (-) (Figure 4D). In order to further understand the relationship between CD56, bile plugs and the pathological classification of BA-E and BA-ML, the percentage of each category was calculated for patients with BA (Table 3). Overall, the percentage of CD56++ was higher for BA-ML than for BA-E (57.1% vs 40.1%) and for sections with bile plugs than those without (51.2% vs 41.7%). Further analysis of the disease subcategories found that BA-E without bile plugs had a higher percentage of CD56- cells than CD56+ cells (50% vs 16.7%) and BA-ML with bile plugs had a higher percentage of CD56+ than CD56- cells (54.4% vs 18.2%). These data suggest a positive correlation between CD56+ cells and disease progression.
Table 3.
Expression of CD56 in biliary atresia n (%)
| Stage of BA | n | CD56++ | CD56+ | CD56- |
| All | 55 | 27 (49.1) | 14 (25.5) | 14 (25.5) |
| BA-E without bile plugs | 6 | 1 (16.7) | 2 (33.3) | 3 (50.0) |
| BA-E with bile plugs | 21 | 10 (47.6) | 5 (23.8) | 6 (28.6) |
| BA-ML without bile plugs | 6 | 4 (66.7) | 1 (16.7) | 1 (16.7) |
| BA-ML with bile plugs | 22 | 12 (54.5) | 6 (27.3) | 4 (18.2) |
| All BA-E | 27 | 11 (40.7) | 7 (25.9) | 9 (33.3) |
| All BA-ML | 28 | 16 (57.1) | 7 (25.0) | 5 (17.9) |
| All without bile plugs | 12 | 5 (41.7) | 3 (25.0) | 4 (33.3) |
| All with bile plugs | 43 | 22 (51.2) | 11 (25.6) | 10 (23.3) |
BA: Biliary atresia; BA-E: Early stage of BA with less tissue fibrosis; BA-ML: Middle or late stage BA with more extensive or dense tissue fibrosis.
Expression of Notch signaling components in patients with BA
Notch signaling is involved in development of the bile duct system and also plays a role in tissue fibrosis. In order to study the relationship between CD56-positive BECs and the Notch signaling pathway, expression of the receptors Notch1 and Notch2 was investigated in patients with BA using immunohistochemistry and double staining. In age-matched liver tissue controls, Notch1 expression was detected in mature bile ducts in the portal area (Figure 5A). Expression of CD56 was rarely detected in normal liver tissue, but, when observed, it was not co-localized with Notch1. In contrast, increased expression of CD56 was found in immature bile ducts in patients with BA, and most of them also expressed Notch1 (Figure 5B). Expression of Notch1 was detected mainly in the lumen of the bile ducts in both normal tissue and tissue from patients with BA, which suggests the presence of the secreted form of the protein. Expression of Notch2 was mainly in the blood vessels, both the hepatic arteries and portal veins, which are situated inside the portal area in healthy subjects and those with BA (Figure 5C-F). No co-localization of CD56+ and Notch2+ cells was observed in the portal tract (Figure 5D). However, increased CD31, one of the endothelial cell markers, was found in tissue from patients with BA, and co-localization with Notch2 was observed. It is suggested that Notch signaling is increased in patients with BA, which may impact progression of tissue fibrosis.
Figure 5.
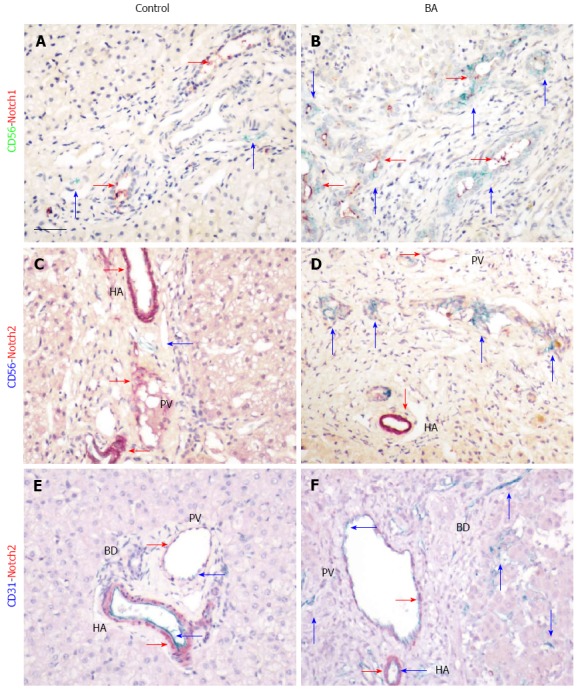
Expression of Notch signaling components in biliary atresia. Co-localization of CD56-positive cells with Notch1 (A and B) and Notch2 (C and D) is illustrated by immunohistochemical double staining with mouse anti-human CD56 and rabbit anti-human Notch1 and Notch2 using the Polink DS-MR-Hu C1 kit. Positive signals are indicated by arrows, with corresponding colors in the side bars. Scale bar represents 50 μm. BD: Bile duct; HA: Hepatic artery; PV: Portal vein.
DISCUSSION
In this study, we compared 10 clinical laboratory parameters in three neonatal cholestatic diseases and found similarities in most of the parameters examined. The levels of TP, ALB, and GLO, which reflect biosynthesis and storage of liver proteins, were similar to normal reference levels. Although there was variation between individual patients, it seems unlikely that these parameters are primarily responsible for severe liver damage. This is understandable, as blockade of bile transport in these three diseases is initially affected, while damage to the hepatocytes occurs only at the end stage of these conditions. Metabolism of bilirubin was the main feature that differentiated CC (including age-matched CC) from BA and NH, with the levels of DBIL being much lower in the CC group. This difference can be explained by the fact that CC mostly affects the extra-hepatic biliary ducts, but also damages the hepatocytes themselves and leakage of bile into the bloodstream is not severe. Similar results were obtained for TBIL and TBA, which indicates that metabolism of bilirubin was more severely impaired in patients with BA and NH than in those with CC. In terms of distinguishing BA and NH based on clinical laboratory parameters, only γ-GT showed different levels. In patients with NH, a 4-fold increase in γ-GT was observed compared to the upper limit of the normal range; however, γ-GT expression was increased 14-fold in patients with BA. Histological and immunohistochemical analyses showed more bile ducts and ductules in patients with BA than in those with NH. As γ-GT was expressed in both hepatocytes and BECs[19], higher numbers of BECs may be the source of increased levels of γ-GT. Our data validate an earlier report in which γ-GT was demonstrated to be a valuable indicator for distinguishing BA from NH[20]. However, as NH is a complex multi-factorial disease with variations in pathology, it is likely that some patients may develop BA in the later phase of the disease, and, therefore, the use of γ-GT as an exclusive clinical indicator requires caution.
The transition from inflammation to fibrosis is a pathological change that occurs in patients with BA, although the time frame for this has not been conclusively defined[2]. Infiltration of inflammatory cells, proliferation of neovascular and neo-biliary epithelial cells, and accumulation of bile play a role in this process, which complicates the understanding of the pathophysiology of the disease. In an attempt to simplify the analysis of the disease processes, we separated liver damage into two stages - BA-E and BA-ML - with tissue fibrosis as the main discriminator. We found that there was no difference in the number of bile plugs in early fibrosis compared to late stage fibrosis.
The number of bile plugs noted in bile ducts, which were occasionally found in hepatocytes, prompted us to study the morphology of the bile ducts. Using three different markers of BECs, we detected the presence of both CD56- and EpCAM-positive cells, which respectively reflect immature and mature BECs, and these two cell populations were a part of the cell population that stained with CK7. These results indicate that pathological changes develop in the biliary system of patients with BA. The expression of CD56 in bile ductules discriminated between BA and both NH and CC, confirming previous observations[10,11,21]. We failed to detect CD56-positive cells in some tissue sections from patients with BA, but the presence of the mature BEC markers may indicate heterogeneity of the maturation process. Whether this reflects the early phase of “ductal reaction” warrants further study. Nevertheless, even in the absence of CD56-positive cells, the possibility of BA cannot be excluded. Our observation that progression of BEC maturation reduced the expression of CD56 also suggests that analyzing the expression of EpCAM might help to clarify further events in the development of BA. As CK7 also stains ductal hepatocytes, or oval cells[22], which are progenitors of BECs, it may aid in understanding the origin of CD56-positive cells. The combined use of these three markers for BECs will extend our understanding of the function of BECs in the BA disease process.
Liver tissue regeneration offers some compensation for lost organ function arising from these diseases, and it has been suggested that the presence of CD56 positivity is a sign of tissue repair[21]. However, in this study, we found that newly formed bile ductules often co-localized with bile plugs. Analysis of the morphology of the ductules with CD56-positive staining often showed no lumen, and as maturation progressed, we noted that the CD56 signal reduced, but increased for EpCAM, which resulted in an increase in the lumen area. However, the high expression of CD56 in BA suggests that there is inhibition of the maturation process. Furthermore, bile accumulation contributes to liver fibrosis, as demonstrated by the finding that ligation of the bile duct can directly induce liver fibrosis[23]. The presence of CD56 implies the progression of BA to liver fibrosis. This, together with evidence for Notch signaling in CD56-positive cells, further increases the possibility of liver fibrosis, as this signaling pathway plays a key role in the development of fibrosis directly within tissues[24] and by the infiltration of inflammatory cells[25]. Our data suggest that the presence of CD56-positive BECs might promote deleterious outcomes in the progression of BA. We also demonstrated that CD56-positive cells are often surrounded by HLA-DR expressing cells, which is in agreement with previous studies[26,27]. Therefore, it is possible that CD56-positive BECs from ductal hepatocytes may interact with the inflammatory cell infiltrate. Our results are compatible with extrahepatic bile duct blockade and inflammatory cellular infiltration acting together to promote the pathogenesis of BA. The presence of CD56-positive cells does not ameliorate the pathological condition but, in contrast, accelerates the development of tissue fibrosis.
In conclusion, our study shows that levels of bilirubin-related parameters, such as TBIL, DBIL, and TBA, are higher in patients with BA and NH and moderately high in patients with CC. Furthermore, γ-GT was significantly increased in patients with BA as compared to those with NH. This change might be related to the increased levels of CD56-positive immature BECs in patients with BA. However, newly formed immature bile ducts or ductules have no bile transport function and bile may accumulate to form bile plugs. Enhanced expression of Notch signaling in CD56-positive cells further increases the possibility of progression to liver cirrhosis. Inhibition of the formation of CD56-positive BECs or the promotion of maturation of these cells may help to reduce BA progression to cirrhosis.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Stacey Cherny (Department of Psychiatry, the University of Hong Kong) for assistance with statistical analysis.
COMMENTS
Background
Clinical laboratory parameters do not correspond fully with the pathological processes in biliary atresia (BA). Therefore, it is necessary to define biliary ductular structure in order to assess the development of fibrotic changes.
Research frontiers
Clinical laboratory parameters, tissue fibrosis, and bile plugs have been examined in many studies, but the different stages of maturation of biliary epithelial cells (BECs) in BA are not well defined and their relationship with fibrosis is unclear.
Innovations and breakthroughs
The expression of CD56+ immature epithelial cells in BA, which was correlated with liver fibrosis, and their expression of fibrogenesis signaling molecule Notch 1 are novel findings in this study.
Applications
CD56+ immunohistochemistry might have additive value in the diagnosis of BA, and studies on the mechanisms of maturation of BECs may provide new insights into the treatment of BA.
Terminology
CD56 is a marker of immature BECs, whereas epithelial cell adhesion molecule (EpCAM) expression is representative of more mature BECs. CK7 labels all BECs.
Peer-review
The authors analyzed the clinical and pathological parameters together with the expression of the neural cell adhesion molecule (CD56) in BA patients. They found that the maturation of biliary epithelial cells and the expression of Notch might play a role in the pathogenesis of BA.
Footnotes
Supported by The grant of State Clinical Key Specialty Construction Project (Pediatric Surgery) 2013, No. GJLCZD1301; and Guangdong Provincial Science and Technology Plan Projects 2014, No. 2014A020212373.
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of Guangzhou Women and Children’s Medical Center, China.
Conflict-of-interest statement: None declared.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 30, 2015
First decision: October 15, 2015
Article in press: December 19, 2015
P- Reviewer: de Silva AP, Dong R, Moralioglu S S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang DN
References
- 1.Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374:1704–1713. doi: 10.1016/S0140-6736(09)60946-6. [DOI] [PubMed] [Google Scholar]
- 2.Bessho K, Bezerra JA. Biliary atresia: will blocking inflammation tame the disease? Annu Rev Med. 2011;62:171–185. doi: 10.1146/annurev-med-042909-093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu BR, Brindley SM, Tucker RM, Lambert CL, Mack CL. α-enolase autoantibodies cross-reactive to viral proteins in a mouse model of biliary atresia. Gastroenterology. 2010;139:1753–1761. doi: 10.1053/j.gastro.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shivakumar P, Sabla G, Mohanty S, McNeal M, Ward R, Stringer K, Caldwell C, Chougnet C, Bezerra JA. Effector role of neonatal hepatic CD8+ lymphocytes in epithelial injury and autoimmunity in experimental biliary atresia. Gastroenterology. 2007;133:268–277. doi: 10.1053/j.gastro.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bezerra JA, Tiao G, Ryckman FC, Alonso M, Sabla GE, Shneider B, Sokol RJ, Aronow BJ. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet. 2002;360:1653–1659. doi: 10.1016/S0140-6736(02)11603-5. [DOI] [PubMed] [Google Scholar]
- 6.Mack CL. The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Semin Liver Dis. 2007;27:233–242. doi: 10.1055/s-2007-985068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cocjin J, Rosenthal P, Buslon V, Luk L, Barajas L, Geller SA, Ruebner B, French S. Bile ductule formation in fetal, neonatal, and infant livers compared with extrahepatic biliary atresia. Hepatology. 1996;24:568–574. doi: 10.1002/hep.510240318. [DOI] [PubMed] [Google Scholar]
- 8.Bellizzi AM, LeGallo RD, Boyd JC, Iezzoni JC. Hepatocyte cytokeratin 7 expression in chronic allograft rejection. Am J Clin Pathol. 2011;135:238–244. doi: 10.1309/AJCPNRXCAP92KNOJ. [DOI] [PubMed] [Google Scholar]
- 9.Joplin R, Kachilele S. Human intrahepatic biliary epithelial cell lineages: studies in vitro. Methods Mol Biol. 2009;481:193–206. doi: 10.1007/978-1-59745-201-4_16. [DOI] [PubMed] [Google Scholar]
- 10.Sira MM, El-Guindi MA, Saber MA, Ehsan NA, Rizk MS. Differential hepatic expression of CD56 can discriminate biliary atresia from other neonatal cholestatic disorders. Eur J Gastroenterol Hepatol. 2012;24:1227–1233. doi: 10.1097/MEG.0b013e328356aee4. [DOI] [PubMed] [Google Scholar]
- 11.Torbenson M, Wang J, Abraham S, Maitra A, Boitnott J. Bile ducts and ductules are positive for CD56 (N-CAM) in most cases of extrahepatic biliary atresia. Am J Surg Pathol. 2003;27:1454–1457. doi: 10.1097/00000478-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Kodama Y, Hijikata M, Kageyama R, Shimotohno K, Chiba T. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology. 2004;127:1775–1786. doi: 10.1053/j.gastro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Zheng S, Qi D, Zheng S, Guo J, Zhang S, Weng Z. Inhibition of Notch signaling by a γ-secretase inhibitor attenuates hepatic fibrosis in rats. PLoS One. 2012;7:e46512. doi: 10.1371/journal.pone.0046512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabris L, Cadamuro M, Guido M, Spirli C, Fiorotto R, Colledan M, Torre G, Alberti D, Sonzogni A, Okolicsanyi L, et al. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am J Pathol. 2007;171:641–653. doi: 10.2353/ajpath.2007.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: who is talking to whom about what? Circ Res. 2007;100:1556–1568. doi: 10.1161/01.RES.0000266408.42939.e4. [DOI] [PubMed] [Google Scholar]
- 17.Moyer K, Kaimal V, Pacheco C, Mourya R, Xu H, Shivakumar P, Chakraborty R, Rao M, Magee JC, Bove K, et al. Staging of biliary atresia at diagnosis by molecular profiling of the liver. Genome Med. 2010;2:33. doi: 10.1186/gm154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weerasooriya VS, White FV, Shepherd RW. Hepatic fibrosis and survival in biliary atresia. J Pediatr. 2004;144:123–125. doi: 10.1016/j.jpeds.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 19.Nemesánszky E, Lott JA. Gamma-glutamyltransferase and its isoenzymes: progress and problems. Clin Chem. 1985;31:797–803. [PubMed] [Google Scholar]
- 20.Rendón-Macías ME, Villasís-Keever MA, Castañeda-Muciño G, Sandoval-Mex AM. Improvement in accuracy of gamma-glutamyl transferase for differential diagnosis of biliary atresia by correlation with age. Turk J Pediatr. 2008;50:253–259. [PubMed] [Google Scholar]
- 21.Okada T, Itoh T, Sasaki F, Honda S, Naito S, Todo S. CD56-immunostaining of the extrahepatic biliary tree as an indicator of clinical outcome in biliary atresia: a preliminary report. Turk J Pediatr. 2008;50:542–548. [PubMed] [Google Scholar]
- 22.Paku S, Dezso K, Kopper L, Nagy P. Immunohistochemical analysis of cytokeratin 7 expression in resting and proliferating biliary structures of rat liver. Hepatology. 2005;42:863–870. doi: 10.1002/hep.20858. [DOI] [PubMed] [Google Scholar]
- 23.Xia JL, Dai C, Michalopoulos GK, Liu Y. Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am J Pathol. 2006;168:1500–1512. doi: 10.2353/ajpath.2006.050747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dees C, Tomcik M, Zerr P, Akhmetshina A, Horn A, Palumbo K, Beyer C, Zwerina J, Distler O, Schett G, et al. Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann Rheum Dis. 2011;70:1304–1310. doi: 10.1136/ard.2010.134742. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi H, Puri P, O’Briain DS, Surana R, Miyano T. Hepatic overexpression of MHC class II antigens and macrophage-associated antigens (CD68) in patients with biliary atresia of poor prognosis. J Pediatr Surg. 1997;32:590–593. doi: 10.1016/s0022-3468(97)90714-4. [DOI] [PubMed] [Google Scholar]
- 27.Gong ZH, Xiao X, Chen L. Hepatic fibrosis with choledochal cyst in infants and children - an immunohistochemical assessment. Eur J Pediatr Surg. 2007;17:12–16. doi: 10.1055/s-2007-964950. [DOI] [PubMed] [Google Scholar]


