Abstract
Introduction
The labyrinthine zone of the placenta is where exchange of nutrients and waste occurs between maternal and fetal circulations. Proper development of the placental labyrinth is essential for successful growth of the developing fetus and abnormalities in placental development are associated with intrauterine growth restriction (IUGR), preeclampsia and fetal demise. Our previous studies demonstrate that Hectd1 is essential for development of the junctional and labyrinthine zones of the placenta. Here we further characterize labyrinthine zone defects in the Hectd1 mutant placenta.
Methods
The structure of the mutant placenta was compared to wildtype littermates using histological methods. The expression of cell type specific markers was examined by immunohistochemistry and in situ hybridization.
Results
Hectd1 is expressed in the labyrinthine zone throughout development and the protein is enriched in syncytiotrophoblast layer type I cells (SynT-I) and Sinusoidal Trophoblast Giant cells (S-TGCs) in the mature placenta. Mutation of Hectd1 results in pale placentas with frequent hemorrhages along with gross abnormalities in the structure of the labyrinthine zone including a smaller overall volume and a poorly elaborated fetal vasculature that contain fewer fetal blood cells. Examination of molecular markers of labyrinthine trophoblast cell types reveals increased Dlx3 positive cells and Syna positive SynT-I cells, along with decreased Hand1 and Ctsq positive sinusoidal trophoblast giant cells (S-TGCs).
Discussion
Together these defects indicate that Hectd1 is required for development of the labyrinthine zone or the mouse placenta.
Keywords: HECT E3 ligase, placenta, labyrinthine layer
Introduction
The chorioallantoic placenta is the primary site of maternal/fetal exchange of oxygen, nutrients and metabolic waste. Defects in placental development cause a spectrum of pregnancy complications including IUGR, preeclampsia and fetal demise. The labyrinthine placenta of the rodent consists of multiple cell layers: the maternal decidua, junctional zone including (trophoblast giant cells (TGCs) and glycogen trophoblasts, spongiotrophoblasts) and the labyrinthine layer. The labyrinthine layer is the site of maternal/fetal exchange. It is comprised of an intricate network of maternal and fetal blood spaces separated by the endothelium of fetal blood vessels and three labyrinthine trophoblast layers: sinusoidal trophoblasts (S-TGC) that line the maternal blood spaces and two syncytial trophoblast cell layers (SynT-I and SynT-II). Labyrinthine trophoblasts develop from the chorionic ectoderm and the fetal endothelial cells from the allantoic mesoderm.
Labyrinth morphogenesis and vascularization are driven by interactions of allantoic mesoderm with chorionic-derived trophoblasts (reviewed in [1]. Labyrinthine layer development begins around E8.0 with collapse of the chorionic cavity resulting in the chorion abutting the ectoplacental cone (EPC). This is followed by chorioallantoic fusion, invagination of the chorion and invasion of fetal vessels into the chorionic trophoblast layer. Interaction of chorionic ectoderm with the fetal vessels results in differentiation of labyrinthine trophoblasts. The elaborate vascular tree forms through branching morphogenesis of villi composed of labyrinthine trophoblasts.
Our previous work demonstrates that Hectd1 is required for development of the placenta. Hectd1 mutant embryos are smaller, show evidence of IUGR and die at mid-gestation [2]. Hectd1opm/opm mutant placentas are smaller than wildtype littermates and show significant defects in development of the junctional zone [2]. Specifically, mutant placentas have reduced numbers of TGCs, glycogen trophoblasts, uterine natural killer cells (uNKs) and spongiotrophoblasts. Here we characterize abnormalities in the labyrinthine layer of the Hectd1 mutant placenta. Our data demonstrate defects in the structure of the placental labyrinth and alterations in the cells that comprise the maternal fetal exchange surface.
Materials and Methods
Mouse Breeding and Placental Tissue Collection
Hectd1opm/+ and Hectd1XC/+ mice were described previously [3]. Placentas were fixed for 24 hours by immersion in 4% paraformaldehyde fixative for in situ analysis or DMSO/Methanol (1:4) fixative for immunohistochemistry. Placentas were embedded in Optimal Cutting Temperature compound (OCT, Tissue-Tek) and serial 20 μm sections cut using a cryostat. Genotyping was performed using the yolk sac as described [4]. All mice were on a congenic 129/SiSv1Mj background (Stock No. 002448, The Jackson Laboratory Repository).
Phenotypic and Histological Analyses
To analyze Hectd1 expression, sectioned Hectd1XC/+ placentas at the indicated stages were subjected to LacZ staining and counterstained with eosin [5]. For immunohistochemistry wildtype placentas were subjected to immunofluorescence confocal microscopy analysis using an antibody against Hectd1 as described [4]. At least two placentas were analyzed at each developmental stage. In situ hybridization on sectioned wildtype and mutant placentas was carried out as described [6]. The following probes were used: Hand1 [7], Ctsq [8], Dlx3 [9], Gcnf/Nr6a1 [10], Esx1 [11], SynA, SynB [12] and Gcm1 [13]. The following antibodies were used: Pecam1 (1:200; BD Pharmingen #550274), Mct1 (1:200; Millipore AB1286I), Mct4 (1:200; Millipore #AB3314P) and Hectd1 (1:100; Novus; #H00025831-M03). Images shown are representative of at least 3 pairs of wildtype and mutant placentas unless otherwise indicated. Measurements of fetal and maternal blood space areas were done in Pecam1 or Mct1 stained sections using Image J software. Only bisected vessels/bold spaces (e.g. roughly circular) were measured in this analysis. Statistical analyses were done using Prism software and the Student’s t-test was used to determine statistical significance between groups.
Stereological analysis of placental structures
Stereological analyses were performed essentially as described [14]. Briefly, three litters were collected at E13.5 and one wildtype and mutant placenta collected from each litter was completely sectioned at 10 μm thickness. Sections were stained by H&E. The Stereo Investigator software system (MBF Biosciences, Vermont, USA) was employed to superimpose probe grids (Cavalieri estimation probe for area and volume; orthogonal intercepts for interhemal membrane thickness) on random fields of view within systematic random cryosections. Volume and surface densities were converted to absolute values by multiplying with the absolute placental volume of the placenta and corrected for tissue shrinkage.
Results
Hectd1 is expressed in the developing labyrinthine layer and in mature SynT-I cells and S-TGCs
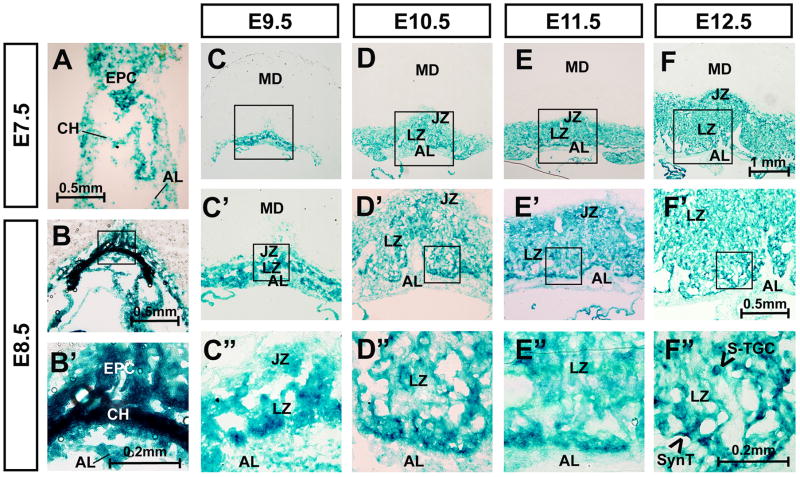
To understand the role of Hectd1 in establishment of the labyrinthine zone, its spatiotemporal expression was examined (Figure 1). For these studies, expression of beta-galactosidase was determined by LacZ staining in heterozygous Hectd1XC/+ genetrap concepti [15]. Samples from crosses between wildtype females and Hectd1XC/+ males were used to label Hectd1 expressing cells derived from the conceptus and not the maternal decidua. LacZ staining is observed throughout placental development (Figure 1). Expression is enriched in the chorion and EPC by E8.5. Between E9.5 and E12.5, LacZ staining is found in almost all trophoblast-derived cells of the placenta. In contrast, cells of the allantoic mesoderm show comparatively weaker staining at E9.5, which decreases further by E12.5. During this time frame, staining is comparatively higher in cells at the chorioallantoic interface. At E12.5, intense LacZ staining is observed in cells that separate maternal and fetal blood spaces. In particular staining is increased in some long flat cells and more rounded cells in the interior of cell clusters that separate maternal and fetal blood spaces.
Figure 1. Hectd1 is expressed throughout development of the labyrinthine zone.
A–F”) Hectd1 expression was monitored by LacZ staining in heterozygous Hectd1XC/+ placentas at E7.5, E8.5, 9.5, 10.5, 11.5 and E12.5 as indicated. Samples from crosses between wildtype females and Hectd1XC/+ males were used to label Hectd1 expressing cells derived from the conceptus and not the maternal decidua. Structures contributing to the placenta are labeled in E7.5 and 8.5 embryos including the ectoplacental cone (EPC), chorion (CH) and allantois (AL). The layers of the E9.5–12.5 placenta are labeled as maternal decidua (MD), junctional zone (JZ), labyrinthine zone (LZ) and allantoic mesoderm (AL). Magnification of boxed region is shown. LacZ staining is greater in the chorion and cells at the border of the chorionic-derived labyrinthine cells and allantoic mesoderm.
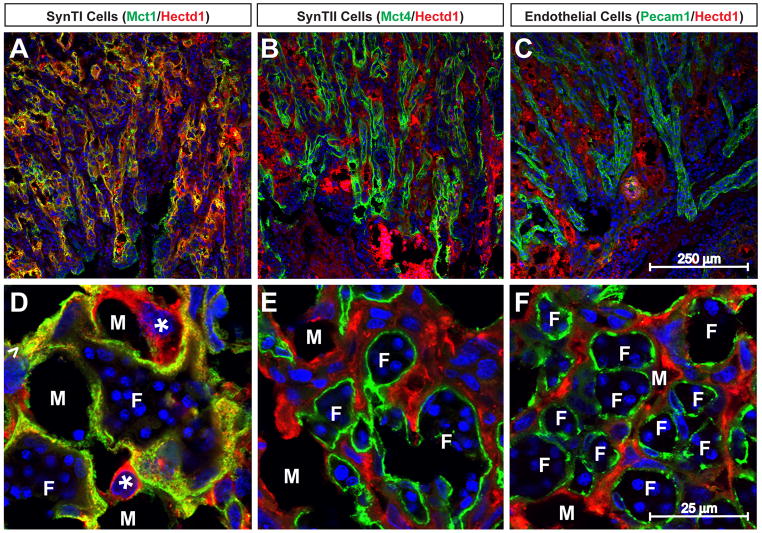
To further characterize the cell types expressing Hectd1 in the mature placenta, we examined expression of Hectd1 protein by immunofluorescence confocal microscopy in E12.5 wildtype placentas. Mct1, Mct4 and Pecam1 were used to label SynT-I, SynT-II and endothelial cells, respectively. Hectd1 protein mainly co-localizes with Mct1 positive SynT-I cells (Figure 2). Expression is also found in cells lining the maternal blood spaces with large nuclei (S-TGCs).
Figure 2. Hectd1 protein is localized to SynT-I and S-TGCs in the labyrinthine zone.
Wildtype placentas were subjected to immunostaining with antibodies against Hectd1 along with Mct1 (A, D), Mct4 (B, E) or Pecam1 (C, F) to label SynT-I, SynT-II and endothelial cells respectively. Placentas in panels AC are transverse and D–F coronal sections. Hectd1 protein co-localizes with Mct1 but not Mct4 or Pecam1. Hectd1 protein is also found in more rounded Mct1-negative S-TGCs that line the maternal blood spaces (*). Examples of fetal vessels (F) and maternal blood spaces (M) are labeled.
Defects in the Hectd1 mutant labyrinthine zone
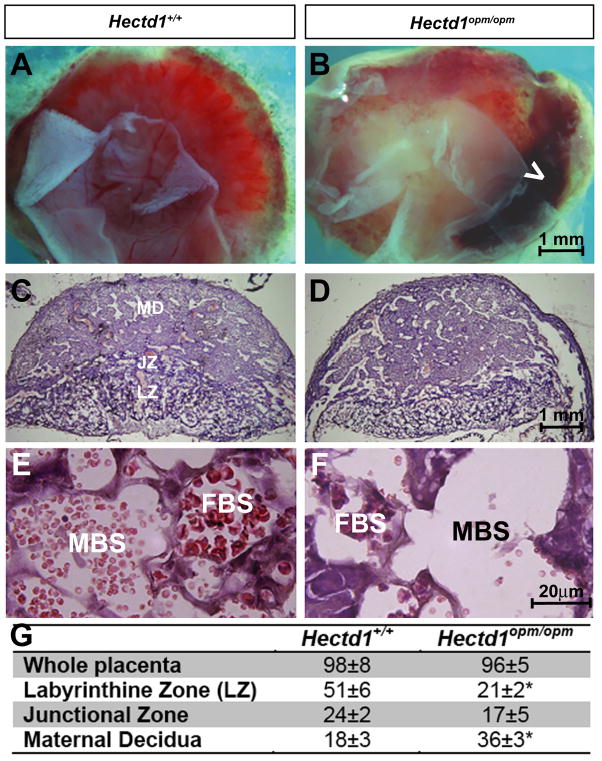
Gross comparison of Hectd1opm/opm mutant placentas with their wildtype littermates reveals that mutant placentas are pale and frequently have large hemorrhages (Figure 3A, B). 54% of the mutant placentas (n=43) analyzed show hemorrhages that occupy 20–30% of the fetal surface of the placenta. Only 7% (n=73) of wildtype littermates showed these hemorrhages. Histological examination reveals that the majority of Hectd1opm mutant placentas are moderately affected with obvious disruption in lamination even in placentas with comparable total thickness (Figure 3C, D). The thickness of the maternal decidua is notably larger with a corresponding decrease in the thickness of the labyrinthine layer. This was quantified by stereology (Figure 3G). Furthermore, while maternal and fetal blood is most often visible in blood spaces in wildtype placentas, mutant placentas retained fewer blood cells in histological sections (Figure 3E,F).
Figure 3. Hemorrhages and altered histological appearance of Hectd1 mutant placentas.
A, B) E12.5 (A) wildtype (Hectd1+/+) and (B) mutant (Hectd1opm/opm) placentas viewed from the fetal side demonstrating the overall pale appearance and large hemorrhages (white arrowhead) in the mutant. C, D) H&E staining of midsagittal section of E12.5 (C) wildtype and (D) mutant placentas. The layers of the placenta are labeled as maternal decidua (MD), junctional zone (JZ) and labyrinthine zone (LZ). E, F) Higher magnification images of the labyrinthine zone of H&E stained wildtype (E) and mutant (F) placentas. Maternal (MBS) and fetal blood spaces (FBS) are labeled that contain anucleated and nucleated red blood cells, respectively. G) Stereological analysis of Hectd1 wildtype and mutant placentas. Three placentas and wildtype siblings at E12.5 from three separate litters were utilized in all groups, mean values ± S.E.M, significant difference assessed by paired t-test between mutant and wildtype (* indicates p < 0.05).
Defects in labyrinthine trophoblasts in the Hectd1 mutant placenta
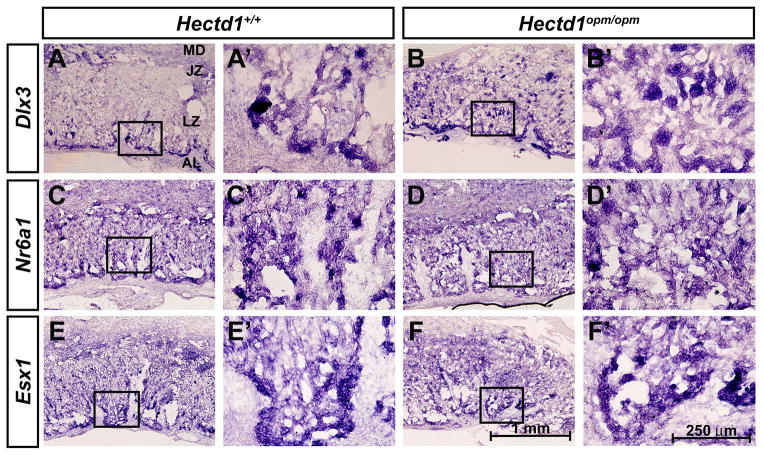
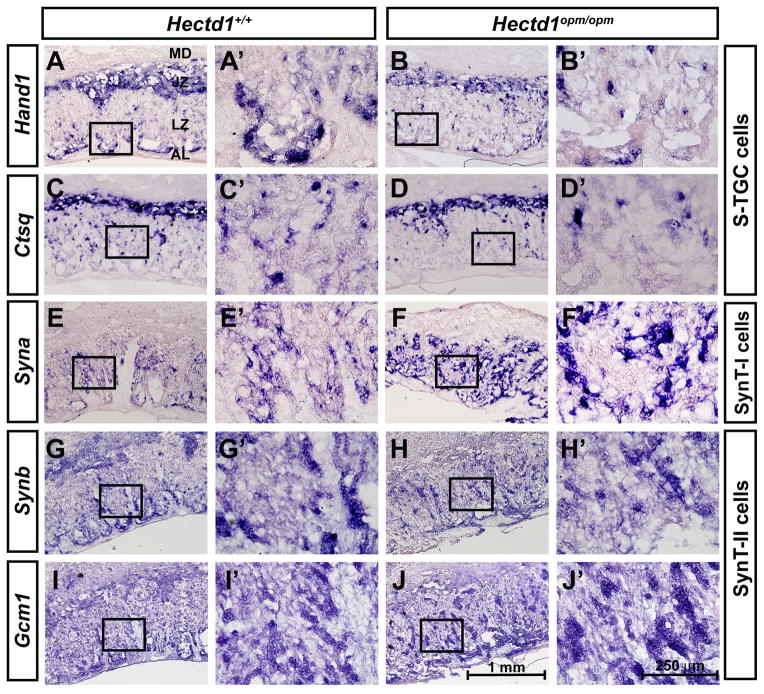
Since Hectd1 is expressed during labyrinthine zone development and alterations in organization of the labyrinthine zone were found, we examined whether labyrinthine trophoblast cells develop normally in the mutant placenta. The expression of Dlx3, Nr6a1/Gcnf and Esx1, expressed in undefined labyrinthine trophoblast subtypes [9, 11, 12, 16, 17], was examined in E12.5 wildtype and mutant placentas. Expression of Dlx3 was increased in the mutant labyrinthine zone (Figure 4A, B), whereas expression of Nr6a1/Gcnf and Esx1 were essentially unchanged (Figure 4C–F). Expression of cell type specific markers was examined to determine if changes in the specific labyrinthine trophoblast cell types are found in Hectd1 mutants. Hand1 and Cathepsin Q (Ctsq) label sinusoidal trophoblast giant cells (S-TGCs) and these cell types are reduced in mutants (Figure 5G–J). The intensity of SynA expression in SynT-I cells is increased in the mutant placenta (Figure 5E, F) and expression of SynB and Gcm1 that mark SynT-II cells were essentially unchanged (Figure 5G–J). These results indicate alterations in development of labyrinthine trophoblast cell types that express Hectd1 protein (Figure 2) in the mutant placenta.
Figure 4. Expression of labyrinthine trophoblast markers in the Hectd1 mutant placenta.
In situ hybridization showing expression of Dlx3 (A, B), Nr6a1/Gcnf (C, D) and Esx1 (E, F) in E12.5 wildtype (A, C, E) and Hectd1opm/opm mutant (B, D, F) placentas. Images are shown at 10X and a 40X magnification of the boxed region.
Figure 5. Altered expression of cell-type specific labyrinthine trophoblast markers in the Hectd1 mutant placenta.
Reduced Hand1 (A, B) and Ctsq (C, D) expressing S-TGCs and expanded expression of Syna (E, F) or Synb (G, H) and Gcm1 (I, J) (SynT-I or SynT-II cells, respectively) in E12.5 wildtype (A, C, E, G, I) and Hectd1opm/opm mutant (B, D, F, H, J) placentas. Images are shown at 10X and a 40X magnification of the boxed region.
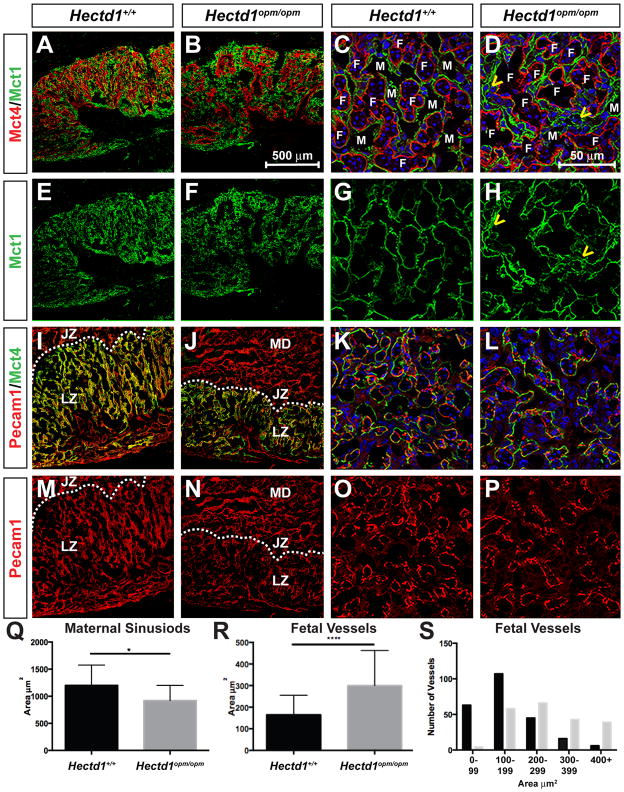
To further examine changes in SynT-I and SynT-II cells in Hectd1 mutants, expression of Mct1 and Mct4 were examined by immunofluorescence confocal microscopy in E12.5 wildtype and mutant placentas (Figure 6). Syncytiotrophoblasts are organized into layers with SynT-I cells adjacent to maternal blood spaces and SynT-II cells adjacent to fetal blood spaces. Mct1 is localized to the apical membrane of SynT-I cells (facing the maternal sinusoid) whereas Mct4 is localized to the basal membrane of SynT-II cells (adjacent to the fetal vasculature [18]. At low magnification of transverse placental sections, Mct4 stained regions of the placenta are fewer and surround larger spaces in the mutant than in the wildtype placenta (Figure 6A, B, E, F). Imaging at high magnification in coronal sections reveals that Mct4 positive cells surround vessels that contain fewer nucleated red blood cells. In the wildtype placenta, Mct1 positive cells are typically next to Mct4 positive cells representing the separation of adjacent maternal and fetal blood spaces by single layers of syncytiotrophoblasts (Figure 6C, D). However, in the mutant placenta there are areas where Mct1 positive cells appear disorganized and show multiple cells layered between fetal vessels (arrowheads in Figure 6D, H).
Figure 6. Altered organization of SynT-I, SynT-II and fetal blood vessels in the Hectd1 mutant placenta.
Expression of Mct1 (SynT-I cells, green) and Mct4 (SynT-II cells, red) were examined by immunofluorescence confocal microscopy in E12.5 wildtype (A, C, E, G) and mutant (B, F, D, H) placentas. Expression of Mct4 (SynT-II cells, green) and Pecam1 (endothelial cells, red) in E12.5 wildtype (I, K, M, O) and mutant (J, L, N, P) placentas. Nuclei are stained with Hoechst and shown in Blue in panels C, D, K and L. Placentas in panels A, B, E, F, I, J, M and N are sectioned in the transverse plane and shown at 10X and C, D, G, H, K, L, O and P in the coronal plane and shown at 60X. Yellow arrowheads in D and H highlight abnormal organization of SynT-I cells in the mutant. Fetal (F) and maternal (M) blood spaces are labeled. In panels I, J, M, N, the layers of the placenta are labeled as maternal decidua (MD), junctional zone (JZ) and labyrinthine zone. The dotted line denotes the boundary between junctional and labyrinthine zones. Q–S) The area of maternal (Q) and fetal (R, S) blood spaces were measured and the average area plotted (Q, R). 208 maternal blood spaces were measured in 13 wildtype and 155 blood spaces were measured in mutant sections from 4 wildtype and 2 mutant placentas. 237 fetal vessels in 12 wildtype and 210 fetal vessels in 18 mutant sections were measured from 4 wildtype and 2 mutant placentas. Significant differences were assessed by paired t-test between mutant and wildtype (* indicates p < 0.05 and *** p < 0.001). (S) The number of fetal vessels with the indicated area for wildtype (black bars) and Hectd1 mutant (grey bars).
To further examine the organization of fetal blood vessels and SynTII cells, transverse and coronal placental sections were co-stained with Pecam1 and Mct4 (Figure 6I-P). Fetal vessels show less elaborate branching in low magnification images of transverse sections. In higher magnification images of coronal sections, the number of blood vessels appears reduced along with increase in diameter of the vessels. This was quantitated by measuring the area of blood vessels in a number of sections of wildtype and mutant placentas, revealing an overall increase in the size of fetal blood vessels along with increased numbers of large vessels with a corresponding decrease in small vessels in the mutant (Figure 6R, S). Similarly, the area of maternal blood spaces was quantitated revealing a slight decrease in the size of maternal blood spaces in the mutant (Figure 6Q).
Discussion
Data presented here and in our previous study demonstrate that Hectd1 is essential for development of the murine placenta [19]. Hectd1 is expressed throughout development of this organ and enriched in some labyrinthine trophoblast populations including SynT-I and S-TGC cells [2]. Mutant placentas are smaller with defects in the junctional zone including decreased TGCs, spongiotrophoblasts and glycogen trophoblasts [2]. Mutant placentas also show increased numbers of immature uterine natural killer (uNK) cells in the maternal decidua [2]. Here we describe in detail defects in development of the labyrinthine zone. These include gross abnormalities in the structure of the labyrinth, with a smaller overall volume. We demonstrate alterations in labyrinthine trophoblast cell types including reduced S-TGCs and increased expression of SynT-I cell markers. Furthermore, SynT-I cell are disorganized with added cell layers between Mct4 labeled membranes. Additionally, the vasculature is less elaborated with increased numbers of larger vessels and a corresponding decrease of small vessels. Together these results indicate altered organization of the cells that separate maternal and fetal circulations.
The severe placental defects described here and in our previous study [2] likely contribute to midgestation lethality and IUGR observed in Hectd1 mutants. Other developmental defects in Hectd1 mutants may also play a role such as complex cardiac defects [20], which could exacerbate or even cause placental defects. Similarly, it is also possible that defects in development of the labyrinthine zone are secondary to abnormal development of the junctional zone [2]. Future experiments utilizing conditional Hectd1 alleles and cell type specific cre drivers will address this issue.
The mechanism by which Hectd1 regulates development of the labyrinthine zone remains to be determined. Our previous studies showed a decrease in overall cell proliferation and increased apoptosis in the labyrinthine zone [2]; however, it remains to be determined if these changes are restricted to specific labyrinthine trophoblast cell types or represent overall changes in apoptosis or proliferation. Furthermore, the molecular pathways controlled by Hectd1 regulating placental development are unknown. Hectd1 is an ubiquitin ligase and Hectd1 dependent ubiquitination does not appear to target proteins to the proteasome but results in altered intracellular localization [19–21]. While the identity of Hectd1 regulated proteins in the placenta are not currently known, Hectd1 can affect ubiquitination of numerous proteins ([21–23] and AAS and IEZ unpublished). Two of these, Hsp90 and APC are associated with development of the labyrinthine zone [1, 24]. Hectd1 ubiquitinates Hsp90alpha regulating its intracellular localization and function [22]. While placental defects have not been described for Hsp90alpha, Hsp90beta is required in the allantoic mesoderm for chorioallantoic attachment [24]. Hectd1 interacts with a conserved domain of Hsp90 [22] raising the possibility that Hectd1 may also regulate Hsp90beta. Furthermore, it is not clear what placental phenotypes would result from altering intracellular localization of Hsp90beta. But as Hsp90 plays an essential role in development of the labyrinthine zone vasculature this remains a viable candidate for mediating Hectd1-dependent placental phenotypes. Hectd1 also inhibits Wnt signaling transduction by ubiquitinating APC to facilitate interaction of Axin and APC [21]. Wnt signaling is required for labyrinthine zone development [1, 25] and is implicated in chorioallantoic attachment [26–28] with less severe mutants showing a poorly developed labyrinthine layer [29–31]. Future experiments will determine which pathways regulated by Hectd1 mediate labyrinthine zone development.
Highlights.
Hectd1 is expressed during development of the labyrinthine placenta
In the mature placenta, Hectd1 protein is localized to SynT-I and S-TGCs.
Mutation of Hectd1 results in a smaller labyrinthine zone.
Hectd1 mutants show reduced S-TGCs and increased expression of SynT-I markers.
The fetal vasculature is less elaborated in the Hectd1 mutant placenta.
Acknowledgments
This study was supported by R01-HD058629 to I.E.Z. Microscopic analysis was carried out at the Children’s Research Institute (CRI) Light Microscopy and Image Analysis Core supported by CRI and NIH grant P30HD040677.
Abbreviation
- HECT
homologous to E6-AP Carboxyl terminus
- Ub
ubiquitin
- IUGR
Intrauterine growth restriction
- TGC
trophoblast giant cell
- H&E
Hematoxylin and eosin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cross JC, Nakano H, Natale DR, Simmons DG, Watson ED. Branching morphogenesis during development of placental villi. Differentiation; research in biological diversity. 2006;74(7):393–401. doi: 10.1111/j.1432-0436.2006.00103.x. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar AA, Nuwayhid SJ, Maynard T, Ghandchi F, Hill JT, Lamantia AS, Zohn IE. Hectd1 is required for development of the junctional zone of the placenta. Dev Biol. 2014;392(2):368–80. doi: 10.1016/j.ydbio.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zohn IE, Anderson KV, Niswander L. The Hectd1 ubiquitin ligase is required for development of the head mesenchyme and neural tube closure. Developmental biology. 2007;306(1):208–21. doi: 10.1016/j.ydbio.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar AA, Zohn IE. Hectd1 regulates intracellular localization and secretion of Hsp90 to control cellular behavior of the cranial mesenchyme. The Journal of cell biology. 2012;196(6):789–800. doi: 10.1083/jcb.201105101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagy A. Manipulating the mouse embryo : a laboratory manual 2003. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; p. x.p. 764. [Google Scholar]
- 6.Zohn IE, Li Y, Skolnik EY, Anderson KV, Han J, Niswander L. p38 and a p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell. 2006;125(5):957–69. doi: 10.1016/j.cell.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Scott IC, Anson-Cartwright L, Riley P, Reda D, Cross JC. The HAND1 basic helix-loop-helix transcription factor regulates trophoblast differentiation via multiple mechanisms. Molecular and cellular biology. 2000;20(2):530–41. doi: 10.1128/mcb.20.2.530-541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons DG, Fortier AL, Cross JC. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Developmental biology. 2007;304(2):567–78. doi: 10.1016/j.ydbio.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Morasso MI, Grinberg A, Robinson G, Sargent TD, Mahon KA. Placental failure in mice lacking the homeobox gene Dlx3. Proc Natl Acad Sci U S A. 1999;96(1):162–7. doi: 10.1073/pnas.96.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Cooney AJ, Wang Y, Law SW, O'Malley BW. Cloning of a novel orphan receptor (GCNF) expressed during germ cell development. Molecular endocrinology (Baltimore, Md. 1994;8(10):1434–44. doi: 10.1210/mend.8.10.7854358. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Lemaire P, Behringer RR. Esx1, a novel X chromosome-linked homeobox gene expressed in mouse extraembryonic tissues and male germ cells. Dev Biol. 1997;188(1):85–95. doi: 10.1006/dbio.1997.8640. [DOI] [PubMed] [Google Scholar]
- 12.Simmons DG, Natale DR, Begay V, Hughes M, Leutz A, Cross JC. Early patterning of the chorion leads to the trilaminar trophoblast cell structure in the placental labyrinth. Development. 2008;135(12):2083–91. doi: 10.1242/dev.020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basyuk E, Cross JC, Corbin J, Nakayama H, Hunter P, Nait-Oumesmar B, Lazzarini RA. Murine Gcm1 gene is expressed in a subset of placental trophoblast cells. Dev Dyn. 1999;214(4):303–11. doi: 10.1002/(SICI)1097-0177(199904)214:4<303::AID-AJA3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 14.Coan PM, Ferguson-Smith AC, Burton GJ. Developmental dynamics of the definitive mouse placenta assessed by stereology. Biology of reproduction. 2004;70(6):1806–13. doi: 10.1095/biolreprod.103.024166. [DOI] [PubMed] [Google Scholar]
- 15.Zohn IE, Anderson KV, Niswander L. The Hectd1 ubiquitin ligase is required for development of the head mesenchyme and neural tube closure. Dev Biol. 2007;306(1):208–21. doi: 10.1016/j.ydbio.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmons DG, Fortier AL, Cross JC. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol. 2007;304(2):567–78. doi: 10.1016/j.ydbio.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Scott IC, Anson-Cartwright L, Riley P, Reda D, Cross JC. The HAND1 basic helix-loop-helix transcription factor regulates trophoblast differentiation via multiple mechanisms. Mol Cell Biol. 2000;20(2):530–41. doi: 10.1128/mcb.20.2.530-541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagai A, Takebe K, Nio-Kobayashi J, Takahashi-Iwanaga H, Iwanaga T. Cellular expression of the monocarboxylate transporter (MCT) family in the placenta of mice. Placenta. 2010;31(2):126–33. doi: 10.1016/j.placenta.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar AA, Nuwayhid SJ, Maynard TM, Ghandchi F, Hill JT, LaMantia AS, Zohn IE. Hectd1 is Required for Development of the Junctional Zone of the Placenta. Dev Biol. doi: 10.1016/j.ydbio.2014.05.007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Klena NT, Gabriel GC, Liu X, Kim AJ, Lemke K, Chen Y, Chatterjee B, Devine W, Damerla RR, Chang C, Yagi H, San Agustin JT, Thahir M, Anderton S, Lawhead C, Vescovi A, Pratt H, Morgan J, Haynes L, Smith CL, Eppig JT, Reinholdt L, Francis R, Leatherbury L, Ganapathiraju MK, Tobita K, Pazour GJ, Lo CW. Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature. 2015 doi: 10.1038/nature14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran H, Bustos D, Yeh R, Rubinfeld B, Lam C, Shriver S, Zilberleyb I, Lee MW, Phu L, Sarkar AA, Zohn IE, Wertz IE, Kirkpatrick DS, Polakis P. HectD1 E3 ligase modifies adenomatous polyposis coli (APC) with polyubiquitin to promote the APC-axin interaction. J Biol Chem. 2013;288(6):3753–67. doi: 10.1074/jbc.M112.415240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar AA, Zohn IE. Hectd1 regulates intracellular localization and secretion of Hsp90 to control cellular behavior of the cranial mesenchyme. The Journal of cell biology. 2012;196(6):789–800. doi: 10.1083/jcb.201105101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Zhou Q, Sunkara M, Kutys ML, Wu Z, Rychahou P, Morris AJ, Zhu H, Evers BM, Huang C. Ubiquitylation of phosphatidylinositol 4-phosphate 5-kinase type I gamma by HECTD1 regulates focal adhesion dynamics and cell migration. Journal of cell science. 2013;126(Pt 12):2617–28. doi: 10.1242/jcs.117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voss AK, Thomas T, Gruss P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development. 2000;127(1):1–11. doi: 10.1242/dev.127.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Sonderegger S, Pollheimer J, Knofler M. Wnt signalling in implantation, decidualisation and placental differentiation--review. Placenta. 2010;31(10):839–47. doi: 10.1016/j.placenta.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 1999;13(6):709–17. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parr BA, Cornish VA, Cybulsky MI, McMahon AP. Wnt7b regulates placental development in mice. Dev Biol. 2001;237(2):324–32. doi: 10.1006/dbio.2001.0373. [DOI] [PubMed] [Google Scholar]
- 28.Aoki M, Mieda M, Ikeda T, Hamada Y, Nakamura H, Okamoto H. R-spondin3 is required for mouse placental development. Dev Biol. 2007;301(1):218–26. doi: 10.1016/j.ydbio.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S, Taketo MM. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128(1):25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- 30.Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122(11):3343–53. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- 31.Lu J, Zhang S, Nakano H, Simmons DG, Wang S, Kong S, Wang Q, Shen L, Tu Z, Wang W, Wang B, Wang H, Wang Y, van Es JH, Clevers H, Leone G, Cross JC, Wang H. A positive feedback loop involving Gcm1 and Fzd5 directs chorionic branching morphogenesis in the placenta. PLoS biology. 2013;11(4):e1001536. doi: 10.1371/journal.pbio.1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]