Abstract
A multivalent magnetic resonance imaging agent based on a 2-hydroxypropyl-β-cyclodextrin (HPCD):Pluronic F127 polyrotaxane carrier has been synthesized and its blood pool contrast properties characterized. This Gd3+-DO3A-HPCD/Pluronic polyrotaxane construct is shown to circulate for more than 30 min and provide >100-fold vascular enhancement relative to the monomeric Gd3+-DO3A-HPCD control that is rapidly cleared via the kidney. The high r1 relaxivity at 37°C (23.83 mM−1s−1 at 1.5T; 34.08 mM−1s−1 at 0.5T), extended blood circulation, well-known pharmacology of the polyrotaxane precursors, and absence of acute toxicity make it a highly attractive blood pool contrast agent candidate.
Keywords: polyrotaxane, 2-hydroxypropyl-β-cyclodextrin, Pluronic, polyrotaxanes, magnetic resonance contrast agent, Gd3+-DO3A, blood pool MR agent, long circulation
TOC Image

Magnetic Resonance Imaging (MRI) is a powerful tool for non-invasive, high resolution three-dimensional (3D) medical imaging of anatomical structures such as organs and tissues within the body. MRI has advantages such as high versatility, high spatial resolution, excellent depth profiling capabilities, and an absence of ionizing radiation as in x-ray and CT imaging.1–4 MRI has extensive applications in the diagnosis of various neurological, cardiovascular and oncological diseases.1 MR contrast agents enhance the image quality by altering the longitudinal (T1) and transverse (T2) relaxation times of the protons in nearby water molecules. Contrast agents can be classified as either T1 agents (e.g., Gd3+ chelates) that increase the T1 relaxation rate and produce positive contrast images, or T2 agents (e.g., superparamagnetic iron oxide nanoparticles) that increase the T2 relaxation rate and produce negative contrast images. The majority of clinically used MRI contrast agents are Gd3+ chelates, which are favored due to their high paramagnetism and excellent relaxation enhancement. Unfortunately, most clinically approved contrast agents suffer from rapid renal clearance and modest contrast enhancement, making them suboptimal for angiographic enhancement and tumor imaging.5 Thus, the use of long circulating high molecular weight contrast agents are attractive due to their greater clinical flexibility arising from improved pharmacokinetics, potential for tissue selectivity through the use of targeting ligands, and capacity for much higher Gd3+ loading to improve contrast and sensitivity.6 For example, a 75 kD Gd3+-DTPA-dextran conjugate with 15 Gd3+ ions was found to enable vascular imaging in rats for up to 1 hr.7
Nanomaterial platforms such as dendrimers,8–10 polymers,10, 11 inorganic particles,12 and other supramolecular assemblies13 have been used as carriers of Gd3+; however, many of these carriers suffer from poor renal filtration, hepatobiliary uptake, and in vivo accumulation. Their high Gd3+ payload can also become a liability if the agent is constructed from non-degradable materials that employ acyclic chelates that are kinetically labile. Contrast agents of this type can leach free Gd3+ ions into the bloodstream, where they can provoke nephrogenic systemic fibrosis (NSF) and other cytotoxic responses as has been observed for low molecular weight acyclic Gd3+-DTPA constructs.14 Additionally, many of these agents possess a highly flexible structure that tends to reduce their relaxivity. We sought to develop a semi-flexible, long circulating contrast agent derived from low toxicity building blocks that could be degraded to rapidly excretable products to obviate these safety and relaxivity problems.
Discher and coworkers have demonstrated that micelles with a flexible rod–like morphology possess longer circulation times than spherical micelles, a property that was attributed to their ability to evade macrophage uptake.15 More recently, Mitragotri, et al.16 and DeSimone, et al.17 have also demonstrated that nanoparticles with rod–like morphologies were internalized by macrophages and HeLa cells, respectively, at a much lower rate than spherical particles. With these prior findings in mind, we designed a multivalent Gd3+ MRI contrast agent based on a flexible rod-like polyrotaxane scaffold. Polyrotaxanes (PR) are non-covalent self-assemblies comprised of cyclic molecules threaded onto a polymer “axle” that retains the macrocycles via bulky endcaps positioned at the termini of the “axle”.18, 19 Herein, we describe a polyrotaxane-based Gd3+-DOTA MRI imaging agent constructed from 2-hydroxypropyl-β-cyclodextrin (HPCD) and Pluronic F127 (Pluronic) (Figure 1), both of which are widely used excipients in FDA approved formulations for human use. Although Gd3+-DOTA chelate-modified cyclodextrins20, 21 and cyclodextrin polymers22–25, have been described previously as MRI contrast agents, few rotaxanated cyclodextrin contrast agents have been reported. This PR was designed based on the hypothesis that its flexible rod–like morphology would confer long circulation properties to the contrast agent. Since HPCD is known to form inclusion complexes with the central poly(propylene oxide) blocks of Pluronic copolymers, this property was utilized to construct a PR bearing carbamate linked endcaps that were attached as described previously,26, 27 except that cholesterol carbonyl chloride was used as endcapping reagent. Analysis by 1H NMR, GPC-MALS/RI, and MALDI-TOF MS indicated that the PR product bore 15 copies of HPCD, with an average molecular weight of 36 kDa and a poly(propylene oxide) block coverage of ~ 46%. The polyrotaxane was also analyzed for presence of free HPCD contamination by RP-HPLC and hydrophilic interaction liquid chromatography; both of these techniques indicated a free HPCD content of <4%. The HPCD/Pluronic PR intermediate was then activated with CDI before modification with an excess of 1,8-diamino 3,6-dioxooctane to increase the water solubility of the material. Finally, this intermediate was coupled with S-2-(4-Isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid (DO3A-Bn-SCN) and then treated with GdCl3 to obtain the Gd3+-DO3A-HPCD/Pluronic PR contrast agent with 14 DO3A attached as determined by 1H NMR and 14 Gd3+ ions as determined by ICP-MS. AFM analysis of dried samples on mica indicated that the polyrotaxane prepared had a rod-like morphology with lengths in the range of 30–40 nm and diameters of ~5 nm, near the threshold dimension than can lead to rapid clearance from the bloodstream due to the 5 nm effective pore size of the glomerular endothelium.28
Figure 1.
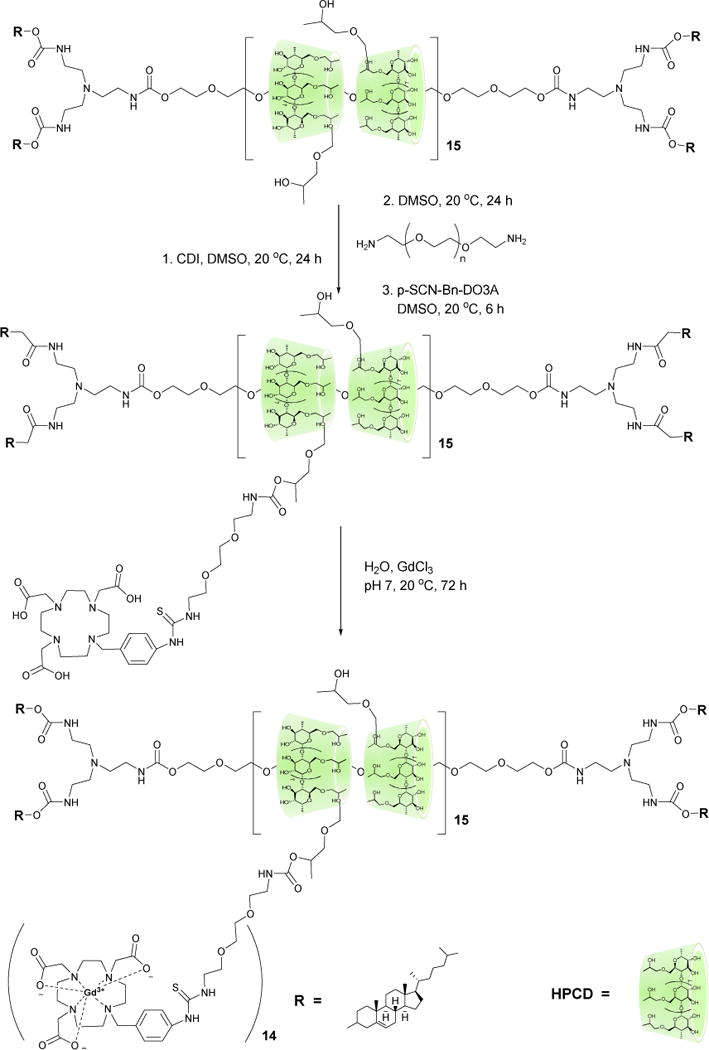
Synthesis of Gd3+-DO3A-HPCD/Pluronic PR.
Relaxivity measurements (Figure 2) of the monomeric and rotaxanated Gd3+ agents gave r1 values of 7.82 and 23.83 mM−1s−1 per Gd chelate for Gd3+-DO3A-HPCD and Gd3+-DO3A-HPCD/Pluronic PR, respectively, at 1.5 T, 37°C (8.46 and mM−1s−1 34.08 at 0.5T, respectively, Supplementary Information). The Gd3+-DO3A-HPCD r1 relaxivity is in good agreement with the ionic relaxivity reported for other cyclodextrin monomeric contrast agents ([Gd3+-DOTA]7-β-CD, r1 = 12.2 mM−1s−1 at 1.5T;20 [Gd3+-DTTA]7-β-CD, r1 = 6.2 mM−1s−1 at 9.4T21) and dextran-DTPA derivative with similar Gd3+ content (r1 = 10.5 mM−1s−1 at 0.25T),7 a [Gd3+-DOTA]8-sucrose derivative (4.1 mM−1s−1),29 all measured at 37 °C. The greater than three fold improvement in ionic relaxivity of the PR construct relative to the Gd3+-DO3A-HPCD monomer is attributed to the increased polymer rigidity imparted by rotaxanation as well as reduced rotational motion of the threaded, hydrogen-bonded nearest-neighbor cyclodextrin units.
Figure 2.
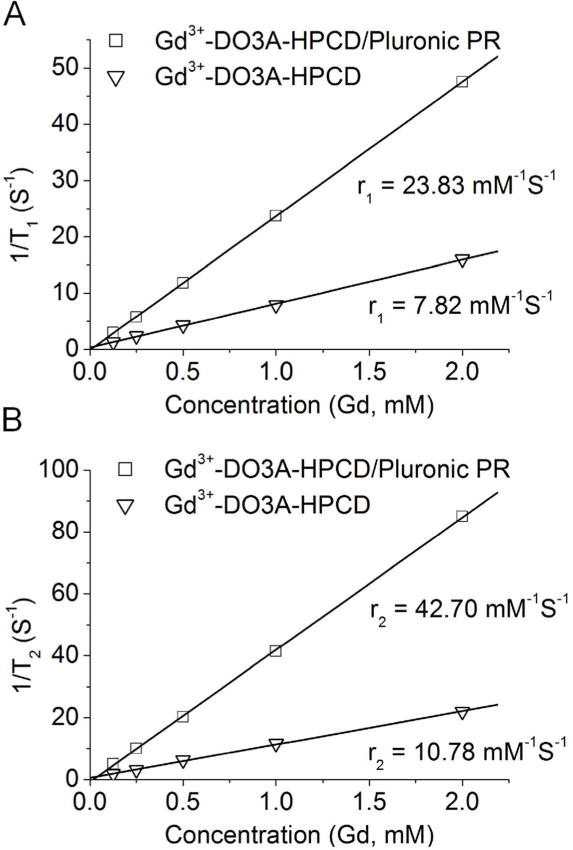
Plots of 1/T1 (A) and 1/T2 (B) versus the concentration of the contrast agents Gd3+-DOTA-HPCD and Gd3+-DOTA-HPCD/Pluronic PR.
We then evaluated the monomeric and PR Gd3+-DO3A agents in Balb/c mice to determine their contrast enhancement capabilities. MR images (Figure 3) revealed that Gd3+-DO3A-HPCD/Pluronic PR has a substantially longer circulation time in mice than the monomeric Gd3+-DO3A-HPCD derivative. Within 5 minutes, the monomer had largely cleared from the blood and accumulated in the kidneys and bladder, with little visible intensity in the heart. The polyrotaxane derivative; however, had a substantially better signal enhancement in the blood of the heart at 5 minutes that only slowly diminished out to the 30 minute timepoint. Improved blood circulation of the Gd3+-DOTA-HPCD/Pluronic PR species enabled the visualization of greater anatomic detail and blood vessel organization. Conversely, the monomeric Gd3+-DO3A-HPCD chelate did not produce significant blood enhancement due to rapid elimination via renal filtration. No acute toxicity was observed in any of the animals tested.
Figure 3.
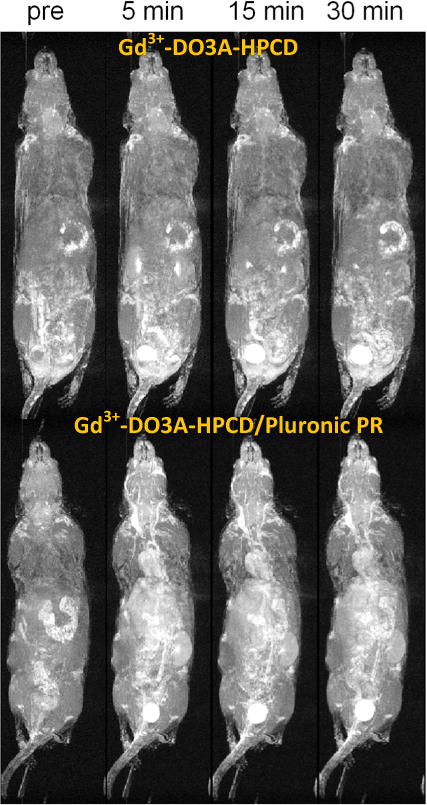
Representative 3D maximum intensity projection images (T1-weighted) of Balb/c mice injected with Gd3+-DOTA-HPCD (top row) or Gd3+-DOTA-HPCD/Pluronic PR (bottom row) at a 0.03 mM-Gd/kg dose. Images are shown for contrast agent distribution before injection (pre) and at 5, 15 and 30 min after tail vein injection of Gd3+ complexes.
Quantitative analysis of the MR images revealed an increased enhancement ratio (ER) in the blood of the heart of approximately two-fold during the first 30 min for the polyrotaxane contrast agent, whereas the ER of the Gd3+-DO3A-HPCD control was 1.25 after 5 min and dropped to background levels within 25 min (Figure 4). Furthermore, the ER observed in the kidneys for the Gd3+-DO3A-HPCD/Pluronic PR gradually decreased from 1.9 to 1.65 between 5–30 min, while that observed with the monomeric Gd3+-DO3A-HPCD control dropped rapidly from 1.55 to 1.1 over the same time period, indicating rapid renal filtration of the control. The superior MR contrast enhancement of the Gd3+-DO3A-HPCD/Pluronic PR derivative in the blood at 30 min relative to the control demonstrates the improved pharmacokinetics of the polyrotaxane motif. Given that the polyrotaxanes were observed to undergo slow clearance through the kidneys, we infer from these findings that the Gd3+-DO3A-HPCD/Pluronic PR contrast agent platform possesses the potential for low in vivo accumulation, either through direct excretion of intact low molecular weight members of the polyrotaxane population through the glomerular membrane or by enzymatic cleavage of the carbamate bonds linking the endcap to the Pluronic core and subsequent dethreading of the rotaxanated HPCD units26, 27 to produce easily eliminated Gd3+-DO3A-HPCD monomers and the F127 Pluronic precursor. Further experiments are needed to discriminate between these possible mechanisms. These findings also suggest that HPCD/Pluronic PR display long circulation properties that may provide significant advantages in the cyclodextrin-mediated mobilization of aberrantly stored cholesterol as occurs in tissues affected by Niemann-Pick Type C disorder.26, 27
Figure 4.
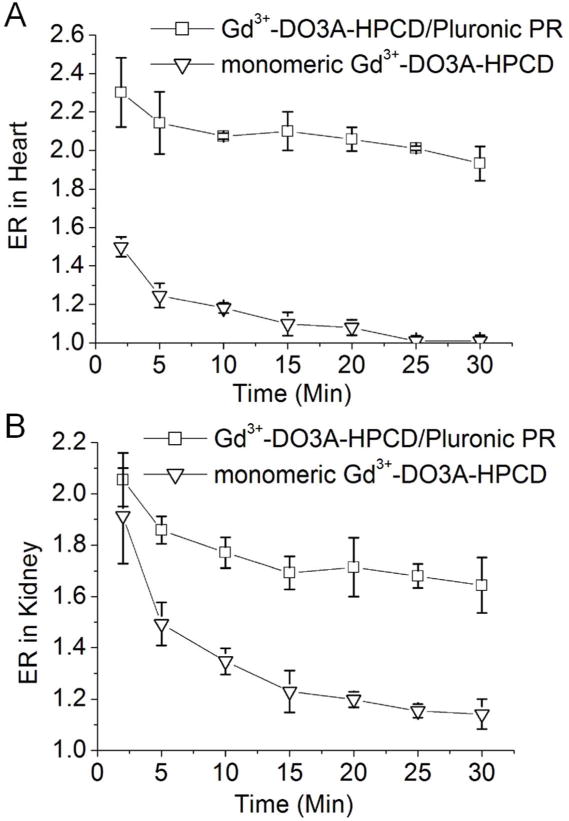
Enhancement ratio (ER = Spost/Spre) of the MR signal post-injection to that of pre-injection in the blood of the heart (A) and in the kidneys (B) (n = 3). Balb/c mice injected at a 0.03 mM-Gd/kg body weight dose with Gd3+-DO3A-HPCD (monomer, square) or Gd3+-DO3A-HPCD/Pluronic PR (PR, triangle).
Conclusions
We report the development of a long circulating, degradable Gd3+-DO3A-HPCD/Pluronic polyrotaxane that has the intravascular imaging capabilities of a macromolecular contrast agent, while retaining the renal elimination properties of a small molecule agent. Taken together, these results suggest that Gd3+-DO3A-HPCD/Pluronic PR may be a promising material for development as a cardiovascular enhancement contrast agent due to its lack of acute toxicity, long circulation properties, and potential for providing a greatly improved safety profile relative to non-degradable polymer contrast agents.
Supplementary Material
Acknowledgments
We gratefully acknowledge the assistance of Mr. Brad Loren in the preparation of this manuscript.
Funding Sources
We would like to express our special thanks for the support of this work by the Smith Family BReaK Thru Fund, the Ara Parseghian Medical Research Foundation, the Indiana Clinical and Translational Sciences Institute Core Pilot Funding Grant # UL1TR001108, and NIH grants GM087016 & EB017921 (DT) and EB000489 (ZL). 1H NMR data were acquired in the Purdue Interdepartmental NMR Facility and MALDI TOF MS data were acquired in the Campus-Wide Mass Spectrometry Center, both of which are supported by the NCI CCSG CA23168 grant to the Purdue Center for Cancer Research. The MRI experiments were conducted in accordance with the animal welfare requirements of Case Western Reserve University.
Abbreviations
- HP-β-CD
2-hydroxypropyl-β-cyclodextrin
- MALS/RI
multiangle light scattering/refractive index detection
- PR
polyrotaxane
Footnotes
Supporting Information.
1H NMR, GPC, MALDI-TOF MS, and AFM data of the PR agent are available in Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
References
- 1.Villaraza AJL, Bumb A, Brechbiel MW. Macromolecules, Dendrimers, and Nanomaterials in Magnetic Resonance Imaging: The Interplay Between Size, Function, and Pharmacokinetics. Chem Rev. 2010;110:2921–2959. doi: 10.1021/cr900232t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Courant T, Roullin VG, Cadiou C, Callewaert M, Andry MC, Portefaix C, Hoeffel C, de Goltstein MC, Port M, Laurent S, Elst LV, Muller R, Molinari M, Chuburu F. Hydrogels Incorporating GdDOTA: Towards Highly Efficient Dual T1/T2 MRI Contrast Agents. Angew Chem Int Ed. 2012;51:9119–9122. doi: 10.1002/anie.201203190. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Z, Lu ZR. Gadolinium-Based Contrast Agents for Magnetic Resonance Cancer Imaging. WIREs Nanomed Nanobiotechnol. 2013;5:1–18. doi: 10.1002/wnan.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang J, Sheng Y, Hu H, Shen Y. Macromolecular MRI Contrast Agents: Structures, Properties and Applications. Prog Polym Sci. 2013;38:462–502. [Google Scholar]
- 5.Mohs AM, Lu ZR. Gadolinium (III)-based Blood Pool Contrast Agents for Magnetic Resonance Imaging: Status and Clinical Potential. Expert Opin Drug Deliv. 2007;4:149–164. doi: 10.1517/17425247.4.2.149. [DOI] [PubMed] [Google Scholar]
- 6.Liu T, Li X, Qian Y, Hu X, Liu S. Multifunctional pH-Disintegratable Micellar Nanoparticles of Asymmetrically Functionalized beta-Cyclodextrin-based Star Copolymer Covalently Conjugated with Doxorubicin and DOTA-Gd Moieties. Biomaterials. 2012;33:2521–2531. doi: 10.1016/j.biomaterials.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Wang SC, Wikstrom MG, White DL. Evaluation of Gd-DTPA Labeled Dextran as an Intravascular MR Contrast Agent - Imaging Characteristics in Normal Rat Tissues. Radiology. 1990;175:483–488. doi: 10.1148/radiology.175.2.1691513. [DOI] [PubMed] [Google Scholar]
- 8.Rudovsky J, Botta M, Hermann P, Hardcastle KI, Lukes I, Aime S. PAMAM Dendrimeric Conjugates with a Gd-DOTA Phosphinate Derivative and Their Adducts with Polyaminoacids: The Interplay of Global Motion, Internal Rotation, and Fast Water Exchange. Bioconjugate Chem. 2006;17:975–987. doi: 10.1021/bc060149l. [DOI] [PubMed] [Google Scholar]
- 9.Boswell CA, Eck PK, Regino CA, Bernardo M, Wong KJ, Milenic DE, Choyke PL, Brechbiel MW. Synthesis, Characterization, and Biological Evaluation of Integrin alphaVbeta3-targeted PAMAM Dendrimers. Molecular Pharm. 2008;5:527–539. doi: 10.1021/mp800022a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu R, Kaneshiro TL, Jeong EK, Parker DL, Lu ZR. Synthesis and Evaluation of Nanoglobule-Cystamine-(Gd-DO3A), a Biodegradable Nanosized Magnetic Resonance Contrast Agent for Dynamic Contrast-enhanced Magnetic Resonance Urography. Int J Nanomed. 2010;5:707–713. doi: 10.2147/ijn.s12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye Z, Jeong EK, Wu X, Tan M, Yin S, Lu ZR. Polydisulfide Manganese(II) Complexes as Non-gadolinium Biodegradable Macromolecular MRI Contrast Agents. J Magn Reson Imaging. 2012;35:737–744. doi: 10.1002/jmri.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santra S, Bagwe RP, Dutta D, Stanley JT, Walter GA, Tan W, Moudgil BM, Mericle RA. Synthesis and Characterization of Fluorescent, Radio-opaque, and Paramagnetic Silica Nanoparticles for Multimodal Bioimaging Applications. Adv Mater. 2005;17:2165–2169. [Google Scholar]
- 13.Chen KJ, Wolahan SM, Wang H, Hsu CH, Chang HW, Durazo A, Hwang LP, Garcia MA, Jiang ZK, Wu L, Lin YY, Tseng HR. A Small MRI Contrast Agent Library of Gadolinium(III)-encapsulated Supramolecular Nanoparticles for Improved Relaxivity and Sensitivity. Biomaterials. 2011;32:2160–2165. doi: 10.1016/j.biomaterials.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frenzel T, Lengsfeld P, Schirmer H, Hutter J, Weinmann HJ. Stability of Gadolinium-Based Magnetic Resonance Imaging Contrast Agents in Human Serum at 37 degrees C. Invest Rad. 2008;43:817–828. doi: 10.1097/RLI.0b013e3181852171. [DOI] [PubMed] [Google Scholar]
- 15.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape Effects of Filaments Versus Spherical Particles in Flow and Drug Delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Champion JA, Mitragotri S. Shape Induced Inhibition of Phagocytosis of Polymer Particles. Pharm Res. 2009;26:244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone J. The Effect of Particle Design on Cellular Internalization Pathways. Proc Natl Acad Sci USA. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wenz G, Han B-H, Muller A. Cyclodextrin Rotaxanes and Polyrotaxanes. Chem Rev. 2006;106:782–817. doi: 10.1021/cr970027+. [DOI] [PubMed] [Google Scholar]
- 19.Loethen S, Kim JK, Thompson DH. Biomedical Applications of Cyclodextrin-based Polyrotaxanes. J Macromol Sci C. 2007;47:383–418. [Google Scholar]
- 20.Song Y, Kohlmeir EK, Meade TJ. Synthesis of Multimeric MR Contrast Agents for Cellular Imaging. J Am Chem Soc. 2008;130:6662–6663. doi: 10.1021/ja0777990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryson JM, Chu WJ, Lee JH, Reineke TM. A beta-Cyclodextrin “Click Cluster” Decorated with Seven Paramagnetic Chelates Containing Two Water Exchange Sites. Bioconjugate Chem. 2008;19:1505–1509. doi: 10.1021/bc800200q. [DOI] [PubMed] [Google Scholar]
- 22.Aime S, Botta M, Frullano L, Geninatti Crich S, Giovenzana GB, Pagliarin R, Palmisano G, Sisti M. Contrast Agents for Magnetic Resonance Imaging: A Novel Route to Enhanced Relaxivities Based on the Interaction of a Gd-III Chelate with Poly-beta-Cyclodextrins. Chem Eur J. 1999;5:1253–1260. [Google Scholar]
- 23.Aime S, Botta M, Fedeli F, Gianolio E, Terreno E, Anelli P. High-relaxivity Contrast Agents for Magnetic Resonance Imaging Based on Multisite Interactions Between a beta-Cyclodextrin Oligomer and Suitably Functionalized Gd-III Chelates. Chem Eur J. 2001;7:5262–5269. doi: 10.1002/1521-3765(20011217)7:24<5261::aid-chem5261>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Aime S, Gianolio E, Uggeri F, Tagliapietra S, Barge A, Cravotto G. New Paramagnetic Supramolecular Adducts for MRI Applications Based on Non-covalent Interactions Between Gd(III)-complexes and beta- or gamma-Cyclodextrin Units Anchored to Chitosan. J Inorg Biochem. 2006;100:931–938. doi: 10.1016/j.jinorgbio.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Battistini E, Gianolio E, Gref R, Couvreur P, Fuzerova S, Othman M, Aime S, Badet B, Durand P. High-relaxivity Magnetic Resonance Imaging (MRI) Contrast Agent Based on Supramolecular Assembly Between a Gadolinium Chelate, a Modified Dextran, and Poly-beta-cyclodextrin. Chem Eur J. 2008;14:4551–4561. doi: 10.1002/chem.200701587. [DOI] [PubMed] [Google Scholar]
- 26.Mondjinou Y, McCauliff LA, Kulkarni A, Paul LN, Hyun SH, Zhang Z, Wu Z, Wirth M, Storch J, Thompson DH. Synthesis of 2-Hydroxypropyl-beta-cyclodextrin/Pluronic-based Polyrotaxanes via Heterogeneous Reaction as Potential Niemann-Pick Type C Therapeutics. Biomacromolecules. 2013;14:4189–4197. doi: 10.1021/bm400922a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins CJ, McCauliff LA, Hyun SH, Zhang Z, Paul LN, Kulkarni A, Zick K, Wirth M, Storch J, Thompson DH. Synthesis, Characterization, and Evaluation of Pluronic-Based beta-Cyclodextrin Polyrotaxanes for Mobilization of Accumulated Cholesterol from Niemann-Pick Type C Fibroblasts. Biochemistry. 2013;52:3242–3253. doi: 10.1021/bi3010889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilum L, Davis SS, Wilson CG, Thomas NW, Frier M, Hardy JG. Blood Clearance and Organ Deposition of Intraveneously Administered Colloidal Particles - The Effects of Particle Size, Nature and Shape. Int J Pharm. 1982;12:135–146. [Google Scholar]
- 29.Martinez GV, Navath S, Sewda K, Rao V, Foroutan P, Alleti R, Moberg VE, Ahad AM, Coppola D, Lloyd MC, Gillies RJ, Morse DL, Mash EA. Demonstration of a Sucrose-derived Contrast Agent for Magnetic Resonance Imaging of the GI Tract. Bioorg Med Chem Lett. 2013;23:2061–2064. doi: 10.1016/j.bmcl.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


