Abstract
Background
Atrial fibrillation (AF) is a common and dangerous paroxysmal rhythm abnormality. Smartphones are increasingly used for mobile health applications by older patients at risk for AF and may be useful for AF screening.
Objectives
To test whether an enhanced smartphone app for AF detection can discriminate between sinus rhythm (SR), AF, premature atrial contractions (PACs) and premature ventricular contractions (PVCs).
Methods
We analyzed 219 2-minute pulse recordings from 121 participants with AF (n=98), PACs (n=15), or PVCs (n=15) using an iPhone 4S. We obtained pulsatile time series recordings in 91 participants after successful cardioversion to sinus rhythm from pre-existing AF. The PULSESMART app conducted pulse analysis using 3 methods [Root Mean Square of Successive RR Differences; Shannon Entropy; Poincare plot]. We examined the sensitivity, specificity, and predictive accuracy of the app for AF, PAC, and PVC discrimination from sinus rhythm using the 12-lead EKG or 3-lead telemetry as the gold standard. We also administered a brief usability questionnaire to a subgroup (n=65) of app users.
Results
The smartphone-based app demonstrated excellent sensitivity (0.970), specificity (0.935), and accuracy (0.951) for real-time identification of an irregular pulse during AF. The app also showed good accuracy for PAC (0.955) and PVC discrimination (0.960). The vast majority of surveyed app users (83%) reported that it was “useful” and “not complex” to use.
Conclusions
A smartphone app can accurately discriminate pulse recordings during AF from sinus rhythm, PACs, and PVCs.
Keywords: Atrial fibrillation, Smartphone, Detection, Technology, Premature beats
Introduction
Atrial fibrillation (AF) is the most common heart rhythm abnormality in the U.S., with an estimated 5–6 million Americans affected.(1) Symptoms of AF can be non-specific and include palpitations, shortness of breath, or fatigue. In contrast to benign causes of palpitations, including premature atrial or ventricular contractions (PACs or PVCs), AF is associated with a 5-fold higher risk for stroke, 2-fold higher risk for heart failure, and reduced survival.(2),(3) Since paroxysms of AF can be brief and infrequent, effective detection of AF is challenging and often requires long-term outpatient cardiac monitoring.(4) On this basis, a call for the development of new approaches for AF screening was issued by a National Institute of Health Heart Lung & Blood Institute Expert panel.(5)
The electrocardiogram remains the gold-standard for the diagnosis of atrial fibrillation. Unfortunately, existing cardiac monitors, with their multiple cables that must remain continuously attached to the body with electrodes, are burdensome, and suffer from poor patient adherence.(4) Implantable monitors are appropriate in high-risk populations (e.g., prior stroke) but remain limited for general use by the fact that they are costly and invasive.(6) As many as 50% of patients with stroke who have paroxysms of AF as the cause may go undetected when screening ends at hospital discharge.(7) Therefore, less burdensome and longer-term methods for AF screening for populations with cryptogenic stroke remain crucial.
Smartphones are becoming increasingly pervasive throughout the general population, including older persons who are most at risk for AF.(8) It is estimated that more than 64% of adults own a smartphone, including 54% of those aged 50–64 years old and more than 27% aged >65 years old.(9) In addition to using smartphones for the Internet and telephone, older persons are also taking advantage of the health monitoring capability of smartphones. For example, a recent Swedish study (STROKESTOP) demonstrated that 50% of the entire 75–76-year-old population screened was willing and able to use a small portable device to screen for AF multiple times per day.(10) In several recent studies conducted in diverse cohorts, the AliveCor system, which pairs a smartphone with proprietary technology enabling single lead ECG recording and rhythm analysis, has proven effective in screening for AF.(11,12)
Although validated AF screening and monitoring smartphone applications exist, benign causes of pulse irregularity such as PACs and PVCs are common in the general population and confound many existing smartphone-based AF monitoring solutions.(13) This limitation leads to false positives and has heretofore limited the widespread acceptance of pulse-based smartphone AF screening.
We sought to test the hypothesis that pulse signals recorded using an out-of-the-box smartphone camera could be analyzed by a real-time realizable software algorithm comprised of 3 statistical methods and motion and noise artifact correction and would be able to distinguish AF from PACs, PVCs, and sinus rhythm.(14) The present study sought to build on our previous work by enriching our existing cohort with a diverse array of benign rhythm abnormalities likely to be encountered in an outpatient setting.(15)
Methods
Study Sample
The details of the original PULSEMART investigation have been published.(16) In brief, the original PULSESMART cohort included 76 participants with AF scheduled to undergo elective cardioversion at the University of Massachusetts Medical Center (UMMC). Original study participants had 2 minute pulse waveforms recorded before and after elective cardioversion by study staff using a labeled study iPhone 4S. Contemporaneous ECG-telemetry data was recorded and used as a gold-standard for rhythm determination. For the present study, we enriched our sample with an additional 55 participants (22 adults with AF, 15 with PACs, and 15 with PVCs) to create a cohort comprised of a more representative array of benign (PAC and PVC) and malignant (AF) causes of an irregular pulse. Differences in the characteristics of participants with AF in the newly recruited cohort (n=22) were compared to those in the original cohort (n=76, Supplemental Table 1). For all participants, after obtaining informed consent, baseline demographic, clinical and laboratory variables were abstracted from participants’ medical records by a trained staff member.
New participants with AF included in this analysis were recruited in the same manner of the original PULSESMART investigation. Two-minute pulse-recordings were obtained before and after cardioversion. Patients with frequent PACs or PVCs were identified from a roster of inpatients on the cardiac telemetry unit at the UMMC. Study staff performed a review of hospital telemetry recordings on a daily basis to identify patients with frequent ectopy. Patients with frequent PACs or PVCs were approached for study participation by study staff. After obtaining informed consent, a single 2-minute pulse recording was obtained from these participants using a study iPhone 4S while contemporaneous telemetry recording was performed. Telemetry strips were printed and reviewed off-line for the presence of PACs or PVCs. The frequency of atrial and ventricular ectopy (ectopic beats/min) was determined post-hoc from available rhythm strips (n=17). All iPhone pulse recordings were downloaded using a de-identified study number to enable post-processing and analysis.
A nested sub-study of app usability consisting of consecutive newly recruited participants not receiving anesthesia (n=65) was conducted using a standardized questionnaire. This study was approved by the Institutional Review Boards of the University of Massachusetts Medical School and Worcester Polytechnic Institute (WPI).(16)
Rhythm determination
Trained physicians reviewed all ECG and/or telemetry data to determine heart rhythm using standard criteria. In cases where reviewers disagreed about the rhythm diagnosis, a “tie-breaker” reader was consulted.
Signal Acquisition and Processing
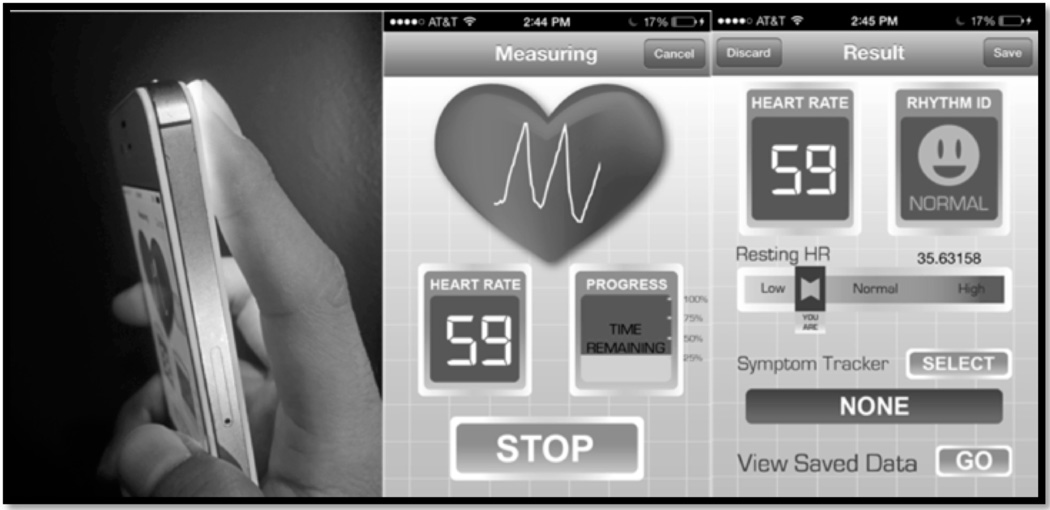
Participants were asked to hold the iPhone 4S in their hand, with their right first or second finger on the standard camera and lamp for 2 minutes, during which time the pulse waveform was recorded (Figure 1). Pulse recordings were obtained with patients in the supine position. A video of user’s fingertip blood flow intensity at 640×480 pixel resolution is sampled at a rate of 30 frames/sec for 2 minutes. An average of the intensity values from the green band from the RGB video is analyzed. Algorithms perform beat-to-beat rhythm analysis from the pulse signal values. If the signal is deemed “too noisy,” to allow meaningful interpretation (e.g., excessive finger movement), the recording is cancelled and the user prompted to restart. As described in our prior work, the app uses a peak detection algorithm that includes motion noise correction, a filter bank with estimates of heart rate, variable cutoff frequencies, rank-order non-linear filters and decision logic.(16) The time required for computational processing of a 64-heartbeat (≈1 minute) pulse recording was ≈20 msec on the iPhone 4S.(14)
Figure 1.
A prototype of the Pulse Waveform Analysis Application running on an iPhone 4S. From left to right: iPhone 4S camera; fingertip applied to iPhone 4S camera; a representative recording from a patient in atrial fibrillation; a representative recording from a patient in normal sinus rhythm.
Pulse Waveform Analysis Approach
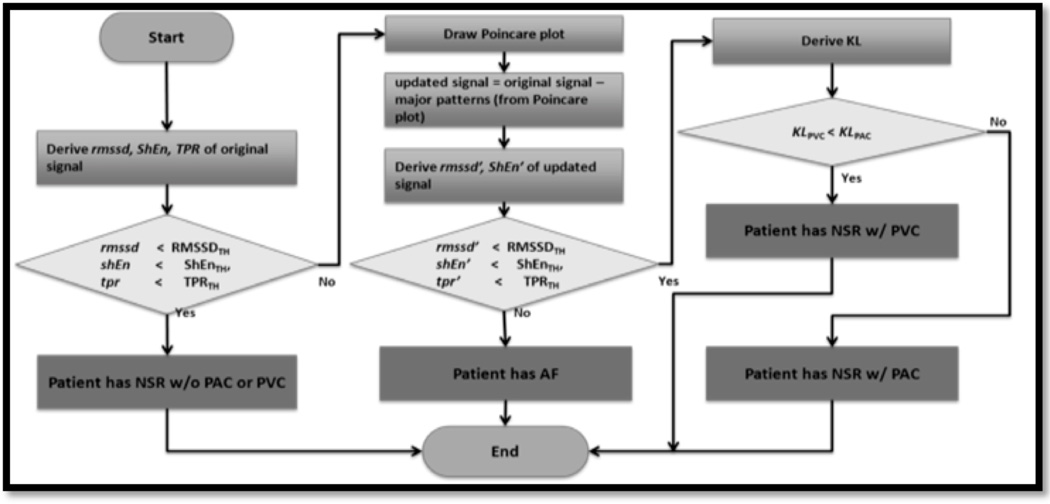
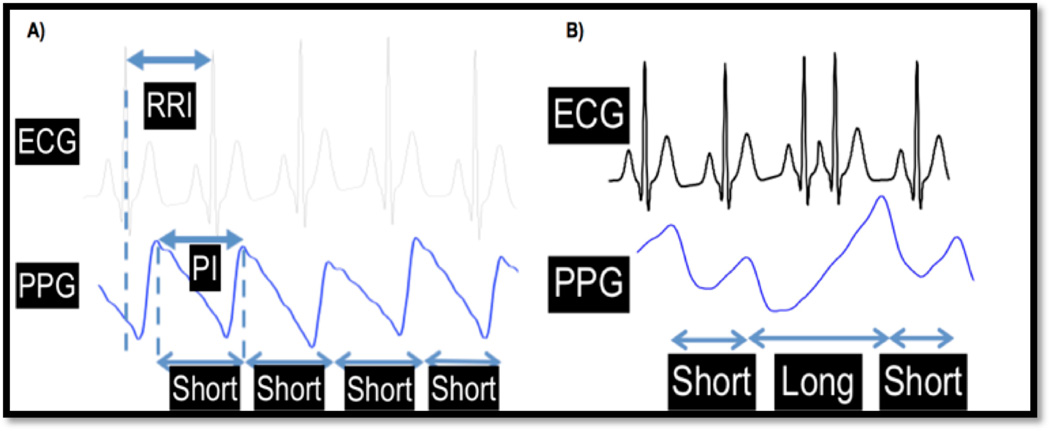
The chaotic atrial electrical activity and resultant rapid, irregular ventricular response that characterizes AF generates a random heart beat sequence with increased beat-to-beat variability. Our approach to rhythm discrimination using the pulse recording is to combine 3 validated statistical techniques (Figure 2).(17),(18) These include Root Mean Square of Successive Difference of RR intervals (RMSSD), Poincare plot (or Turning Point Ratio), and Shannon Entropy (ShE). RMSSD quantifies RR variability, ShE characterizes its complexity, and the Poincare plot can help distinguish AF from ectopic atrial or ventricular beats (PACs, PVCs, Figure 3).(18) Threshold values for RMSSD, ShE, and TPR were derived using the MIT-BIH AF and NSR (normal sinus rhythm) databases as well as our own previously published data.(16) We used threshold values of RMSDD = 0.1093, ShE = 0.4890, Poincare Plot = 0.2, since these values corresponded to the largest area under ROC curves for each respective measure.(14)
Figure 2.
A flowchart of the Pulse Waveform Analysis Algorithm
Figure 3.
a): Comparison of ECG RR intervals to pulse intervals obtained from an iPhone; b): an example illustrating how a premature atrial contraction results in a longer duration pulse interval and larger amplitude pulse beat when compared to a normal pulse beat.(12)
Usability Questionnaire
In a subset of 65 English-literate inpatients at the UMMC who did not receive sedation and who consented separately for a usability assessment of the smartphone app, we administered a questionnaire focused on assessing the participants’ perception of PULSESMART app’s ease of use (e.g., “Overall, do you find the application to be [very difficult to very easy],” “In comparison to other heart rhythm monitors, do you consider the app to be [very difficult to very easy],”) and importance to their personal health (“If this app could determine your heart rhythm, how important could this be for you: [not important-very important], “The app gives me reassurance: [agree-disagree]”). Participants responded to the questions using a 5-point Likert scale. The internal consistency of the perceived importance component was excellent (cronbach’s α = 0.88; 8 items); the perceived difficulty subcomponent had only three items and therefore measurement of internal consistency was not appropriate.(19)
Data analysis
We analyzed all 219 pulse recordings derived from participants in the original PULSESMART cohort as well as from newly recruited participants with AF, PACs, or PVCs. We compared the characteristics of participants by rhythm status (pre-cardioversion AF, post-cardioversion sinus rhythm, PACs and PVCs). We then calculated the mean, median, and interquartile range for RMSSD and ShE, which were calculated for all participants. We then calculated test characteristics, including sensitivity, specificity, and accuracy, for the automated smart-phone based detection algorithm for AF, PACs, and PVCs when compared with the expert reviewer diagnosis (criterion standard) of sinus rhythm based on 12-lead ECG, using the threshold values of RMSSD, ShE and TPR/Poincare plot, respectively.
Participants perception of the app was described using univariate dot plots since the instrument was developed specifically for this technology and the interpretation of summary data may not be as meaningful as the distribution of the data (20)
Results
The characteristics of the 121 participants included in our study are shown in Table 1. The mean age of the cohort was 66 years, 7% were non-white, and 18% were women. There was a high burden of comorbid cardiovascular diseases (e.g., hypertension, coronary artery disease, stroke) in the cohort and neither the demographic nor clinical characteristics appeared to vary significantly across rhythm categories (AF, PAC, or PVC). The 22 newly recruited participants with AF did not differ significantly with respect to demographic characteristics or burden of comorbid illnesses to those recruited for our original investigation (Supplemental Table 1). Participants with PACs and PVCs included in our post-hoc analysis had 11.1 PACs/minute and 17.5 PVCs/minute, respectively. The relative proportion of patients with benign (PAC and PVC) vs. malignant (AF) arrhythmias would be representative of high-risk populations, including those with cryptogenic stroke.
Table 1.
PULSESMART participant characteristics
| Characteristics | AF (n=98) |
PAC (n=15) |
PVC (n=15) |
Sinus Rhythm (n=91)* |
|---|---|---|---|---|
| Age, years, M (SD) | 65.9 (12.2) | 73.1 (5.9) | 62.8 (13.8) | 66 (11.9) |
| Sex, male, n (%) | 70 (71.4) | 11 (73.3) | 9 (60) | 63 (69.2) |
| White Race, n (%) | 91 (92.9) | 14 (93.3) | 13 (86.7) | 86 (94.5) |
| Body Mass Index, kg/m2, M (SD) | 31.6 (8.5) | 27.7 (8.4) | 32.1 (8.6) | 31.6 (8.1) |
| Medical Characteristics | ||||
| Hypertension | 70 (71.4) | 14 (93.3) | 13 (86.7) | 63 (69.2) |
| Hyperlipidemia | 59 (60.2) | 10 (66.7) | 10 (66.7) | 56 (61.5) |
| Current smoking | 8 (8.2) | 2 (13.3) | 4 (26.7) | 7 (7.7) |
| Diabetes mellitus | 26 (26.5) | 6 (40) | 4 (26.7) | 25 (27.5) |
| Coronary artery disease | 24 (24.5) | 4 (26.7) | 4 (26.7) | 23 (25.3) |
| Congestive heart failure | 27 (27.6) | 7 (46.7) | 8 (53.3) | 22 (24.2) |
| Sleep Apnea | 17 (17.3) | 2 (13.3) | 3 (20) | 14 (15.4) |
| Stroke | 10 (10.2) | 4 (26.7) | 3 (20) | 8 (8.8) |
| Medical treatment | ||||
| Beta-blocker | 64 (65.3) | 8 (53.3) | 9 (60) | 61 (67) |
| Calcium channel blocker | 27 (27.6) | 5 (33.3) | 1 (6.7) | 23 (25.3) |
| Anti-arrhythmic drug | ||||
| Class I | 8 (8.2) | 0 (0) | 0 (0) | 8 (8.8) |
| Class III | 29 (29.6) | 2 (13.3) | 2 (13.3) | 26 (28.6) |
| Digoxin | 7 (7.1) | 1 (6.7) | 1 (6.7) | 5 (5.5) |
Legend. AF = atrial fibrillation. PAC = premature atrial contraction. PVC = premature ventricular contraction.
91 of the 98 participants with AF were successfully converted to sinus rhythm.
App Performance In a Sample with Diverse Arrhythmias
Using established threshold values of 0.1093 for RMSSD, 0.4890 for ShE, and 2.0 for Turning Point Ratio, we observed that the algorithm comprised of all three statistical methods exhibited excellent sensitivity (0.97), specificity (0.935), and diagnostic accuracy (0.951) for the detection of an irregular pulse from AF when compared to the gold-standard diagnosis of AF by 12-lead ECG (Table 2). Using the same methods and threshold values, the algorithm combining RMSSD, ShE and TPR showed modest sensitivity for PAC (0.667) and PVC detection (0.733), respectively. However, false positives were rare (only 1 PAC and 1 PVC mischaracterized as AF by the application), and the specificity of the app for PAC (0.98), and PVC (0.976) detection was excellent.
Table 2.
Test Characteristics* for our Arrhythmia Detection Algorithm in 104 Subjects with Atrial Fibrillation, 91 with Sinus Rhythm, 14 with Premature Atrial Contraction, and 16 with Premature Ventricular Contractions.
| Algorithm | Sensitivity | Specificity | Accuracy |
|---|---|---|---|
| Atrial Fibrillation | 0.970 | 0.935 | 0.951 |
| Premature atrial Contraction | 0.667 | 0.980 | 0.955 |
| Premature ventricular contraction | 0.733 | 0.976 | 0.960 |
Test Characteristics of Statistical Methods Established using the threshold values of RMSDD = 0.1093, Shannon Entropy = 0.4890, Poincare Plot = 0.2. RMSSD = Root Mean Square of Successive RR Differences
Nested usability assessment
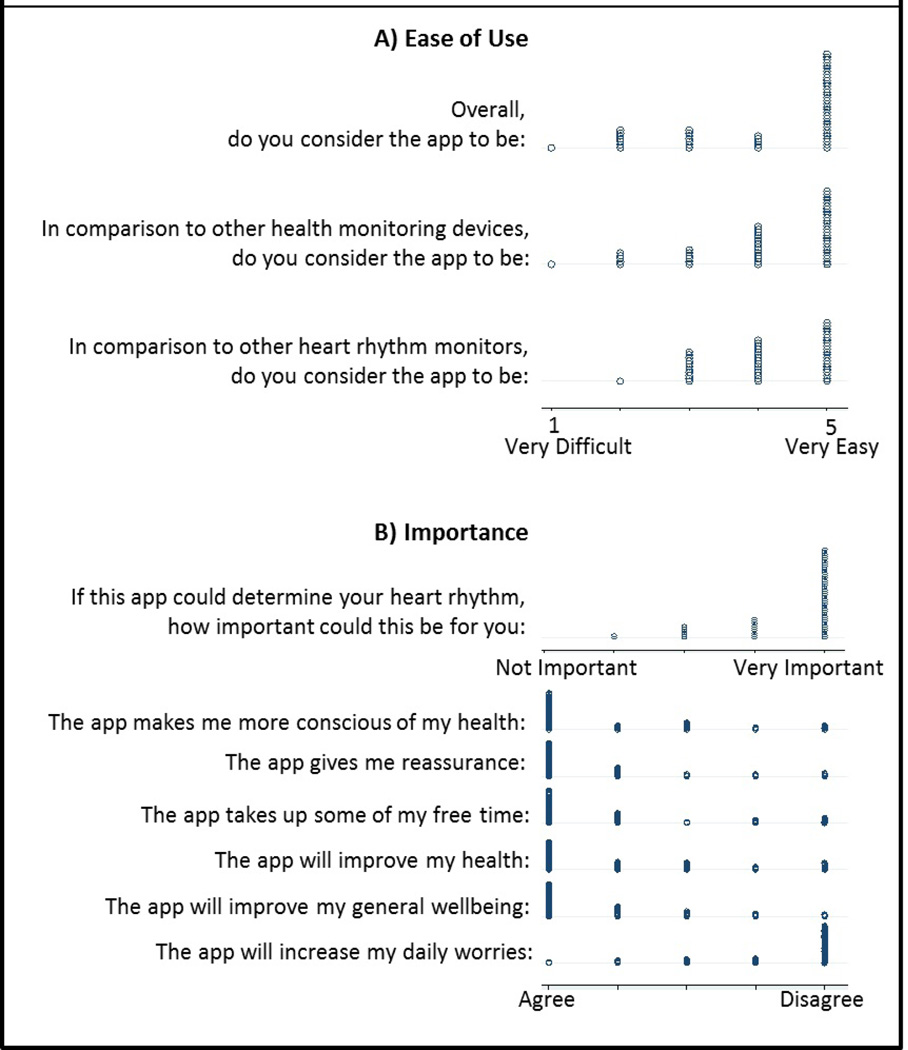
The demographic characteristics of the 65 participants that completed a usability assessment were similar to the overall study population. The majority of participants reported that the heart rhythm detection tool, or app, was “easy” or “very easy to use” (63%) and participants felt that the app could be “important” or “very important” (88%) to them if it could determine of their heart rhythm (Figure 4). Participants predominantly reported that app use was “reassuring,” “improve[d] my general well-being,” and could fit “very well” into their daily life (Figure 4).
Figure 4.
Distribution of the nested (n=65) usability assessment for A) Perceived ease of use and B) Importance.
Discussion
In this NHLBI-funded, prospective clinical investigation, we demonstrate that a novel arrhythmia detection application can reliably distinguish an irregular pulse from AF from sinus rhythm and other benign causes of cardiac rhythm abnormalities, including PVCs and PACs. The frequency of ectopic atrial beats and ventricular beats was high among participants during the period when rhythm analysis was conducted, equating to ≈16,000 and ≈25,000 ectopic beats per day, respectively. False positives due to PACs and PVCs bedevil current smartphone-based monitoring solutions, including ECG-based systems such as Alivecor.(21) False positive detection of AF due to PACs and PVCs can create undue patient anxiety as well as have important clinical implications. Our findings suggest that use of motion and noise artifact plus statistical methods for analyzing pulse variability can eliminate a key source of false positives. Our work provides strong evidence that “out-of-the-box” smartphone hardware can be harnessed for AF screening in a manner that is not intimidating to older, at-risk potential users.
AF is the world’s most common serious heart rhythm problem, affecting over 5 million Americans, and the prevalence of AF in the U.S. is expected to rise to 12 million in 2050.(2) In its early stages, most cases of AF are paroxysmal, making them difficult to identify early in the course of their disease. Excess annual national cost from treating AF patients is $26 billion - and much of this cost relates to cardiac rhythm monitoring as well as treatment of secondary complications of AF, complications that are preventable if an early AF diagnosis is made and prophylactic therapies initiated.(22,23)
Nationwide efforts are underway to promote AF screening, particularly in patients with risk factors (67 million Americans) to avert strokes, heart failure, and death,(24) but the most commonly prescribed non-invasive AF monitors, including Holter and 30-day event monitors, have severe methodological shortcomings that result in low long-term adherence, delays in diagnosis, high cost, and adverse patient outcomes.(25) Monitors with automated AF detection capabilities (e.g., Medtronic REVEAL XT or LinQ systems) are approved for clinical use, but the invasive nature, cost, and technical limitations of this AF monitoring solution have limited their widespread application.(6)
The ideal AF screening tool would be low-cost and would provide accurate and real-time realizable rhythm discrimination. Such a tool would have tremendous value for AF screening, particularly in rural areas and in the developing world.(26) Several mobile health solutions have been proposed to leverage the increasing use of smartphones among older persons,(26) in part driven by the ubiquity of these powerful devices worldwide as well as increasing interest in use of smartphones for health monitoring.(26) A recent study involving elderly patients demonstrated a willingness (>50% of those screened) and capacity to adhere to use of mobile health devices for AF screening among asymptomatic individuals. Not surprisingly, the vast majority of older patients who screened positive for AF were prescribed anticoagulants on the basis of these findings, impacting AF outcomes.(10)
Several heart rate apps are available, but these apps cannot analyze cardiac rhythm because they cannot distinguish between motion noise artifact and AF.(13),(27),(28) Another smartphone-based rhythm monitoring technology, AliveCor, is FDA-approved for single-lead ECG-based manual and automated rhythm analysis.(11,12) In contradistinction to other available smartphone AF monitoring solutions, including AliveCor, our app does not require additional hardware and is powered by patented motion noise correction software.(24) Given that now at least 64% of the U.S. adult population already owns a smartphone, the majority of Americans already possess the potential hardware needed for such an app.(9)
Several prior studies from our group introduced the concept of using a smartphone camera to record the pulse, extract peak-to-peak intervals, and developed threshold values for RMSDD, ShE, and TPR.(14,16,29) In a prior clinical investigation, we reported the performance of our app comprised of 2 algorithms for AF detection, but this study excluded patients with common benign arrhythmias such as PACs or PVCs, thereby limiting its generalizability.(16) In this larger clinical study, we report the sensitivity, specificity and accuracy of a novel 3-phase real-time algorithm in a larger, more generalizable cohort and show that the app can accurately discriminate between AF, PACs, and PVCs.
Design of an app for AF detection has to be directed toward older persons, who, as technology “immigrants,” tend to not be ‘native’ to mobile technology use.(30) Although smartphone use is increasingly common (10% increase in the last 3 years) in seniors,(9) research indicates that physical difficulties, skeptical attitudes and difficulty leaning new technologies are barriers in this population(8), but that once they adopt new technologies, these often become integral to their lives.(30) Our finding that the vast majority of participants surveyed found the use of the app more useful than other health or heart rhythm devices highlights the simplicity of our approach. Moreover, they found the app “reassuring to [their] general sense of well-being,” and made them “conscious of [their] health,” suggesting that app users found the app empowering.
Future implementation research is needed to outline the ideal process associated with use of our app. We envision that use will differ across diverse healthcare environments, patient populations, and geographic regions. However, we expect that a “screen-positive” result from our app would be confirmed through the use of the gold-standard 12-lead ECG or ECG-based cardiac monitoring. If used in this fashion, we believe this software-based AF screening solution is well positioned to facilitate large scale AF screening across a diverse array of environments, driving app users with rhythm irregularity to engage proactively in their own health and rhythm evaluation.
Strengths and Limitations
Pulse recording was conducted in a standardized, temperature and light-controlled environment.(16) It is possible that exposure to extreme temperatures could result in peripheral vasoconstriction, thereby deleteriously affecting pulse recording and app performance. In a similar manner, darker skin color might reduce the quality of the pulse recording and app performance. Despite the fact that ectopy was frequent among participants with PACs and PVCs, it is possible that very frequent atrial or ventricular ectopic beats could diminish app performance. Our application has not been tested in patients with atrial flutter or atrial tachycardia and although the non-random characteristics of these other atrial dysrhythmias makes it unlikely that the app would characterize them as AF, it may not be able to distinguish between them and sinus rhythm. Bright ambient light might also adversely affect the performance of our AF detection app by introducing noise, potentially leading to lower accuracy outside of the hospital setting. Further testing of the app in larger and ethnically diverse cohorts and a more diverse array of real-world settings is necessary.
Conclusions
In this novel analysis of 219 pulse waveform recordings, we show that we have developed an app capable of transforming a smartphone into a device capable of screening for AF in real-time in a cohort with a diverse array of benign arrhythmias likely to cause false positives using pre-existing systems. We have also demonstrated that our app running on “out-of-the-box” hardware is highly acceptable to older users. To our knowledge, no existing smartphone-based app for AF detection uses the combination of pattern recognition, computational speed, and robust noise cancellation routines that our app employs while requiring no additional hardware (i.e., special phone case or Bluetooth sensor). On this basis, we believe PULSESMART represents an exciting new platform to enhance AF screening. Plans for future development include an open trial and formative qualitative work with older users so that the app and its interfaces meet their needs.
Supplementary Material
Acknowledgments
This work was funded in part by NIH grant 1R15HL121761, as well as the office of Naval Research work unit N00014-12-1-0171. DDM’s time was funded by NIH grant KL2RR031981. Dr. Saczynski was supported in part by funding from the National Institute on Aging (K01AG33643). Drs. McManus and Saczynski were supported in part by funding from the National Heart Lung and Blood Institute (U01HL105268). Dr. Boyer was supported by 1K24DA037109.
Dr. Chon has a patent on the algorithm described in the manuscript.
Abbreviations
- ECG
electrocardiogram
- AF
atrial fibrillation
Footnotes
Other authors: No disclosures.
REFERENCES
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 4.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D. American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke J Cereb Circ. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Chen P-S, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TSM, Wagoner DR Van, Waldo AL, Wyse DG. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pürerfellner H, Sanders P, Pokushalov E, Bacco M Di, Bergemann T, Dekker LRC Reveal LINQ Usability Study Investigators. Miniaturized Reveal LINQ insertable cardiac monitoring system: First-in-human experience. Heart Rhythm Off J Heart Rhythm Soc. 2015 doi: 10.1016/j.hrthm.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Sposato LA, Cipriano LE, Saposnik G, Ruíz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14:377–387. doi: 10.1016/S1474-4422(15)70027-X. [DOI] [PubMed] [Google Scholar]
- 8.Barret L. Health and Caregiving among the 50+: Ownership, Use and Interest in Mobile Technology [Internet] AARP Research and Strategic Analysis. 2011 Available from: http://assets.aarp.org/rgcenter/general/health-caregiving-mobile-technology.pdf. [Google Scholar]
- 9.Pew Research Center. The Smartphone Difference [Internet] Pew Research Center. 2015 Available from: http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015. [Google Scholar]
- 10.Friberg L, Engdahl J, Frykman V, Svennberg E, Levin L-Å, Rosenqvist M. Population screening of 75- and 76-year-old men and women for silent atrial fibrillation (STROKESTOP) Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol. 2013;15:135–140. doi: 10.1093/europace/eus217. [DOI] [PubMed] [Google Scholar]
- 11.Haberman ZC, Jahn RT, Bose R, Tun H, Shinbane JS, Doshi RN, Chang PM, Saxon LA. Wireless Smartphone ECG Enables Large-Scale Screening in Diverse Populations. J Cardiovasc Electrophysiol. 2015;26:520–526. doi: 10.1111/jce.12634. [DOI] [PubMed] [Google Scholar]
- 12.Tarakji KG, Wazni OM, Callahan T, Kanj M, Hakim AH, Wolski K, Wilkoff BL, Saliba W, Lindsay BD. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm. 2015;12(3):554–559. doi: 10.1016/j.hrthm.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Ho C-L, Fu Y-C, Lin M-C, Chan S-C, Hwang B, Jan S-L. Smartphone applications (apps) for heart rate measurement in children: comparison with electrocardiography monitor. Pediatr Cardiol. 2014;35:726–731. doi: 10.1007/s00246-013-0844-8. [DOI] [PubMed] [Google Scholar]
- 14.Chong JW, Esa N, McManus D, Chon K. Arrhythmia Discrimination using a Smart Phone. IEEE J Biomed Health Inform. 2015 doi: 10.1109/JBHI.2015.2418195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Reyes BA, McManus DD, Maitas O, Mathias O, Chon KH. Atrial fibrillation detection using an iPhone 4S. IEEE Trans Biomed Eng. 2013;60:203–206. doi: 10.1109/TBME.2012.2208112. [DOI] [PubMed] [Google Scholar]
- 16.McManus DD, Lee J, Maitas O, Esa N, Pidikiti R, Carlucci A, Harrington J, Mick E, Chon KH. A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm Off J Heart Rhythm Soc. 2013;10:315–319. doi: 10.1016/j.hrthm.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tateno K, Glass L. Automatic detection of atrial fibrillation using the coefficient of variation and density histograms of RR and deltaRR intervals. Med Biol Eng Comput. 2001;39:664–671. doi: 10.1007/BF02345439. [DOI] [PubMed] [Google Scholar]
- 18.Dash S, Chon KH, Lu S, Raeder EA. Automatic real time detection of atrial fibrillation. Ann Biomed Eng. 2009;37:1701–1709. doi: 10.1007/s10439-009-9740-z. [DOI] [PubMed] [Google Scholar]
- 19.Strickland OL. When is internal consistency reliability assessment inappropriate? J Nurs Meas. 1999;7:3–4. [PubMed] [Google Scholar]
- 20.Tukey JW, Tukey PA. Strips displaying empirical distributions: I. Textured dot strips. Bellcore Technical Memorandum. 1990 [Google Scholar]
- 21.Williams J, Pearce K, Bennet I. The effectiveness of a mobile ECG device in identifying AF: sensitivity, specificity and predictive value. [cited 2015 Apr 27];Br J Cardiol. 2015 [Internet]. Available from: http://bjcardio.co.uk/2015/04/the-effectiveness-of-a-mobile-ecg-device-in-identifying-af-sensitivity-specificity-and-predictive-value/ [Google Scholar]
- 22.Fak AS, Küçükoğlu MS, Fak NA, Demir M, Ağir AA, Demirtaş M, Köse S, Özdemir M. Expert panel on cost analysis of atrial fibrillation. Anadolu Kardiyol Derg AKD Anatol J Cardiol. 2013;13:26–38. doi: 10.5152/akd.2013.004. [DOI] [PubMed] [Google Scholar]
- 23.Kim MH, Johnston SS, Chu B-C, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 24.Know Your Pulse [Internet]. Available from: http://www.heartrhythmcharity.org.uk/www/673/0/know_your_pulse/ [Google Scholar]
- 25.Ricci RP. Disease management: atrial fibrillation and home monitoring. Eur Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol. 2013;15(Suppl 1):i35–i39. doi: 10.1093/europace/eut114. [DOI] [PubMed] [Google Scholar]
- 26.Haberman ZC, Jahn RT, Bose R, Tun H, Shinbane JS, Doshi RN, Chang PM, Saxon LA. Wireless Smartphone ECG Enables Large-Scale Screening in Diverse Populations. J Cardiovasc Electrophysiol. 2015;26:520–526. doi: 10.1111/jce.12634. [DOI] [PubMed] [Google Scholar]
- 27.Scully CG, Lee J, Meyer J, Gorbach AM, Granquist-Fraser D, Mendelson Y, Chon KH. Physiological parameter monitoring from optical recordings with a mobile phone. IEEE Trans Biomed Eng. 2012;59:303–306. doi: 10.1109/TBME.2011.2163157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petterson MT, Begnoche VL, Graybeal JM. The effect of motion on pulse oximetry and its clinical significance. Anesth Analg. 2007;105:S78–S84. doi: 10.1213/01.ane.0000278134.47777.a5. [DOI] [PubMed] [Google Scholar]
- 29.Harrington JL, Chong JW, Li J, Esa N, Pidikiti R, Maitas O, McManus D, Chon KH. THE DETECTION AND DIFFERENTIATION OF ARRRHYTHMIAS USING A SMARTPHONE: A CLINICAL STUDY OF PATIENTS WITH ATRIAL FIBRILLATION, PREMATURE ATRIAL AND PREMATURE VENTRICULAR CONTRACTIONS. [cited 2015 Apr 27];J Am Coll Cardiol. 2013 61 [Internet]. Available from: http://dx.doi.org/10.1016/S0735-1097(13)60362-9. [Google Scholar]
- 30.Smith A. Older Adults and Technology Use [Internet] [cited 2015 Apr 27];Pew Res. Cent. Internet Am. Life Proj. 2015 Available from: http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.