Abstract
Background
Chemotherapy-associated neutropenia has been reported to be a pharmacodynamic marker of response in some advanced solid tumors. Factors that accelerate drug clearance lead to lower plasma concentrations and toxicity, including neutropenia. Smoking accelerates themetabolism of several drugs, including chemotherapy. We sought to study the effects of smoking on gemcitabine-induced neutropenia in this retrospective study.
Methods
Smoking status and neutropenia along with other clinical parameters were recorded in 151 patients receiving first-line gemcitabine-based chemotherapy for advanced solid tumors.
Results
Tumor types included breast (9.3%), lung (4.6%), pancreatobiliary (70.9%), or other/unknown primary cancer (15.2%). Logistic regression showed that never smokers had increased neutropenia versus current smokers (odds ratio:3.5; 95% confidence interval, CI: 1.1–11.4). A 5-unit increase in pack-years reduced the odds of having higher neutropenia toxicity by 6.3% (95% CI 12 to 1%; p = 0.036).
Conclusion
Smokers had less neutropenia than nonsmokers, a finding that was more pronounced with increasing pack-years. This pharmacodynamic marker of gemcitabine-induced neutropenia may result in less efficacy of gemcitabine. Future prospective trials should correlate smoking, metabolizing phenotype, neutropenia, and response to gemcitabine therapy.
Keywords: Chemotherapy, Neutropenia, Pharmacodynamics, Smoking
Introduction
Cigarette smoking is a major health concern and has been linked to a multitude of malignancies, leading to significant morbidity and mortality. The constituents of tobacco smoke are known to affect drug metabolism by both pharmacokinetic and pharmacodynamic mechanisms, causing alterations in drug clearance, drug concentrations, toxicity, and, potentially, drug efficacy. Polycyclic aromatic hydrocarbons present in tobacco smoke are thought to induce certain key enzymes responsible for drug metabolism, including cytochrome P450 1A1 and 1A2 (CYP1A1 and CYP1A2), and, to a lesser extent, CYP2E1 and some UDP-glucuronyltransferases [1, 2]. The effect of this enzymatic induction and downstream effects are well studied with respect to many medications, including theophylline, caffeine, paracetamol, clozapine, olanzapine, fluvoxamine, codeine, propranolol, and inhaled insulin [3–5].
While tobacco smoke is a known carcinogen implicated in the development of many cancers, investigation into the effect of smoking on the metabolism and pharmacokinetics of chemotherapeutic agents is still evolving. Prior studies have studied this interaction for a handful of chemotherapy agents, including erlotinib, irinotecan, taxanes, and gemcitabine. Smokers receiving erlotinib have more rapid drug clearance than nonsmokers, and require a higher dose to reach an equivalent area under the concentration curve in the therapeutic range [6, 7]. Similarly, smokers receiving irinotecan demonstrate increased clearance, lower area under the concentration curve, lower systemic exposure to the active drug metabolite, and less hematologic toxicity [8]. While the pharmacokinetics of taxanes were not altered with smoking, taxane-associated neutropenia was less pronounced in smokers versus nonsmokers [9]. Kanai et al. [10] have similarly reported on an inverse relationship between gemcitabine and higher grades of neutropenia in Asian smokers treated for lung cancer. Importantly, these effects may be noted even in former smokers, as epigenetic effects of smoking can lead to prolonged alterations in certain oxidative enzymes, for example, MAO-dependent
5-HT catabolism [11]. Others have demonstrated overexpression of placental CYP1A1 while exposed to maternal smoke; this overexpression of CYP1A1 was associated with hypomethylation of the CYP1A1 promoter [12]. Therefore, the metabolic ‘phenotype’ involving the cytochrome P450 enzymes may in fact be quite different in current and former smokers compared with nonsmokers. These findings support the hypothesis that cigarette smoking may accelerate chemotherapy metabolism and result in lower plasma concentrations of the chemotherapeutic agent, as demonstrated from multiple pharmacokinetic studies carried out in a number of drugs, including those previously mentioned [6–9, 13]. Reduced drug levels may lead to undertreatment in smokers, and, conversely, increased treatment-related neutropenia in non-smokers.
Based on the retrospective review by Kanai et al. [10] demonstrating that smoking emerged as an independent inverse predictor of gemcitabine-induced neutropenia, we performed our own retrospective review of 151 patients with solid tumor malignancies who received gemcitabine at the University of Michigan Comprehensive Cancer Center over a 2-year period.
Patients and Methods
After obtaining approval from the University of Michigan Institutional Review Board, the University of Michigan Comprehensive Cancer Center pharmacy database was queried for those patients receiving gemcitabine alone or in combination with oral chemotherapy agents from July 1, 2009, to June 30, 2011. For these patients, we retrospectively obtained data on demographics, tumor type, smoking history, and neutropenia throughout the entire treatment course from the University of Michigan’s electronic medical record system. Hematological results were graded according to the Common Terminology Criteria (CTC) for Adverse Events (AE), version 4.0.
Smoking History
Information about the patients’ smoking history was obtained by review of the electronic medical records and was typically found in the initial clinic note recorded upon diagnosis of the patient’s malignancy. Patients were classified as smokers and nonsmokers based on self-reported information that was recorded in the medical record. Former smokers were subsequently classified according to pack-year history: 1–24, 25–49, and ≥50 pack-years in their lifetime.
Statistical Methods
Unless otherwise stated, categorical variables are presented using frequencies and percentages, and continuous variables are presented using mean and standard deviations. Cumulative logistic regression was used to assess the association between graded gemcitabine-induced neutropenia toxicity and smoking status, gender, age, Eastern Cooperative Oncology Group (ECOG) performance status (PS), disease stage, tumor type, prior chemotherapy, and current therapy regimen. The cumulative logistic model reported analyzed grades of neutropenia by smoking status, pack-year history, and controlled for ECOG PS, prior chemotherapy, and current regimen (monotherapy with gemcitabine or combinations). In a separate model, the effect of the quantity of smoking in all patients who ever smoked as a continuous variable on neutropenia grade toxicity was analyzed and predicted probabilities were re- ported graphically. All statistical analyses were completed using SAS version 9.3 (SAS Institute, Cary, N.C., USA).
Results
Patient and Treatment Characteristics
Data for 151 patients who received gemcitabine chemotherapy alone or in combination with oral chemotherapy agents (e.g. erlotinib in a subset of pancreatic cancer patients) from July 1, 2009, to June 30, 2011, were analyzed. Patient characteristics of interest are summarized in table 1. Pancreatobiliary malignancies (cholangiocarcinoma, pancreas, ampullary, and hepatocellular carcinomas) comprised the largest cohort of patients who received gemcitabine (n = 107, 70.9%). Other diagnoses included breast cancer (n = 14, 9.3%), lung cancer (n = 7, 4.6%),and other types or unknown cancer(n = 23, 15.2%), which included carcinoma of unknown primary, ovarian cancer, cutaneous T-cell lymphoma, angioimmunoblastic T-cell lymphoma, and glioma. Sixty-three patients were classified as ‘never’ smokers, 76 patients were classified as former smokers, and 12 patients were classified as current smokers based on data recorded from the medical record. The majority of patients were classified as having stage 3–4 disease (n = 118, 78.1%), while 26 had stage 1–2 disease, and 7 were classified as unknown stage. Within the pancreatobiliary cohort, for example, 77.6% of patients had stage 3–4 disease.
Table 1.
Association between patient characteristics and grade of neutropenia
| Characteristics | CTC-AE grade of neutropenia
|
OR (95% CI) | p value | ||
|---|---|---|---|---|---|
| none (n = 55) | grade 1–2 (n = 50) | grade 3–4 (n = 46) | |||
| Smoking status | 0.039 | ||||
| Never smoker (n = 63) | 20 (31.75%) | 20 (31.75%) | 23 (36.51%) | 3.6 (1.1, 11.2) | |
| Former smoker | |||||
| <25 pack-years (n = 35) | 11 (31%) | 11 (31%) | 13 (37%) | 3.6 (1.1, 12.2) | |
| 25–49 pack-years (n = 27) | 7 (26%) | 14 (52%) | 6 (22%) | 2.9 (0.8, 10.4) | |
| >49 pack-years (n = 12) | 8 (67%) | 3 (25%) | 1 (8%) | 0.7 (0.2, 3.6) | |
| Current smoker (n = 12) | 9 (64%) | 2 (14%) | 3 (21%) | reference | |
| Gender | 0.35 | ||||
| Female (n = 85) | 36 (42.35%) | 22 (25.88%) | 27 (31.76%) | 1.3 (0.7, 2.3) | |
| Male (n = 66) | 19 (28.79%) | 28 (42.42%) | 19 (28.79%) | reference | |
| Age (mean ± SD), years | 61.85±14.58 | 64.42 ± 14.56 | 62.43 ± 11.24 | 1.0 (0.98, 1.03) | 0.78 |
| ECOGPS | 0.01 | ||||
| 1–2 (n= 133) | 43 (32.33%) | 47 (35.34%) | 43 (32.33%) | reference | |
| 3–4 (n = 18) | 12 (66.67%) | 3 (16.67%) | 3 (16.67%) | 0.27 (0.10, 0.73) | |
| Stage | 0.89 | ||||
| 1–2 (n = 26) | 8 (30.77%) | 12 (46.15%) | 6 (23.08%) | reference | |
| 3–4 (n= 118) | 46 (38.98%) | 37 (31.36%) | 35 (29.66%) | 0.95 (0.43, 2.07) | |
| Unknown/other (n = 7) | 1 (14.29%) | 1 (14.29%) | 5(71.43%) | N/A | |
| Tumor type | 0.075 | ||||
| Pancreatobiliary (n = 107) | 39 (36.45%) | 33 (30.84%) | 35 (32.71%) | reference | |
| Breast (n = 14) | 7 (50.00%) | 6 (42.86%) | 1 (7.14%) | 0.44 (0.15, 1.27) | |
| Lung (n = 7) | 4 (57.14%) | 3 (42.86%) | 0 (0%) | 0.32 (0.07, 1.48) | |
| Other/unknown (n = 23) | 5 (21.74%) | 8 (34.78%) | 10 (43–48%) | 1.79(0.77, 4.13) | |
ORs with 95% CIs and type 3 p values are reported from separate cumulative logistic models.
Treatment history and type varied among tumor types and is described in table 2. Within the entire cohort, 81 patients (54.6%) were chemo-naïve, while 70 patients (46.4%) had received treatment prior to gemcitabine. Of those patients who received prior chemotherapy, agents included platinums, anthracyclines, taxanes, 5-fluorouracil, and gemcitabine, with some patients having received multiple agents (table 2). The majority of patients were on gemcitabine alone (n = 108, 71.5%), while a subset of patients with pancreatobiliary malignancies concurrently received oral targeted agents such as erlotinib, capecitabine, or sorafenib. Use of concurrent oral targeted therapies has been controlled for upon multivariate analysis.
Table 2.
Treatment description
| None (n = 55) | Grade 1–2 (n = 50) | Grade 3–4 (n = 46) | p value (lonckheere-Terpstra test) | |
|---|---|---|---|---|
| Chemo-naïve | 33 (60%) | 21 (42%) | 27 (58.7%) | 78 |
| Prior chemotherapy | ||||
| Platinums | 4 (7%) | 2 (4%) | 3 (6.5%) | 0.83 |
| Anthracyclines | 8 (14.6%) | 7 (14.0%) | 4 (8.7%) | 0.40 |
| Taxanes | 7 (12.7%) | 5 (10.0%) | 3 (6.5%) | 0.30 |
| 5-Fluorouracil | 6 (10.9%) | 7 (14.0%) | 4 (8.7%) | 0.77 |
| Gemcitabine | 12 (21.8%) | 14 (28%) | 13 (28.3%) | 0.44 |
| Other | 10 (18.2%) | 13 (26%) | 5 (10.9%) | 0.42 |
| Gemcitabine regimen | 0.36 | |||
| Gem. alone | 40 (72.7%) | 38 (76%) | 30 (65%) | |
| Gem. + other chemotherapy | 7 (12.7%) | 8 (16%) | 10 (21.7%) | |
| Gem. + non -chemotherapy | 7 (12.7%) | 4 (8%) | 6 (13%) | |
| Gem. + other | 1 (1.8%) | 0 | 0 | |
| Cycles received | 3 (1,11) | 4 (1,14) | 4 (1,15) | <0.000l |
| Dose of gemcitabine | 0.0075 | |||
| 1,000 mg/m2 | 45 (81.8%) | 45 (90%) | 45 (97.8%) | |
| 750mg/m2 | 3 (5.5%) | 3 (6%) | 1 (2.2%) | |
| Other | 7 (12.7%) | 2 (4%) | 0 (0%) | |
| Schedule | 0.40 | |||
| Days 1 and 8: 3-week cycle | 31 (56.4%) | 25 (50%) | 30 (65.2%) | |
| Days 1,8 and 15:4-week cycle | 17 (30.9%) | 21 (42%) | 12 (26.1%) | |
| Every other week | 6 (10.9%) | 3 (6%) | 1 (2.2%) | |
| Other | 1 (1.8%) | 1 (2%) | 3 (6.5%) | |
| GCSF treatment | 0 (0%) | 0 (0%) | 3 (6.5%) | 0.03 |
| Gem. delayed/held | 0 (0%) | 2 (4%) | 28 (61%) | <0.0001 |
| Gem. dose adjusted | 0 (0%) | 3 (6%) | 29 (63.0%) | <0.000l |
| Gem. stopped early | 44 (80%) | 35 (70%) | 24 (52.2%) | 0.003 |
Patients received a median of 4 (minimum 1, maximum 15) cycles of gemcitabine. The majority of patients (n = 135, 89.4%) were given gemcitabine at a dose of 1,000 mg/m2 infused over 30 min. Most patients (n = 136, 90.6%) were treated on days 1 and 8 of a 21-day cycle or days 1, 8, and 15 of a 28-day cycle. Granulocyte-colony stimulating factor was used in 3 patients (2%) for growth factor support. Gemcitabine was held or delayed in 30 patients (19.9%), the dose was adjusted in 32 patients (21.2%), and treatment was discontinued early in 103 patients (68.2%).
Smoking History and Gemcitabine-Induced Neutropenia
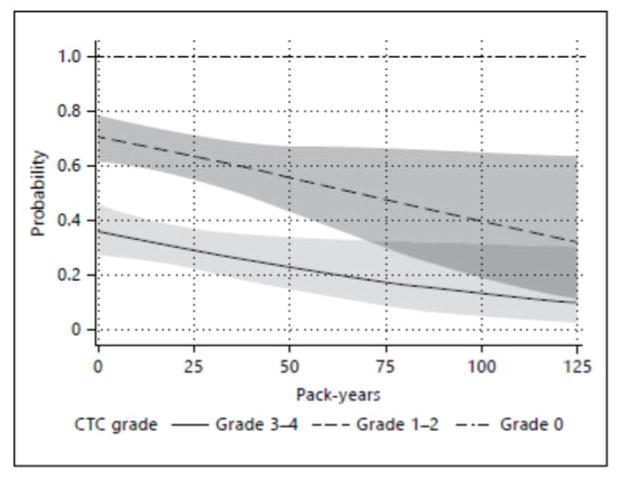
Cumulative logistic regression showed that never smokers had increased CTC-AE-graded neutropenia toxicity versus current smokers (odds ratio, OR: 3.5; 95% confidence interval, CI: 1.1–11.4; table 3). Among former smokers, higher pack-year histories were associated with lower odds of higher grades of neutropenia toxicity compared to current smokers. Former smokers with <25 pack-year history had odds of neutropenia toxicity similar to never smokers (OR: 3.4; 95% CI: 1.0–11.7); former smokers with higher pack-year histories (25–49 years and >49 years) had lower odds of higher toxicity with reference to current smokers (OR: 3.0 and 0.7, respectively; see table 3 for 95% CIs). Predicted probabilities from the cumulative logistic model including only patients who were current or former smokers over the range of pack-years were calculated and represented in figure 1. A 5-unit increase in pack-years reduced the odds of having higher neutropenia toxicity by 6.3% (95% CI 12 to 1%; p = 0.036). Baseline neutrophil counts in current smokers were higher compared with nonsmokers (8.3 vs. 5.5, respectively; p = 0.03; table 4). This study had a diverse range of malignancy types and severity of disease. Subset analyses were completed in pancreatobiliary malignancy since it was the largest malignancy cohort. Similarly, a subset analysis of stage 4 malignancy was accomplished. Both subset analyses show similar results (data not shown) and thus the entire cohort model is chosen to represent the study.
Table 3.
Cumulative logistic model estimating OR of neutropenia grade (high, low, none)
| Effect | OR | 95% Wald CIs | P value |
|---|---|---|---|
| Smoking status | 0.045 | ||
| Never smoker vs. current smoker | 3.5 | 1.1–11.4 | |
| Former smoker | |||
| <25 pack-years vs. current smoker | 3.4 | 1.0–11.7 | |
| 25–49 pack-years vs. current smoker | 3.0 | 0.8–10.7 | |
| ≥50 pack-years vs. current smoker | 0.7 | 0.1–3.5 | |
| ECOG | 0.018 | ||
| 1–2 vs. 3–4 | 3.5 | 1.2–9.9 | |
| Therapy regimen | 0.42 | ||
| Gem. + chemotherapy vs. Gem. only | 1.7 | 0.7–4.2 | |
| Gem. + other vs. Gem. only | 0.9 | 0.3–2.4 | |
| Prior Chemotherapy | 0.93 | ||
| Chemonaïve vs. prior chemotherapy | 1.0 | 0.5–1.9 |
Fig 1.
Representation of the predicted probabilities from the cumulative logistic model over the range of pack-years in the cohort (with 95% CIs)
Table 4.
Mean baseline neutrophil counts
| Groups | Neutrophil counts
|
p value vs. current smokersa | |
|---|---|---|---|
| mean | SD | ||
| Never smoker | 5.5 | 3.7 | 0.03 |
| Former smoker | |||
| <25 pack-years | 5.2 | 2.1 | 0.02 |
| 25–49 pack-years | 5.2 | 2.7 | 0.03 |
| >49 pack-years | 5.8 | 1.1 | 0.26 |
| Current smoker | 8.3 | 4.6 | reference |
Overall p = 0.0326 (ANOVA type 3 p value).
Pairwise p value with a Tukey correction for multiple testing.
Discussion
Gemcitabine has a wide spectrum of activity across a variety of tumor types, which is similarly reflected in the diverse sample in our retrospective review. As demonstrated in prior pharmacokinetic and toxicity studies, pack-year histories. The 95% Wald CIs were broad in this study. However, this correlation with pack-years was accentuated as pack-years increased in analysis among current and former smokers only, possibly indicating a dose- response effect in patients with heavier smoking histories.
Additionally, predicted probabilities from the cumulative logistic model over the range of pack-years in the cohort were calculated, and a 5-unit increase in pack-years was found to reduce the odds of having higher hematologic toxicity by 6.3% (95% CI 12 to 1%; p = 0.036), a finding that was surprising, yet statistically significant. This finding is in keeping with earlier data supporting the hypothesis that smoking is inversely correlated with hematologic toxicity, particularly neutropenia, as similar effects on hematologic toxicity have been observed with taxanes and irinotecan [8, 9]. The cumulative logistic model also provides a pooling of the ORs from two logistic models, a model with grade 3–4 versus grade 0–2 and a model with grades 1–4 versus none, to provide one OR estimate for the smoking covariate. The assumption that these two models must be proportional was tested and met. We found that never smokers have 3.5 greater odds of higher grade toxicity compared to smokers. This is a summary of both grade 3–4 toxicity compared to none and any grade toxicity compared to none. Hence, smoking history reduces all grades of neutropenia as well as grade 3–4 neutropenia.
It is unclear, however, what impact dose alterations according to toxicity may have on survival, if any. There are data (both preclinical as well as clinical data) to support the premise that higher doses of gemcitabine are associated with greater responses. In a preclinical study by Von Hoff [14], a concentration of 22 μg was associated with higher clonogenic cell kill compared with 2 μg, and phase I data have illustrated evidence of increasing response at least up to 2,200 mg/m2 [15]. It is clear that additional prospective trials are needed in order to answer the question of whether dose alterations according to metabolic phenotype impact response and survival.
As mentioned, former heavy smokers with higher pack-year histories (25–49 years and >49 years) had lower odds of higher toxicity with reference to current smokers. While the literature on this topic is sparse, some data suggest that this phenomenon may be due to epigenetic effects of smoking on the CYP isoenzymes that keep them persistently activated for some time, even after smoking cessation [11]. Similarly, Spira et al. [16] studied gene expression signatures of current, non- and former smokers, albeit in airway epithelial cells. Thirteen genes did not return to normal levels in former smokers, even in those who had discontinued smoking 20–30 years before testing (p < 9.8 × 10−4; threshold determined by permutation analysis). These genes included a number of potential tumor suppressor genes, e.g., TU3A and CX3CL1, which are permanently decreased, and several putative oncogenes, e.g., CEACAM6 and HN1, which are permanently increased [16]. Thus, even former smokers may continue to exhibit evidence of epigenetic alterations on key metabolizing enzymes years after smoking cessation.
Interestingly, baseline neutrophil levels were higher in current smokers versus nonsmokers, and in those former smokers with a shorter history of smoking. In terms of the exact effect of smoking on neutrophils, few prospective studies in the literature exist to answer this question. van Eeden and Hogg [17] examined a cohort of 38 healthy chronic smokers (23 ± 5 pack-years) and 15 age- and sex- matched nonsmoking controls, with the goal of measuring several aspects of white cell parameters. The total white cell and polymorphonuclear leukocyte cell counts were higher in smokers as were the percentage and total number of band cells. In our study, baseline neutrophil counts in current smokers were higher compared with nonsmokers (8.3 vs. 5.5, p = 0.03), which we acknowledge may potentially contribute to the degree of nadir neutropenia.
Limitations of our study include the retrospective nature and its attendant drawbacks such as heterogeneity of the population as well as varying practice patterns. We expect that the wide CIs in the statistical models are due to the heterogeneous population and small sample sizes in the subgroups of the population. Intervariability and differing practice patterns among different physicians are also worth noting and are difficult to control for in a retrospective review, as different practitioners may have different thresholds for holding chemotherapy in response to neutropenia versus dose adjustment alone. Additionally, we acknowledge that there are many other variables that can affect chemotherapy tolerability aside from smoking history, e.g. whether the patient had received previous chemotherapy or PS, for example. In the future, studies could focus solely on one tumor type, e.g. thoracic or pancreatobiliary malignancies only, thereby potentially eliminating any source of bias, as practice patterns and gemcitabine administration also differ across tumor types, as well as response to therapy. Similarly, we focused on the incidence of treatment-related neutropenia only and did not examine the incidence of thrombocytopenia and correlation with smoking status, which may be a future area of investigation.
Future Directions
Retrospective chart reviews including ours carry obvious and inherent drawbacks and thereby have limited applicability to clinical practice. Additionally, there are little data on how smoking may affect drug efficacy and progression-free and overall survival. Thus, we propose a cross-sectional prospective study of non-small cell lung cancer patients who are initiating single-agent gemcitabine as either first- or second-line chemotherapy, at the discretion of their treating physician. We plan to measure baseline CDA expression in peripheral blood, and gemcitabine and difluorodeoxyuridine concentrations in plasma at specified time points (before and 15 min after starting infusion, and then 15, 30, and 90 min after completing infusion). Our study will inform us if there exists a difference in pharmacokinetics of gemcitabine between smokers and nonsmokers, and a follow-up study will be designed to titrate the dose of gemcitabine based on base-line CDA mRNA expression, individualizing the dose to the metabolizing enzyme genotype.
Conclusion and Summary
Cigarette smoking and the constituents in cigarette smoke are known to accelerate the metabolism of certain drugs, particularly those metabolized by CYP1A1 and CYP1A2, and to a lesser extent CYP2E1 and some UDP-glucuronosyltransferases, leading to both pharmacokinetic and pharmacodynamic effects in smokers compared to nonsmokers. In smokers, this induction of metabolism has been shown to increase drug clearance and decrease toxicity (particularly treatment-induced neutropenia).
While this phenomenon is fairly well described in the literature for non-chemotherapy medications, the data investigating the effect on chemotherapy drugs is limited and primarily limited to retrospective data and pharmacokinetic studies. There are 13 million former smokers in the United States, and lung cancer is the leading cause of cancer death for both men and women. Outcomes in lung cancer for both never smokers and current or former smokers are equally dismal, with a 5-year relative survival rate for lung cancer of approximately 15% [18]. It can be posited that reduced levels of a chemotherapy drug due to increased catabolism may lead to reduced efficacy of the drug in smokers and former smokers, and, conversely, increased treatment-related neutropenia in never smokers. Smokers, therefore, may require a higher dose of the drug to overcome the enzyme Kd, and thereby drive the reaction to the right. To best answer this question, a prospective trial comparing chemotherapy pharmacokinetics in smokers versus nonsmokers must be designed, and will potentially help to answer this question.
In summary, pharmacokinetic and pharmacodynamic interactions between cigarette smoking and chemotherapy drugs may significantly impact drug clearance, delivery, toxicity, and efficacy. Cigarette smoking history must be carefully considered as a patient-specific factor capable of impacting treatment and, potentially, outcome. Optimally, clinical trials should take this factor into consideration and potentially use it as an independent predictive variable when designing studies that include smokers. Similarly, it is also recommended that clinicians take a careful smoking history prior to starting chemotherapy, and become aware of smoking history as a potential factor impacting treatment, toxicity, and ultimately response.
References
- 1.Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 2.Hukkanen J, Jacob P, III, Peng M, Dempsey D, Benowitz NL. Effects of nicotine on cytochrome P450 2A6 and 2E1 activities. Br J Clin Pharmacol. 2010;69:152–159. doi: 10.1111/j.1365-2125.2009.03568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirota T, Takane H, Higuchi S, Ieiri I. Epigenetic regulation of genes encoding drug-metabolizing enzymes and transporters; DNA methylation and other mechanisms. Curr Drug Metab. 2008;9:34–38. doi: 10.2174/138920008783331130. [DOI] [PubMed] [Google Scholar]
- 4.Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64:1917–1921. doi: 10.2146/ajhp060414. [DOI] [PubMed] [Google Scholar]
- 5.Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update Clin Pharmacokinet. 1999;36:425–438. doi: 10.2165/00003088-199936060-00004. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton M, Wolf JL, Rusk J, Beard SE, Clark GM, Witt K, et al. Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res. 2006;12:2166–2171. doi: 10.1158/1078-0432.CCR-05-2235. [DOI] [PubMed] [Google Scholar]
- 7.Hughes AN, O’Brien ME, Petty WJ, Chick JB, Rankin E, Woll PJ, et al. Overcoming CYP1A1/1A2 mediated induction of metabolism by escalating erlotinib dose in current smokers. J Clin Oncol. 2009;27:1220–1226. doi: 10.1200/JCO.2008.19.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Bol JM, Mathijssen RH, Loos WJ, Fri berg LE, van Schaik RH, de Jonge MJ, et al. Cigarette smoking and irinotecan treatment: pharmacokinetic interaction and effects on neutropenia. J Clin Oncol. 2007;25:2719–2726. doi: 10.1200/JCO.2006.09.6115. [DOI] [PubMed] [Google Scholar]
- 9.de Graan AJ, Loos WJ, Friberg LE, Baker SD, van der Bol JM, van Doorn L, et al. Influence of smoking on the pharmacokinetics and toxicity profiles of taxane therapy. Clin Cancer Res. 2012;18:4425–4432. doi: 10.1158/1078-0432.CCR-12-0728. [DOI] [PubMed] [Google Scholar]
- 10.Kanai M, Morita S, Matsumoto S, Nishimura T, Hatano E, Yazumi S, et al. A history of smoking is inversely correlated with the incidence of gemcitabine-induced neutropenia. Ann Oncol. 2009;20:1397–1401. doi: 10.1093/annonc/mdp008. [DOI] [PubMed] [Google Scholar]
- 11.Launay JM, Del Pino M, Chironi G, Callebert J, Peoc’h K, Megnien JL, et al. Smoking induces long-lasting effects through a mono-amine-oxidase epigenetic regulation. PLoS One. 2009;4:e7959. doi: 10.1371/journal.pone.0007959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suter M, Abramovici A, Showalter L, Hu M, Shope CD, Varner M, et al. In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism. 2010;59:1481–1490. doi: 10.1016/j.metabol.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu JF, Eppler SM, Wolf J, Hamilton M, Rakhit A, Bruno R, et al. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin Pharmacol Ther. 2006;80:136–145. doi: 10.1016/j.clpt.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 14.VonHoff D. Activity of gemcitabine in a human tumor cloning assay as a basis for clinical trials with gemcitabine. San Antonio Drug Development Team Invest New Drugs. 1996;14:265–270. doi: 10.1007/BF00194529. [DOI] [PubMed] [Google Scholar]
- 15.Levitt ML, Kassem B, Gooding WE, Miketic LM, Landreneau RJ, Ferson PF, et al. Phase I study of gemcitabine given weekly as a short infusion for non-small cell lung cancer: results and possible immune system-related mechanisms. Lung Cancer. 2004;43:335–344. doi: 10.1016/j.lungcan.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, et al. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci USA. 2004;101:10143–10148. doi: 10.1073/pnas.0401422101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Eeden SF, Hogg JC. The response of human bone marrow to chronic cigarette smoking. Eur Respir J. 2000;15:915–921. doi: 10.1034/j.1399-3003.2000.15e18.x. [DOI] [PubMed] [Google Scholar]
- 18.Meguid RA, Hooker CM, Harris J, Xu L, Westra WH, Sherwood JT, et al. Long-term survival outcomes by smoking status in surgical and nonsurgical patients with non-small cell lung cancer: comparing never smokers and current smokers. Chest. 2010;138:500–50. doi: 10.1378/chest.08-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]