Abstract
Current models entail that transport through the Golgi — the main sorting compartment of the cell — occurs via cisternal progression/maturation, and that Ypt/Rab GTPases regulate this process. However, there is very limited evidence that cisternal progression is regulated, and no evidence for involvement of Ypt/Rab GTPases in such a regulation. Moreover, controversy about the placement of two of the founding members of the Ypt/Rab family, Ypt1 and Ypt31, to specific Golgi cisternae interferes with addressing this question in yeast, where cisternal progression has been extensively studied. Here, we establish the localization of Ypt1 and Ypt31 to opposite faces of the Golgi, early and late, respectively. Moreover, we show that they partially overlap on a transitional compartment. Finally, we determine that changes in Ypt1 and Ypt31 activity affect Golgi cisternal progression, early-to- transitional and transitional-to-late, respectively. These results show that Ypt/Rab GTPases regulate two separate steps of Golgi cisternal progression.
Introduction
In the exocytic pathway, cargo is transported from the endoplasmic reticulum (ER), through the Golgi, to the plasma membrane (PM), whereas in the endocytic pathway, cargo is transported from the PM through endosomes to the lysosome, a major degradative compartment. The Golgi is the major sorting compartment of the cell. At its entry side, cis, cargo from the ER is sorted for forward and retrograde transport. At its exit side, trans, cargo is sorted for secretion to the PM or for delivery to endosomal compartments. Traditionally, the Golgi is considered to have three-stacked functional cisternae, cis, medial and trans, and two networks on each side (Shorter and Warren, 2002). While a number of models exist regarding transport through the Golgi, the current view is that Golgi cisternae are transient, and forward transport probably occurs through cisternal progression/maturation (Glick and Luini, 2011). The question is what regulates Golgi cisternal progression?
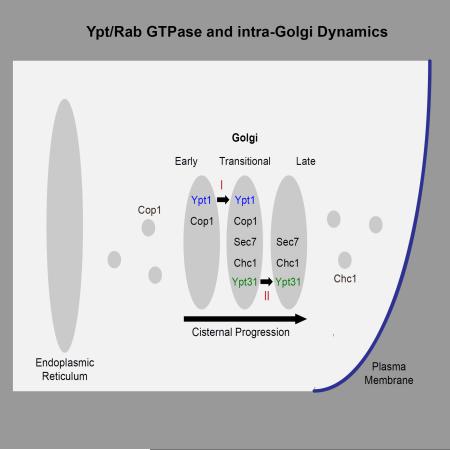
In budding yeast, the Golgi cisternae are not stacked but dispersed, which provides a convenient model system for studying cisternal maturation (Suda and Nakano, 2012). Markers are established for the early (cis) and late (trans) compartments of the yeast Golgi, whereas the nature of the intermediate compartment is not clear (Papanikou and Glick, 2014). Here, we propose that the intermediate Golgi cisterna is a transitional compartment on which early and late Golgi markers coincide.
Evidence for Golgi cisternal progression comes mostly from yeast and is based on observing dynamic switching of early and late Golgi markers on individual cisternae using time-lapse live-cell microscopy (Losev et al., 2006; Matsuura-Tokita et al., 2006). However, information about the mechanisms and regulation of Golgi cisternal progression is very scarce. Recently, a role for Arf1 GTPase, a component of COPI vesicles, was proposed in early-to-late Golgi transition, based on slower Golgi maturation in arf1Δ mutant cells (Bhave et al., 2014). While Ypt/Rab GTPases were proposed to play a role in this process (Glick and Luini, 2011; Suda and Nakano, 2012), there is currently no experimental data supporting this idea. Here, we provide evidence that Ypt/Rabs regulate Golgi cisternal progression.
The conserved Ypt/Rab GTPases regulate all vesicle-mediated transport steps of the exocytic (secretory) and endocytic pathways. These GTPases are stimulated by nucleotide exchangers termed GEFs and, when in the GTP-bound form, interact with their multiple downstream effectors. These effectors then mediate the multiple steps of vesicular transport, from vesicle formation and motility to their tethering and fusion with the acceptor compartment (Segev, 2001a). Recently, Ypt/Rab GTPases have also emerged as candidates for coordination of intra-cellular transport steps, with Ypt/Rab cascades or conversion as an example of coordination that drive compartment maturation (Segev, 2011). An open question in the field is the nature of Ypt/Rab specificity: Are they specific to a particular transport pathway and/or a cellular organelle?
Our previous work has established that in budding yeast, two Ypts regulate Golgi entry and exit: Ypt1 regulates ER-to-cis Golgi transport and the functional pair Ypt31/Ypt32 regulates trans Golgi-to-PM transport (Jedd et al., 1997; Jedd et al., 1995; Segev, 1991). The human functional homolog of Ypt1, Rab1, also regulates ER-to-Golgi transport (Haubruck et al., 1989; Pind et al., 1994).
While Ypt/Rab GTPases are considered to be specific to cellular compartments (Pfeffer, 2005; Pfeffer, 2013; Zerial and McBride, 2001), currently there is controversy about the localization and function of Ypt1 and Ypt31/32 and their GEFs, the TRAPP complexes. Based on our cumulative data, we proposed that TRAPP I acts as the GEF for Ypt1 to regulate ER-to-Golgi traffic and TRAPP II stimulates Ypt31/32 to mediate traffic at the trans Golgi (Lipatova et al., 2015). However, based on ~60% co-localization of mCherry-tagged Ypt1 with Sec7, which is considered a late-Golgi marker (Sclafani et al., 2010; Suda et al., 2013), assignment of a role for Ypt1 in late Golgi (Sclafani et al., 2010), and different specificity of GEF activity assays (Cai et al., 2008), a different view exists in the field. This view entails that both TRAPP I and TRAPP II complexes act as Ypt1 GEFs and Ypt1 acts throughout the Golgi (Barrowman et al., 2010). We have recently shown that Ypt1 does not function at the late Golgi (Lipatova et al., 2013), and here we address its Golgi distribution compared to that of Ypt31/32. The uncertainty about the placement of Ypt1 and Ypt31 to specific Golgi cisternae hampered the ability to determine their possible role in cisternal progression in yeast, where cisternal progression was extensively studied (Suda and Nakano, 2012).
In this manuscript, we define a set of markers for the early and late Golgi cisternae, determine their co-localization with the Ypts, and test the effect of altering the level and/or activity of the Ypts on the co-localization of the Golgi markers with each other. Our localization analysis provides evidence that Ypt1 and Ypt31 exhibit inverse polarization on the Golgi and overlap on a transitional compartment supporting the Ypt/Rab compartment specificity idea. The activity alteration analysis provides evidence for a role of Ypt/Rab GTPases in Golgi cisternal progression.
Results
Establishing marker pairs for early and late Golgi cisternae
To determine the distribution of Ypt1 and Ypt31 on the Golgi, we first established a set of four markers; we used two markers for each side of the Golgi: a vesicle coat subunit and an integral-membrane or membrane-associated protein. For the early Golgi, we used Cop1, a subunit of the COPI vesicle coat that mediates retrograde Golgi to ER transport, and Vrg4, an integral membrane GDP-mannose transporter with a role in glycosylation. Fluorescently tagged Cop1 and Vrg4 were used previously as markers for early Golgi (Huh et al., 2003; Losev et al., 2006). For the late Golgi we used Sec7, a membrane-associated Arf GEF, and Chc1, the heavy chain subunit of the clathrin vesicle coat. Fluorescently tagged Sec7 and Chc1 were previously used as markers for the late Golgi (Huh et al., 2003; Losev et al., 2006; Matsuura-Tokita et al., 2006).
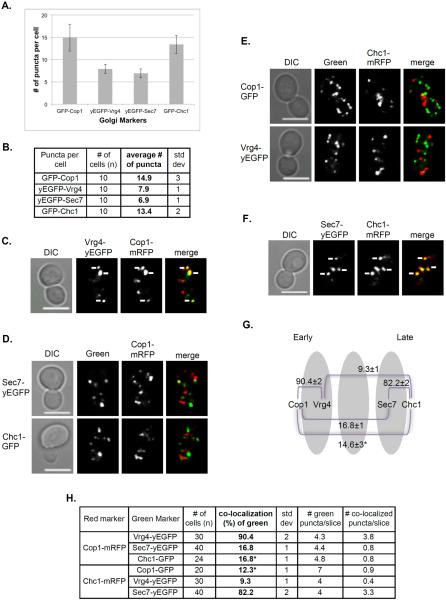
When tagged with GFP, there are twice more puncta per cell for vesicle-coat subunits, Cop1 and Chc1 (~15), than for Golgi membrane proteins, Vrg4 and Sec7 (~7-8) (Figure 1A–B). This supports the idea that vesicle-coat proteins exist both on the Golgi and on vesicles, and agrees with the estimate of 6–10 early and late Golgi cisternae (Papanikou and Glick, 2009).
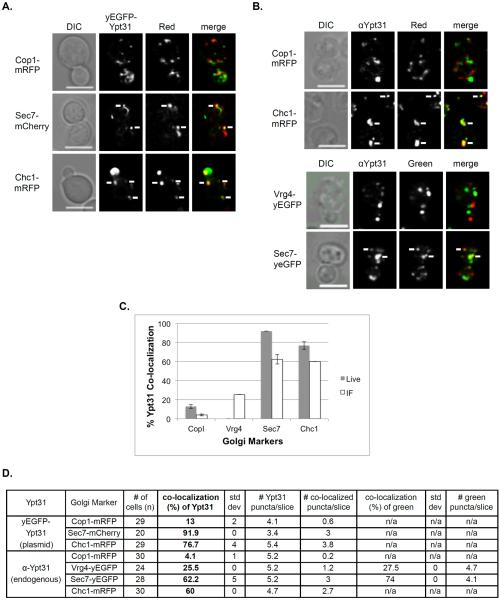
Figure 1. Co-localization pattern of early and late Golgi markers.
Four Golgi markers were tagged with GFP or RFP at their C-termini at their endogenous loci and visualized using live-cell confocal microscopy. A. Bar graph showing the number of GFP-tagged Golgi markers per cell. There are more puncta of vesicle subunit proteins (14–15) than Golgi membrane proteins (7-8) even though they are tagged with GFP and yEGFP, respectively. B. Table showing quantification from two independent experiments used for panel A. C–H: Co-localization of marker pairs was determined as follows: C. Early Golgi markers Cop1-mRFP with Vrg4-yEGFP; D. Early Golgi marker Cop1-mRFP with late Golgi markers Sec7-yEGFP or Chc1-GFP; E. Late Golgi marker Chc1-mRFP with early Golgi markers Vrg4-yEGFP and Cop1-GFP; and F. Late Golgi markers Sec7-yEGFP and Chc1-mRFP. C–F, Shown from left to right: DIC, GFP, RFP, merge (yellow). White arrows point to co-localized signal. Bar, 5 μm. G. Diagram showing the relative distribution of Golgi markers used here. Whereas the ~90% of the two early markers and ~80% of the late markers co-localize with each other, early and late markers exhibit only 10-15% co-localization. H. Table showing quantification from two independent experiments for panels C–F, bolded numbers were used for the diagram in panel G (asterisk in G is an average of values marked by asterisks in H. Error bars and +/− represent STDEV.
The four Golgi markers were tagged at their C-termini with GFP or RFP and their co-localization with each other in different combinations was determined by live-cell confocal microscopy (Figure 1). As expected, the two early Golgi markers, Cop1 and Vrg4, exhibited 90% co-localization, and the two late Golgi markers, Sec7 and Chc1, showed >80% co-localization. The co-localization of early and late markers was ~10-15% (Figure 1G). We propose that the co-localization of early and late Golgi markers reflects their transient overlap on a transitional cisterna as discussed below.
Polarized localization of Ypt1 and Ypt31 to early and late Golgi, respectively
To compare the distribution of Ypt1 and Ypt31 on the Golgi cisternae, we used live-cell and immuno-fluorescence (IF) microscopy. For live-cell microscopy, the two Ypts were tagged with a fluorescent moiety at their N, but not C-termini, because the latter has to be lipidated for membrane attachment and functionality (Segev, 2001b). For this analysis, we wished to use tagged versions of Ypt1 and Ypt31 expressed from a CEN plasmid under their own promoter and terminator, which are capable of functioning as a sole copy. To determine functionality, the ability of a tagged Ypt expressed from a plasmid to support the growth of cells deleted for their endogenous Ypt, and carrying a URA3 plasmid for expression of untagged Ypt1, was tested using the 5FOA assay. For Ypt1, we tested two versions: mCherry-Ypt1, whose localization was reported by Sclafani et al. (Sclafani et al., 2010), and Ypt1 tagged with yeast codon-optimized enhanced Venus (yEVenus), yEVenus-Ypt1. Both versions show clear fluorescent puncta when expressed in cells that also express endogenous Ypt1 (Figure S1A). However, the functionality assay showed that whereas yEVenus-Ypt1 could support cell growth as a sole Ypt1 copy, mCherry-Ypt1 could not do that (Figure S1B). Ypt31 tagged with yeast codon-optimized enhanced GFP (yEGFP), yEGFP-Ypt31, could function as a sole Ypt31/32 copy in cells deleted for both Ypt31 and Ypt32 (Figure S1C).
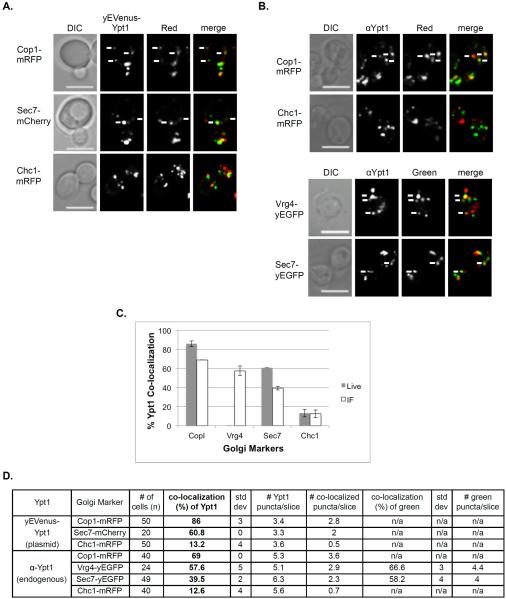
The co-localization of yEVenus-Ypt1 with red Golgi cisternal markers, Cop1-mRFP, Sec7-mCherry, and Chc1-mRFP, was determined using live-cell microscopy (Vrg4 tagged with a red fluorescent moiety was too dim for this analysis). The highest co-localization of the yVenus-Ypt1 was with the early Golgi Cop1 (>85%), ~60% co-localized with Sec7, and <15% co-localized with the late Golgi marker Chc1 (Figure 2A and 2C). IF microscopy showed a similar distribution pattern, with the highest co-localization of Ypt1 with the early Golgi markers Cop1 and Vrg4 (~70 and 60%, respectively), ~40% with Sec7 and <15% with Chc1 (Figure 2B and 2C). While the polarized localization of the Ypt1 to the early Golgi is similar in both the live-cell and IF microscopy, the levels of co-localization are higher in the live-cell microscopy (also true for Ypt31, see below). We interpret this phenomenon to be the result of higher levels of Ypts expressed from a plasmid than their endogenous level (see below).
Figure 2. Polarized distribution of Ypt1 from early to late Golgi.
A. Co-localization of Ypt1 using live cell microscopy. Cells expressing a Golgi marker tagged with red fluorescence were transformed with a CEN plasmid for expression of yEVenus-Ypt1. Co-localization was determined using live-cell confocal microscopy. The Golgi markers shown from top to bottom: Cop1-mRFP, Sec7-mCherry and Chc1-mRFP. Shown from left to right: DIC, Ypt1 (green), Golgi marker (red) and merge (yellow). B. Co-localization of Ypt1 using IF microscopy. Cells expressing fluorescently tagged Golgi markers were processed for IF analysis using anti-Ypt1 antibodies. The secondary antibody was conjugated with green (FITC) or red (Texas Red) fluorescent dye depending on the tag of the Golgi marker. Co-localization was determined using confocal microscopy. Shown from left to right: DIC, Ypt1, Golgi marker and merge (yellow). Top panels: Red Golgi markers Cop1-mRFP and Chc1-mRFP. Bottom panels: Green Golgi markers Vrg4-yEGFP and Sec7-yEGFP. For panels A–B: White arrows point to co-localized signals; Bar, 5 μm. C. Bar graph summarizing the quantification of Ypt1 co-localization with the different Golgi markers using live-cell (panel A, grey bars) and IF (panel B, white bars) microscopy. Left to right: Ypt1 co-localize with decreasing frequencies with Cop1, Vrg4, Sec7 and Chc1. D. Table shows quantification from two independent experiments of panels A–B; bolded numbers were used for graph in panel C. Error bars represent STDEV.
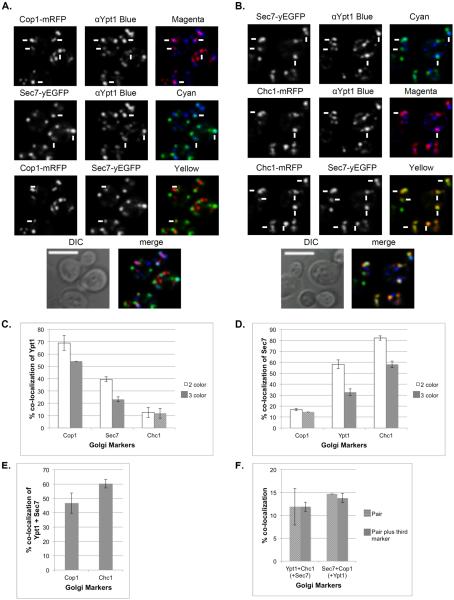
The co-localization of Ypt1 with Sec7 was previously taken as evidence for the presence of Ypt1 on late Golgi (Sclafani et al., 2010). To better understand this observation, the co-localization of Ypt1 with Sec7 was further analyzed using three-color IF microscopy using anti-Ypt1 antibody and Sec7-yEGFP, with the third color being mRFP-tagged Cop1 or Chc1 (Figure 3A–B). The pairwise co-localization patterns in the triple-color analyses were similar to those observed in the double-color analyses, albeit with lower levels: ~55% of the Ypt1 puncta co-localize with Cop1, ~25% with Sec7 and 12% with Chc1 (Figure 3C). Therefore, the triple-color IF corroborates the double-color IF observation that Ypt1 co-localizes best with the early Golgi marker Cop1, less with Sec7, and very little with Chc1. Similarly, the triple-color IF corroborates the double-color IF observation that Sec7 co-localizes mostly with Chc1, less with Ypt1, and very little with Cop1 (Figure 3D). Analyses of triple-color IF indicate that the Ypt1-Sec7 puncta also contain Cop1 and/or Chc1 (Figures 3E). Even though only a small fraction of Sec7 co-localizes with Cop1 (~15%), Ypt1 completely overlaps with this compartment. Similarly, even though only a small fraction of Ypt1 co-localizes with Chc1 (~15%), Sec7 completely overlaps with this compartment (Figure 3F). Thus, we propose that the co-localization of Ypt1 with Sec7 represents localization of Ypt1 to a transitional compartment marked by Sec7, Cop1 and/or Chc1, and not to late Golgi marked only by Sec7 and Chc1.
Figure 3. Distribution of Ypt1 on the Golgi using three-color IF microscopy.
A. Three-color fluorescence microscopy of Sec7, Ypt1 and Cop1. IF microscopy was performed with cells expressing Sec7-yEGFP and Cop1-mRFP, using anti-Ypt1 antibodies (secondary antibodies conjugated with α-Alexa-fluor 647, false colored blue). B. Three-color fluorescence microscopy of Sec7, Ypt1 and Chc1. IF microscopy was preformed with cells expressing Sec7-yEGFP and Chc1-mRFP, using anti-Ypt1 antibodies (secondary antibodies conjugated with α-Alexa-fluor 647, false colored blue). A–B, shown from top to bottom: 3 pairwise co-localizations (single colors, and 2-color merge: magenta for red and blue; cyan for green and blue; yellow for red and green), DIC and 3-color merge (white). White arrows point to co-localized signal in the 2-color merge; bar, 5μm. C. Ypt1 co-localizes mostly with Cop1. Pairwise co-localization of Ypt1 (%) with the Golgi markers is compared between two-color (from Figure 2, white bars) and three-color (this figure, grey bars) IF analyses. The two analyses show similar co-localization patterns. D. Increasing co-localization levels of Sec7 with Cop1, Ypt1 and Chc1. Pairwise co-localization of Sec7 (%) with the Cop1, Ypt1 and Chc1 is compared between two-color (from Figure 1 and Figure 2, white bars) and three-color (from this figure, grey bars) microscopy analyses. The two analyses show similar co-localization patterns. E. Ypt1-Sec7 puncta co-localize with Cop1 or Chc1. Three-color analysis shows that 47 and 60% of the Ypt1-Sec7 puncta also contain Cop1 and Chc1, respectively. F. Ypt1-Sec7: The slight co-localization of Sec7 with Cop1 and Ypt1 with Chc1 (~15%, striped bars in panels C and D, respectively) fully overlaps the other protein, Ypt1 and Sec7, respectively. Three-color analyses of Sec7-Cop1 with Ypt1 and Ypt1-Chc1 with Sec7 were performed. White bars show pairwise co-localization and grey bars show triple co-localization with the third marker: Ypt1 (left) and Sec7 (right). Error bars represent STDEV. Quantifications from two independent experiments are detailed in Table S1.
The co-localization of Ypt31 with Golgi cisternal markers was also determined using live-cell and IF microscopy. In live-cell microscopy analysis, yEGFP-Ypt31, expressed from a CEN plasmid, showed >90 and >75% co-localization with the late Golgi markers Sec7 and Chc1, respectively, and <15% co-localization with the early Golgi marker Cop1 (Figure 4A and 4C). The IF microcopy showed a co-localization pattern similar to that of the live-cell microscopy, with lower numbers: ~60% with the late Golgi markers Sec7 and Chc1, 25% with Vrg4 and <5% with Cop1 (Figure 4B and 4C).
Figure 4. Polarized distribution of Ypt31 towards the late Golgi.
A. Co-localization of Ypt31 using live cell microscopy. Cells expressing a Golgi marker tagged with red fluorescence were transformed with a CEN plasmid for expression of yEGFP-Ypt31. Co-localization was determined using live-cell confocal microscopy. The Golgi markers shown from top to bottom: Cop1-mRFP, Sec7-mCherry and Chc1-mRFP. Shown from left to right: DIC, Ypt31 (green), Golgi marker (red) and merge (yellow). B. Co-localization of Ypt31 using IF microscopy. Cells expressing fluorescently tagged Golgi markers were processed for IF analysis using anti-Ypt31 antibodies. The secondary antibody was conjugated with green (FITC) or red (Texas Red) fluorescent dye depending on the tag of the Golgi marker. Co-localization was determined using confocal microscopy. Shown from left to right: DIC, Ypt31, Golgi marker and merge (yellow). Top panels: Red Golgi markers Cop1-mRFP and Chc1-mRFP. Bottom panels: Green Golgi markers Vrg4-yEGFP and Sec7-yEGFP. For panels A–B: white arrows point to co-localized signals; Bar, 5μm. C. Bar graph summarizing the quantification of Ypt31 co-localization with the different Golgi markers using live-cell (panel A, grey bars) and IF (panel B, white bars) microscopy. Left to right: Ypt31 co-localize in increasing frequencies with Cop1, Vrg4, Sec7 and Chc1. D. Table shows quantification from two independent experiments for panels A–B; bolded numbers were used for graph in panel C. Error bars represent STDEV.
Together, this localization analysis establishes that Ypt1 and Ypt31 are polarized to opposite sides of the Golgi, early and late, respectively (Figure 5A).
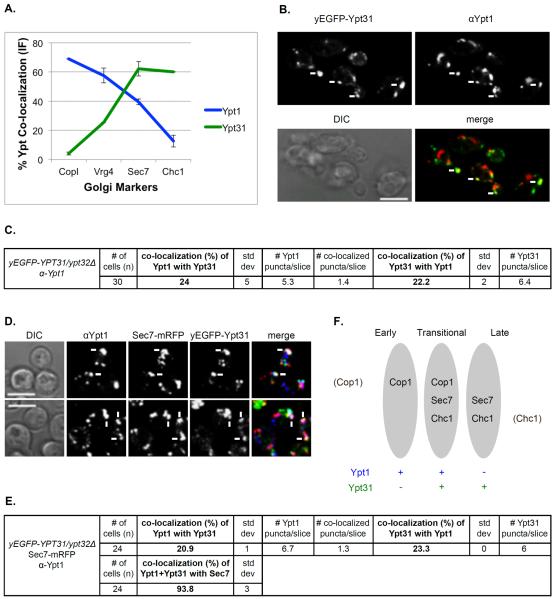
Figure 5. Ypt1 and Ypt31 co-localize on a Sec7-marked Golgi compartment.
A. Both Ypt1 and Ypt31 show intermediate levels of co-localization with Sec7. Summary of IF analyses of Ypt1 (Figure 2B, red line) and Ypt31 (Figure 4B, green line) co-localization with the Golgi markers. B. About 20–25% of Ypt1 and Ypt31 co-localize with each other. Cells deleted for YPT32 and expressing yEGFP-Ypt31 from its endogenous locus were processed for IF microscopy using anti-Ypt1 antibodies (secondary antibody conjugated with red (Texas Red) fluorescent dye). Shown: top panels: Ypt31 (green) and Ypt1 (red); bottom panels: DIC and merge (yellow). C. Table shows quantifications from two independent experiments for panel B; bolded numbers show the co-localization of the two Ypts. D. >90% of the Ypt1-Ypt31 puncta also contain Sec7 in a three-color IF microscopy. Cells deleted for YPT32 and expressing yEGFP-Ypt31 and Sec7-mRFP from their endogenous loci were processed for IF microscopy using anti-Ypt1 antibodies (secondary antibody conjugated with α-Alexa-fluor 647, false colored blue). Shown from left to right: DIC, Sec7, Ypt31, Ypt1 and merge of 3 colors (white). Panels B and D: white arrows point to co-localized signal; bar, 5μm. E. Table shows quantifications from two independent experiments for panel D; bolded numbers show the 2 and 3-color co-localizations. F. Ypt1 and Ypt31 co-localize in a transitional Golgi compartment marked by Cop1, Sec7 and Chc1. A diagram showing three Golgi compartments: early, transitional and late and the distribution of Ypt1 (blue) and Ypt31 (green) in these compartments.
Ypt1 and Ypt31 co-localize on the Sec7-marked Golgi cisterna
The localization of Ypt1 and Ypt31 to the late and early Golgi compartments, respectively, is very low. However, both Ypt1 and Ypt31 showed significant co-localization with one Golgi marker, Sec7, 40% and 60%, respectively (by two-color IF, Figure 5A). Therefore, we wished to determine whether the two Ypts co-localize with each other, and if they do, on which Golgi compartment it happens.
Endogenous Ypt31 was tagged at its N-terminus with yEGFP in cells in which the YPT32 gene was deleted. The localization of Ypt1 in these cells was determined using IF microscopy and anti-Ypt1 antibodies. Approximately 20-25% of the Ypt1 and Ypt31 puncta co-localized with each other (Figure 5B–C). To determine on which Golgi cisterna this co-localization occurs, three-color IF experiment was done in cells that also express Sec7-mRFP. Approximately 95% of the puncta on which Ypt1 and Ypt31 co-localized, also contained Sec7 (Figure 5D–E). This indicates that Ypt1 and Ypt31, which are polarized to the two sides of the Golgi, overlap on the Sec7 marked Golgi cisterna. Based on these results and on the fact that all the Ypt1-Sec7 puncta co-localize also with Cop1 or Chc1 (see above), we propose that the Golgi compartment on which Ypt1 and Ypt31 co-localize is a transitional Golgi compartment that contains all these proteins (Figure 5F).
Effect of Ypt1 and Ypt31 level and/or activity on the Golgi
While the idea that cisternal maturation underlies transport through the Golgi is largely accepted in the field, it is currently not clear what drives it. We hypothesized that Ypts have a role in this process. To test this hypothesis, the effect of altering the level and/or activity of Ypt1 and Ypt31 on the Golgi was determined using static fluorescence microscopy.
Determination of the levels of Ypt1 and Ypt31 expressed from a CEN plasmid, either tagged with yEVenus/yEGFP or not, showed that they are 10-and 5-fold higher than the endogenous levels, respectively (Figures 6A and 7A). This can explain the higher levels of co-localization of Ypt1 and Ypt31 with Golgi markers in live-cell microscopy than in IF (Figures 2 and 4). To determine the effect of higher Ypt levels on the Golgi, the wild type and activated (GTP-bound) untagged versions of Ypt1 (Q67L) and Ypt31 (Q72L) were expressed also from 2μ plasmids, and 15- and 45-fold increases were observed, respectively (Figures 6B and 7B). Cells expressing a combination of green and red Golgi markers were transformed with one of the above plasmids and the effect of the higher levels of the Ypts on their co-localization was determined by live-cell microscopy (Figures 6–7 and Figures S2–3). While overexpression of Ypt1 or Ypt31 did not affect the number of Sec7 puncta per cell (Figure 6E), it did affect the co-localization of Sec7 with Cop1 and Chc1 in different ways.
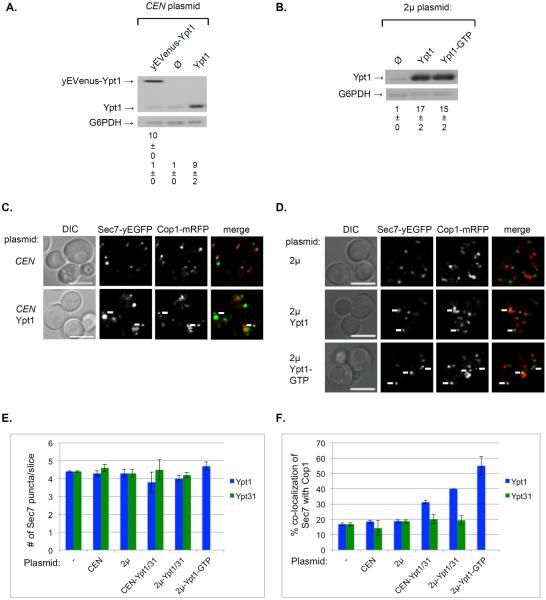
Figure 6. Greater level of co-localization of Sec7 with Cop1 upon increased level and activity of Ypt1, but not Ypt31.
A. Expression of Ypt1 from a CEN plasmid results in a 10-fold increase of its level. The levels of Ypt1 protein in cells expressing it from its endogenous locus or a CEN plasmid were determined using immuno-blot analysis and anti-Ypt1 antibodies. Cells were transformed with a CEN plasmid expressing from left to right: yEVenus-Ypt1, empty plasmid (Ø), and Ypt1 (from its native promoter and terminator). Shown top to bottom: yEVenus-Ypt1, Ypt1, G6PDH (loading control), quantification of yEVenus-Ypt1 (left lane), and Ypt1 expressed as fold of endogenous level. B. Expression of Ypt1 from a 2μ plasmid results in a ~15-fold increase of its level. The level of Ypt1 was determined as described for panel A. Cells were transformed with a 2μ plasmid expressing from left to right: empty plasmid (Ø), Ypt1 and Ypt1-GTP (Ypt1-Q67L). Shown top to bottom: Ypt1, G6PDH (loading control), quantification of Ypt1 expressed as fold of endogenous level. C. The effect of expression of Ypt1 from a CEN plasmid on the co-localization of Cop1 and Sec7. Cells expressing Cop1-mRFP and Sec7-EGFP from their endogenous loci were transformed with a CEN plasmid (from panel A): empty (top) and for Ypt1 expression (bottom) and visualized by live-cell microscopy. Shown from left to right: DIC, Sec7, Cop1, and merge (yellow). D. The effect of expression of Ypt1 from a 2μ plasmid on the co-localization of Cop1 and Sec7. Cells expressing Cop1-mRFP and Sec7-EGFP from their endogenous loci were transformed with a 2μ plasmid (from panel B): empty (top), Ypt1 (middle) and Ypt1-GTP (bottom), and visualized by live-cell microscopy. Shown from left to right: DIC, Cop1, Sec7 and merge (yellow). Panels C–D: white arrows point to co-localized signal; bar, 5μm. E. The number of Sec7 puncta does not change upon overexpression of Ypt1 (blue bars) or Ypt31 (green bars). F. Co-localization (%) of Cop1 and Sec7 increases upon overexpression of Ypt1 (blue bars) but not Ypt31 (green bars). Shown from left to right in panels E–F: no plasmid (−), empty CEN and 2μ plasmids, expression of wild-type Ypt from CEN, 2μ, and Ypt-GTP from 2μ plasmids. Error bars and +/− represent STDEV. Information about Ypt1 is from this figure, and Ypt31 is from Figure S3A. Quantifications from two independent experiments for Ypt1 and Ypt31 are detailed in Figures S2C and S3C, respectively
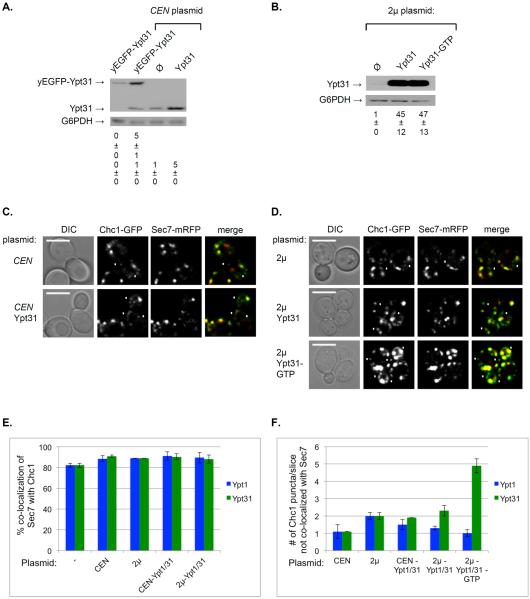
Figure 7. Higher number of Chc1 puncta that do not co-localize with Sec7 upon increased level and activity of Ypt31.
A. Expression of Ypt31 or yEGFP-Ypt31 from a CEN plasmid results in a 5-fold increase of its endogenous level. The levels of Ypt31 and yEGFP-Ypt31 proteins in cells expressing it from its endogenous locus or a CEN plasmid were determined using immuno-blot analysis and anti-Ypt31 antibodies. Left lane: ypt32Δ cells expressing yEGFP-Ypt31 from its endogenous promoter (used in Figure 5); CEN plasmid lanes: Cells (wild type) were transformed with a CEN plasmid expressing (from left to right): yEGFP-Ypt31, empty plasmid (Ø), and Ypt31 (from its native promoter and terminator). Shown top to bottom: yEGFP-Ypt31, Ypt31, G6PDH (loading control), quantification of yEGFP-Ypt31 (left lanes), and Ypt31 expressed as fold of endogenous level. B. Expression of Ypt31 from a 2μ plasmid results in a ~45-fold increase of its level. The level of Ypt31 was determined as described for panel A. Cells were transformed with a 2μ plasmid expressing from left to right: empty plasmid (Ø), Ypt31 and Ypt31-GTP (Ypt31-Q72L). Shown top to bottom: Ypt31, G6PDH (loading control), quantification of Ypt31 expressed as fold of endogenous level. C. The effect of expression of Ypt31 from a CEN plasmid on the co-localization of Sec7 and Chc1. Cells expressing Sec7-mRFP and Chc1-GFP from their endogenous loci were transformed with a CEN plasmid (from panel A): empty (top) and for Ypt31 expression (bottom) and visualized by live-cell microscopy. Shown from left to right: DIC, Chc1, Sec7 and merge (yellow). D. The effect of expression of Ypt31 from a 2μ plasmid on the co-localization of Sec7 and Chc1. Cells expressing Sec7-mRFP and Chc1-GFP from their endogenous loci were transformed with a 2μ plasmid (from panel B): empty (top), Ypt31 (middle) and Ypt31-GTP (bottom), and visualized by live-cell microscopy. Shown from left to right: DIC, Chc1, Sec7 and merge (yellow). Panels C–D: white arrowheads point to Chc1-GFP puncta that do not co-localize with Sec7-mRFP; bar, 5μm. E. The % co-localization of Sec7 with Chc1 does not change upon overexpression of Ypt1 (blue bars, from Figure S2A) or Ypt31 (green bars, Figure S3B). F. The number of Chc1 puncta that do not co-localize with Sec7 increases upon overexpression of Ypt31 (green bars, from this figure), but not Ypt1 (blue bars, Figure S2B). Shown from left to right in panels E–F: no plasmid (−), empty CEN and 2μ plasmids, expression of wild-type Ypt from CEN and 2μ, and Ypt-GTP from 2μ plasmids. Error bars and +/− represent STDEV. Quantifications from two independent experiments are detailed in Figures S2C and S3C.
For the Cop1 and Sec7 pair (Figures 6C–D and S3A), increased levels of Ypt1, but not Ypt31, resulted in a gradual increase of their co-localization: from <20% (no overexpression) to 30, 40 and 55% for wild-type Ypt1 overexpressed from CEN and 2μ plasmids, and Ypt1-GTP overexpressed from 2μ plasmid, respectively (Figure 6F). This reflects ~3-fold increase for the Cop1-Sec7 compartment upon overexpression of activated Ypt1.
The loss-of-function ypt1ts mutation resulted in an opposite effect on Cop1-Sec7 co-localization. Specifically, whereas activation of Ypt1 results in increase of co-localization of Cop1 with Sec7 (Figure 6F), there is a significant increase of Sec7-free Cop1 puncta (~30%) in ypt1ts mutant cells (Figure S4A–B). Neither activation nor inhibition of Ypt31 function affects this transport step. Together, the effects of increase and decrease in Ypt1 activity suggest that it regulates the recruitment of Cop1 to the Sec7-marked Golgi cisterna.
For the Sec7 and Chc1 pair (Figures 7C–D and S2), while their co-localization level did not change (Figure 7E), increased levels of Ypt31, but not Ypt1, resulted in a gradual increase in the number of Chc1 puncta that did not overlap with Sec7 (Figure 7F). This suggests that increase in Ypt31 activity does not affect the late Golgi, but the release of Chc1-vesicles from it.
The loss-of-function ypt31Δ/ypt32ts mutation resulted in an opposite effect on Sec7-Chc1 co-localization. Specifically, whereas activation of Ypt31 results in increase of the number of Chc1 puncta that do not co-localize with Sec7 (Figure 7F), there is a significant reduction (25%) in the number of Chc1 puncta that co-localize with Sec7 in ypt31Δ/ypt32ts mutant cells (Figure S4C–D). Neither activation nor inhibition of Ypt1 function affects this transport step. Together, the effects of increase and decrease in Ypt31 activity suggest that it regulates the recruitment of Chc1 to the Sec7-marked Golgi cisterna.
To support the idea that Ypt1 controls the formation of the transitional Golgi cisterna, the 3-color co-localization of Ypt1 and Ypt31 on the Sec7 compartment was determined when the GTP-restricted form of either Ypt1 or Ypt31 were expressed from a CEN plasmid. Triple-IF analysis of Ypt1, Ypt31 and Sec7 showed that the two Golgi Ypts co-localize on the Sec7 cisterna (Figure 5). As expected from the co-localization results of Golgi marker (Figures 6–7), when Ypt1, but not Ypt31, is activated there is a highly significant increase (67%) of its co-localization with Sec7. There is also a significant increase (~50%) in the co-localization of Ypt31 and Sec7 and co-localization of all three proteins when Ypt1 is activated (Figure S5). These results support the idea of a Ypt1-to-Ypt31 exchange in the Sec7-marked transitional Golgi cisterna.
Effect of overexpressed hyperactive Ypt1 and Ypt31 on the Golgi cisternal progression
The results of the static microscopy suggest that the overexpression of active Ypts affect the Golgi cisternal progression. To test this idea directly, we used time-lapse microscopy. The dynamics of two pairs of Golgi markers, Cop1-Sec7 and Sec7-Chc1, was determined in cells expressing tagged markers from their endogenous loci and transformed with a 2μ plasmid for overexpression of Ypt1-GTP or Ypt31-GTP (empty vector used as a control) (Figures 8 and S6).
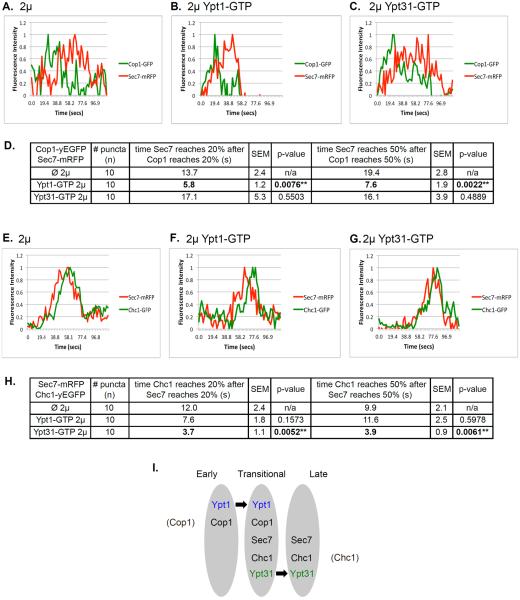
Figure 8. The effect of overexpression of activated Ypt1 and Ypt31 on Golgi cisternal progression.
Time-lapse fluorescence microscopy was done with two pairs of Golgi markers: Cop1-Sec7 (A–C) and Sec7-Chc1 (E–G). A–C: Overexpression of Ypt1-GFP, but not Ypt31-GFP, results in ~2.5 fold increase in the rate of Cop1-to-Sec7 conversion. Cells expressing Cop1-GFP and Sec7-mRFP from their endogenous loci were transformed with a 2μ plasmid (pRS425): empty (A), Ypt1-GTP (B), or Ypt31-GTP (C). D. Table shows quantifications from two independent experiments for panels A–C; bolded numbers show the significant change in Cop1-to-Sec7 conversion upon overexpression of Ypt1-GTP. E–G: Overexpression of Ypt31-GFP, but not Ypt1-GFP, results in ~2.5 fold increase in the rate of Sec7-to-Chc1 conversion. Cells expressing Sec7-mRFP and Chc1-GFP from their endogenous loci were transformed with a 2μ plasmid (pRS425): empty (E), Ypt1-GTP (F), or Ypt31-GTP (G). H. Table shows quantifications from two independent experiments for panels E–G; bolded numbers show the significant change in Sec7-to-Chc1 conversion upon overexpression of Ypt31-GTP. Cells were analyzed by time-lapse live-cell microscopy. Graphs show normalized fluorescence intensity of representative switching puncta over time (secs); bottom: average of time between markers reaching 20 and 50% of their total average fluorescence level (n=10); +/− represent STDEV, (**, p value <0.01). Three-channel kymographs of puncta used for panels A–C and E–G are shown in Figure S6. I. Model summarizing the roles of Ypt1 and Ypt31 on Golgi cisternal progression. Based on results presented here we propose that Ypt1 regulates early-to-transitional cisternal progression whereas Ypt31 facilitates transitional-to-late cisternal maturation (see text for discussion).
Conversion of early to late Golgi markers was previously observed using tagged Vrg4 or Sed5 and Sec7, respectively (Losev et al., 2006; Matsuura-Tokita et al., 2006). In wild type cells (without overexpression of a Ypt), green Cop1 puncta converts to red Sec7 with a clear separation between the peaks (Figure 8A). Overexpression of Ypt1-GTP, but not Ypt31-GTP, results in ~2.5-fold decrease in the gap between the curves (Figure 8B–C). This result reflects a faster conversion of a Cop1- to Cop1-Sec7-marked compartment, and is in agreement with the ~3-fold increase in the co-localization of Cop1 and Sec7 upon overexpression of Ypt1-GTP observed in the static microscopy analysis (Figure 6).
Conversion of Sec7 to Chc1 has not been not previously reported. When their individual dynamics was compared to that of Gga2, they both peaked at the same time as Gga2 (Daboussi et al., 2012). Here, we followed their dynamics directly and observed a short gap between their appearance. In wild type cells (without overexpression of a Ypt), red Sec7 puncta acquire green Chc1 with a short gap of ~10 seconds, which is ~20% of their co-localization time (average ~50 sec) (Figure 8E). This result is in agreement with ~25% higher overlap of Ypt1 with Sec7 than with Chc1 (Figure 5A) and with the idea that Ypt1 co-localizes with these markers at the transitional compartment. Overexpression of Ypt31-GTP, but not Ypt1-GTP, results in ~2.5-fold decrease in the gap between the curves (Figure 8F–G). This result reflects a faster conversion of the Sec7- to Sec7-Chc1-marked compartment. While this faster conversion does not significantly affect the Sec7-Chc1 co-localization, which is already high (80%), we observed ~2.5-fold increase in the release of Chc1 vesicles form the Golgi in the static microscopy analysis (Figure 7). Moreover, the decrease in Chc1-Sec7 co-localization in ypt31Δ32ts mutant cells (Figure S4) further supports the idea that Ypt31 regulates Sec7-to-Chc1 conversion.
Together, the static and time-lapse microscopy experiments show that overexpression of activated Ypt1 and Ypt31 affect two separate steps of Golgi cisternal progression: early-to-transitional and transitional-to-late.
Discussion
In this study we settle a long-standing controversy regarding the Golgi localization of two founding members of the Ypt/Rab GTPase family, Ypt1 and Ypt31. Our findings support a basic paradigm about compartment specificity of members of this family and allow us to determine a role for these GTPases in Golgi cisternal progression.
Ypt/Rabs and compartment specificity
Using live-cell and IF microscopy, we clarify two important points about the distribution of Ypt1 and Ypt31/32 on the Golgi. First, Ypt1 and Ypt31 exhibit inverse polarized distribution to opposite sides of the Golgi, early and late, respectively. Second, the two Ypts display 20% co-localization with each other, and this co-localization overlaps with Sec7. We term the compartment on which these two Ypts and Sec7 overlap “transitional Golgi” and show that it also contains early and late Golgi markers, Cop1 and Chc1 (Figure 5F).
Two recent studies undermine the two other claims that form the basis for the idea that TRAPP I and TRAPP II complexes converge through a common Rab, Ypt1, which functions throughout the Golgi (Barrowman et al., 2010). First, we have shown that Ypt1 mutants used to implicate Ypt1 in late-Golgi transport (Sclafani et al., 2010) are instead defective in autophagy (Lipatova et al., 2013). Second, in vitro and in vivo studies support a role for TRAPP II at the late Golgi as a GEF for the S. cerevisiae Ypt31 (Morozova et al., 2006) and its Aspergillus nidulans ortholog RabERAB11 (Pinar et al., 2015). Thus, our and others cumulative data reinforce the idea that TRAPP I-stimulated Ypt1 regulates transport at the early Golgi, whereas TRAPP II-activated Ypt31/32 regulate transport at the late Golgi. This placement agrees with the established roles of Ypt1 and Ypt31 on the two sides of the Golgi (Lipatova et al., 2015). It also supports the idea that although Ypt/Rab GTPases can regulate multiple transport steps (Lipatova and Segev, 2014), they are specific to intracellular compartments (Zerial and McBride, 2001).
What is the regulatory basis for Ypt1 versus Ypt31 localization? Mutations that deplete Ypt/Rab GEF activity affect the cellular localization of their Ypt/Rab substrate. For example, the TRAPP II-specific trs130ts mutation results in a diffuse distribution of Ypt31, but not Ypt1 (Morozova et al., 2006). Thus, we propose that TRAPP I and TRAPP II, which localize to early and late Golgi, respectively (Cai et al., 2005; Sacher et al., 2000), regulate the localization of Ypt1 and Ypt31 to the two faces of the Golgi.
How do we reconcile the controversy about the previously reported localization of Ypt1 to the late Golgi (Sclafani et al., 2010; Suda et al., 2013) and its documented function in the early Golgi (Jedd et al., 1995; Segev, 1991)? First, the localization of Ypt1 to the late Golgi was based on 60% co-localization of tagged-Ypt1 with Sec7. We consider this an overestimate resulting from overexpression of the tagged Ypt1 (Figure 2), even though it was expressed from a low-copy CEN plasmid. Based on IF microscopy, we estimate that the Ypt1-Sec7 co-localization is 25-40%. Moreover, based on the finding that Ypt1 co-localizes less with Chc1, another late Golgi marker, we propose that the co-localization of Ypt1 and Sec7 reflects the presence of Ypt1 on a transitional compartment, and not at the late Golgi, where only Sec7 and Chc1 co-localize.
What is the transitional Golgi compartment? We propose that it is a transient compartment on which early and late Golgi markers and Ypts transiently overlap. The reason that we do not use the term “medial” is because it has been traditionally used to define a Golgi compartment in which specific cargo modifications reactions occur (Nilsson et al., 2009).
Several lines of evidence presented here support the existence of the transitional Golgi compartment. First, early and late Golgi markers exhibit 10-15% co-localization, which we suggest occurs on the transitional compartment (Figure 1). Moreover, the frequency of the Cop1-Sec7 puncta and the rate of Cop1-to-Sec7 conversion increase by 2.5–3 fold upon overexpression of activated Ypt1 (Figures 6–8). These findings indicate that the Cop1-Sec7 co-localization reflects a distinct compartment after the early Golgi, marked by Cop1, Sec7 and Ypt1. Second, while Ypt1 shows 25-40% co-localization with Sec7, it shows only 15% co-localization with another late Golgi marker, Chc1 (Figure 2). This suggests that Ypt1 and Sec7 co-localize on a compartment distinct from the late Golgi marked by Sec7, Chc1 and Ypt31. Third, all the Ypt1-Sec7 puncta contain Cop1 and/or Chc1 (Figure 3E), Ypt1 is always present on the infrequent Cop1-Sec7 puncta, and Sec7 is always present on the infrequent Ypt1-Chc1 puncta (Figure 3F). This suggests that all these proteins are present, at least transiently, on one compartment. Fourth, 20-25% of Ypt1 and Ypt31 puncta overlap on a Sec7 marked compartment (Figure 5), which we consider the transitional compartment. Perhaps most importantly, progression into and out of the Sec7-marked transitional compartment is regulated independently by two different Ypts, Ypt1 and Ypt31, respectively (Figure 8).
Our combined evidence of the two-color and three-color IF and time-lapse microscopy suggest that Ypt1 localizes first to early Golgi marked with Cop1 and Vrg4, then to an transitional compartment marked by Sec7, Cop1 (Vrg4) and Chc1. Ypt31 also localizes to this transitional compartment, were it overlaps with Ypt1 and then to the late Golgi marked by Sec7 and Chc1 (Figure 5F). This data suggest the following dynamics: early Golgi that contains Ypt1 converts to an transitional compartment by acquiring Sec7, followed by recruitment of Ypt31 and late Golgi markers. Subsequent loss of Ypt1 and early Golgi markers indicates the transitional-to-late Golgi switch. These findings are in agreement with previous studies that showed that Sec7 appears on Ypt1-marked puncta, whereas Ypt31/32 appear on Sec7-marked puncta just before the disappearance of the Sec7 (McDonold and Fromme, 2014; Suda et al., 2013). Moreover, the localization of Ypt1 and Ypt31 to opposite sides of the Golgi reported here, highlights the relevance of the Ypt1-to-Sec7 and Sec7-to-Ypt31 order previously reported by McDonald and Fromme to Golgi dynamics.
An interesting question is what regulates this early-to-transitional and transitional-to-late Golgi transitions.
Implications on Ypt/Rab GTPases and Golgi cisternal progression
Concrete localization of Ypt1 and Ypt31 to opposite sides of the Golgi and characterization of a transitional Golgi compartment allowed us to study the effect of these Ypts on the dynamics of Golgi cisternal progression (Figure 8I). We show that increased levels and activity of the Ypts can stimulate the conversion rate of Golgi markers (Figure 8A–D). Increase in the activity of Ypt1, which functions at and localizes mostly to the early Golgi, resulted in an increase of conversion of Cop1-marked early Golgi to Cop1/Sec7-marked transitional compartment and propagation of the latter (Figure 6). On the other hand, increase in the activity of Ypt31, which functions at and localizes mostly to the late Golgi, resulted in a faster conversion of transitional Golgi to the Sec7-Chc1-marked late Golgi (Figure 8E–H), and increased formation of Chc1-marked vesicles (Figure 7). In contrast to the effect of Ypt activation, the effects of loss-of-function ypt1 and ypt31/32 mutations is in agreement with a decrease in early-to-transitional and transitional-to-late Golgi switching, respectively (Figure S4). Although Ypt/Rab GTPases were proposed to regulate Golgi cisternal maturation (Suda and Nakano, 2012), to our knowledge, this is the first evidence that substantiates a role for Ypt/Rab GTPases in this process.
Two Ypt GAP cascades have been reported. The first, a Ypt1-Gyp1-Ypt32 cascade was not anchored to specific Golgi cisterna (Rivera-Molina and Novick, 2009) and the second, a Ypt6-Gyp6-Ypt32 cascade, was proposed to act during endosome-to-Golgi transport (Suda et al., 2013). Neither cascade provides evidence for the role of Ypts in Golgi cisternal maturation. In both cases, the second Ypt was proposed to recruit a GAP for the first Ypt to ensure that only one Ypt is active at a certain time and place. For both cascades, additional genetic evidence is needed to support this idea. For example, the gyp1Δ mutation used in the first report affects not only Ypt localization, but also results in permanent changes in Golgi morphology, e.g., increase in co-localization of Sec7 with Cog3, a subunit of a complex that mediates retrograde transport within the Golgi and endosome-to-Golgi transport (Rivera-Molina and Novick, 2009). Regardless, the localization of Ypt1 and Ypt31/32 reported here provides context for the Ypt1-GAP-Ypt32 cascade to concrete Golgi cisterna.
Importantly, our findings add to the currently very limited genetic support for the existence of Golgi cisternal maturation. Recently, it has been reported that deletion of Arf1, a COPI component, resulted in slower and less frequent conversion of early, Vrg4-marked, puncta to Sec7-marked puncta (Bhave et al., 2014). We propose that this reflects a role of Arf1 in conversion of early (Vrg4) to transitional (Sec7) cisternal progression. Here, we show that Golgi cisternal progression can be accelerated when Ypt1 and Ypt31 are activated. Moreover, we show that two steps of cisternal maturation can be uncoupled: early (Cop1)-to-transitional (Sec7), and transitional (Sec7)-to-late (Chc1). Whereas Ypt1 increases the rate of first, but not the second, Ypt31 increases the rate of the second, but not the first. Interestingly, even though the Golgi Ypts exert their functions through effectors, an increase in their activity alone is enough to accelerate these conversions. This suggests that Ypt/Rabs GTPases regulate Golgi cisternal progression, whereas accessory proteins that mediate this process are readily available.
Future perspectives
What might be the mechanism by which Ypt1 and Ypt31 regulate Golgi cisternal maturation? One possible mechanism is that the Ypts regulate Golgi dynamics through their interaction with Sec7. Based on genetic interactions, we have previously proposed that a cascade of alternating Ypts and Arf GEFs regulate the yeast secretory pathway. Specifically, we proposed that Sec7 functions in a step between Ypt1 and Ypt31/32 and that they affect each other (Jones et al., 1999). Recently, biochemical evidence confirmed that both Ypt1 and Ypt31 interact with Sec7, and affect its localization and Arf GEF activity, respectively (McDonold and Fromme, 2014). Based on our data and others, we propose that Ypt1 recruits Sec7 to the early Golgi, which is the step of early-to-transitional Golgi conversion, whereas Ypt31 activates Sec7-regulated recruitment of Chc1, which constitutes transitional-to-late Golgi conversion, followed by Arf-mediated formation of Chc1-coated trans-Golgi vesicles.
The conservation of the Ypt/GTPases specifically, and the machinery components of intracellular trafficking in general, suggests that principles learned here with the yeast Ypts will pertain to human cells where Rabs were shown to be important for human health and disease (Mitra et al., 2011).
Materials and Methods
Strains, plasmids, and reagents
Yeast strains and plasmids used in this paper as well as strain and plasmid construction is detailed in Supplemental Experimental Procedures. The sources of antibodies used in this study are detailed below in the sections for their specific use. All reagents were purchased from Fisher Scientific (Bridgewater, NJ) except for: media components other than amino acids from US Biological (Swampscott, MA); ProtoGel for immuno-blots from National Diagnostics (Atlanta, GA); detection reagents for immuno-blots from GE Healthcare Life Sciences (Buckinghamshire, UK); film from Denville Scientific (Holliston, MA); glass beads from BioSpec Products (Bartlesville, OK); PCR reagents, restriction enzymes and buffers from New England BioLabs (Ipswich, MA); hygromycin B (Hyg) and geneticin (G418) from Invitrogen (Carlsbad, CA); and nourseothricin (Nat) from Jena Bioscience (Jena, Germany).
Protein level analysis
Levels of Ypt1 and Ypt31 in cell lysates were determined from exponentially growing cells normalized to the same OD600 as previously described (Lipatova et al., 2012). Lysates were subjected to immuno-blot analysis using affinity-purified rabbit anti-Ypt1 or anti-Ypt31 antibodies (Jedd et al., 1997). Loading control was determined using rabbit anti-G6PDH antibodies (Sigma). Quantification of bands was performed with ImageJ software (National Institute of Health).
Fluorescence microscopy
Fluorescence microscopy of cells grown to mid-log phase (in flasks) was done using a Zeiss Confocal LSM700 microscope controlled by Zen software. Images were captured using a 100x/1.45 NA objective. Laser lines used: 488nm for GFP/YFP/FITC, 555nm for mCherry/mRFP/Texas Red, and 639nm for Alexa Fluor® 647. To limit photo bleaching, all images were taken as fast as possible and within 10 minutes of slide preparation and using minimal laser strength.
For live-cell imaging Z-stacks were taken at 0.35 μm increments. Only Figure S1A microscopy was performed on a deconvolution Zeiss Axiovision microscope (Carl Zeiss, Thornwood, NY) with fluorescein isothiocyanate (yEVenus) and Texas Red (mCherry) filters. Co-localization was quantified by counting puncta that do or do not overlap through several z-stacks and the stack with the highest amount of co-localization was selected. Random images of Golgi marker co-localization from Figure 1 were processed with the JACoP plugin to determine the Pearson's coefficient of co-localization (by pixels). Pearson's coefficient confirmed the relative map of the Golgi proteins determined by counting puncta.
Immunofluorescence was done with Ibidi μ-slide 8-well coated (Poly-L-Lysine) microscopy chambers using affinity-purified anti-Ypt1 and anti-Ypt31 antibodies as previously described (Jedd et al., 1997). The following dye-conjugated secondary antibodies were purchased from Jackson Immunoresearch: Texas Red® dye-conjugated AffiniPure Goat Anti-Rabbit IgG; Alexa Fluor® 647-conjugated AffiniPure Goat Anti-Rabbit IgG; Fluorescein (FITC)-conjugated AffiniPure Goat Anti-Rabbit IgG. Co-localization was quantified by counting puncta that do or do not overlap on a single focal plane.
Time-lapse microscopy was performed using the Zen software. One focal plane was followed for approximately 2 minutes. The resulting movies were analyzed using Fiji software (Schindelin et al., 2012). Images were measured for photobleach-correction and then adjusted with Gaussian filters. Single puncta were selected to track over time and measured for average fluorescence intensity. The resulting curves were adjusted to the same minimum/maximum range (0–1) using the equation previously detailed (Daboussi et al., 2012). Puncta were chosen as being a single color followed closely by the second color in the order of anterograde traffic as previously described (Losev et al., 2006). Statistical significance of the time-lapse curve calculations were tested using the unpaired, Student's t-Test with GraphPad Software (http://www.graphpad.com/quickcalcs/ttest1/?Format=50).
Supplementary Material
Research Highlights.
Golgi cisternal progression is regulated by Ypt/Rab GTPases
Ypt1 and Ypt31 GTPases localize to opposite sides of the yeast Golgi
Ypt1 and Ypt31 co-localize on a transitional Golgi cisterna defined here
Two distinct progression steps are regulated by Ypt1 and Ypt31
Acknowledgements
We thank B. Glick and members of the Segev lab for useful discussions, B. Grewe for critical reading of the manuscript, V. Nazarko for text editing, Z. Almeida for technical support, and P. Toth from UIC RRC for help with Fiji software. This research was supported by grant GM-45444 from NIH to N. Segev.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors Contributions Conceptualization, Methodology and Resources, J.J.K., Z.L., and N.S.; Investigation, J.J.K., Z.L., and U.M.; Visualization and Formal Analysis, J.J.K., and N.S.; Writing – Original Draft, N.S.; Writing – Review and Editing, J.J.K., Z.L., and N.S.; Funding Acquisition and Supervision, N.S.
References
- Barrowman J, Bhandari D, Reinisch K, Ferro-Novick S. TRAPP complexes in membrane traffic: convergence through a common Rab. Nat Rev Mol Cell Biol. 2010;11:759–763. doi: 10.1038/nrm2999. [DOI] [PubMed] [Google Scholar]
- Bhave M, Papanikou E, Iyer P, Pandya K, Jain BK, Ganguly A, Sharma C, Pawar K, Austin J, 2nd, Day KJ, et al. Golgi enlargement in Arf-depleted yeast cells is due to altered dynamics of cisternal maturation. J Cell Sci. 2014;127:250–257. doi: 10.1242/jcs.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Zhang Y, Pypaert M, Walker L, Ferro-Novick S. Mutants in trs120 disrupt traffic from the early endosome to the late Golgi. J Cell Biol. 2005;171:823–833. doi: 10.1083/jcb.200505145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Chin HF, Lazarova D, Menon S, Fu C, Cai H, Sclafani A, Rodgers DW, De La Cruz EM, Ferro-Novick S, et al. The structural basis for activation of the Rab Ypt1p by the TRAPP membrane-tethering complexes. Cell. 2008;133:1202–1213. doi: 10.1016/j.cell.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daboussi L, Costaguta G, Payne GS. Phosphoinositide-mediated clathrin adaptor progression at the trans-Golgi network. Nat Cell Biol. 2012;14:239–248. doi: 10.1038/ncb2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS, Luini A. Models for Golgi traffic: a critical assessment. Cold Spring Harb Perspect Biol. 2011;3:a005215. doi: 10.1101/cshperspect.a005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubruck H, Prange R, Vorgias C, Gallwitz D. The ras-related mouse ypt1 protein can functionally replace the YPT1 gene product in yeast. Embo J. 1989;8:1427–1432. doi: 10.1002/j.1460-2075.1989.tb03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O'Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G, Richardson C, Litt R, Segev N. The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway. J Cell Biol. 1995;131:583–590. doi: 10.1083/jcb.131.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Jedd G, Kahn RA, Franzusoff A, Bartolini F, Segev N. Genetic interactions in yeast between Ypt GTPases and Arf guanine nucleotide exchangers. Genetics. 1999;152:1543–1556. doi: 10.1093/genetics/152.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatova Z, Belogortseva N, Zhang XQ, Kim J, Taussig D, Segev N. Regulation of selective autophagy onset by a Ypt/Rab GTPase module. Proc Natl Acad Sci U S A. 2012;109:6981–6986. doi: 10.1073/pnas.1121299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatova Z, Hain AU, Nazarko VY, Segev N. Ypt/Rab GTPases: Principles learned from yeast. Crit Rev Biochem Mol Biol. 2015:1–9. doi: 10.3109/10409238.2015.1014023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatova Z, Segev N. Ypt/Rab GTPases regulate two intersections of the secretory and the endosomal/lysosomal pathways. Cell Logist. 2014;4:e954870. doi: 10.4161/21592780.2014.954870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatova Z, Shah AH, Kim JJ, Mulholland JW, Segev N. Regulation of ER-phagy by a Ypt/Rab GTPase module. Mol Biol Cell. 2013;24:3133–3144. doi: 10.1091/mbc.E13-05-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS. Golgi maturation visualized in living yeast. Nature. 2006;441:1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441:1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- McDonold CM, Fromme JC. Four GTPases differentially regulate the Sec7 Arf-GEF to direct traffic at the trans-golgi network. Dev Cell. 2014;30:759–767. doi: 10.1016/j.devcel.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Cheng KW, Mills GB. Rab GTPases implicated in inherited and acquired disorders. Semin Cell Dev Biol. 2011;22:57–68. doi: 10.1016/j.semcdb.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova N, Liang Y, Tokarev AA, Chen SH, Cox R, Andrejic J, Lipatova Z, Sciorra VA, Emr SD, Segev N. TRAPPII subunits are required for the specificity switch of a Ypt-Rab GEF. Nat Cell Biol. 2006;8:1263–1269. doi: 10.1038/ncb1489. [DOI] [PubMed] [Google Scholar]
- Nilsson T, Au CE, Bergeron JJ. Sorting out glycosylation enzymes in the Golgi apparatus. FEBS Lett. 2009;583:3764–3769. doi: 10.1016/j.febslet.2009.10.064. [DOI] [PubMed] [Google Scholar]
- Papanikou E, Glick BS. The yeast Golgi apparatus: insights and mysteries. FEBS Lett. 2009;583:3746–3751. doi: 10.1016/j.febslet.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikou E, Glick BS. Golgi compartmentation and identity. Curr Opin Cell Biol. 2014;29:74–81. doi: 10.1016/j.ceb.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. A model for Rab GTPase localization. Biochem Soc Trans. 2005;33:627–630. doi: 10.1042/BST0330627. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol. 2013;25:414–419. doi: 10.1016/j.ceb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinar M, Arst HN, Jr., Pantazopoulou A, Tagua VG, de los Rios V, Rodriguez-Salarichs J, Diaz JF, Penalva MA. TRAPPII regulates exocytic Golgi exit by mediating nucleotide exchange on the Ypt31 ortholog RabERAB11. Proc Natl Acad Sci U S A. 2015;112:4346–4351. doi: 10.1073/pnas.1419168112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pind SN, Nuoffer C, McCaffery JM, Plutner H, Davidson HW, Farquhar MG, Balch WE. Rab1 and Ca2+ are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J Cell Biol. 1994;125:239–252. doi: 10.1083/jcb.125.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Molina FE, Novick PJ. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proc Natl Acad Sci U S A. 2009;106:14408–14413. doi: 10.1073/pnas.0906536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M, Barrowman J, Schieltz D, Yates JR, 3rd, Ferro-Novick S. Identification and characterization of five new subunits of TRAPP. Eur J Cell Biol. 2000;79:71–80. doi: 10.1078/S0171-9335(04)70009-6. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Chen S, Rivera-Molina F, Reinisch K, Novick P, Ferro-Novick S. Establishing a role for the GTPase Ypt1p at the late Golgi. Traffic. 2010;11:520–532. doi: 10.1111/j.1600-0854.2010.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev N. Mediation of the attachment or fusion step in vesicular transport by the GTP-binding Ypt1 protein. Science. 1991;252:1553–1556. doi: 10.1126/science.1904626. [DOI] [PubMed] [Google Scholar]
- Segev N. Ypt and Rab GTPases: insight into functions through novel interactions. Curr Opin Cell Biol. 2001a;13:500–511. doi: 10.1016/s0955-0674(00)00242-8. [DOI] [PubMed] [Google Scholar]
- Segev N. Ypt/rab gtpases: regulators of protein trafficking. Sci STKE. 2001b;2001:re11. doi: 10.1126/stke.2001.100.re11. [DOI] [PubMed] [Google Scholar]
- Segev N. Coordination of intracellular transport steps by GTPases. Semin Cell Dev Biol. 2011;22:33–38. doi: 10.1016/j.semcdb.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J, Warren G. Golgi architecture and inheritance. Annu Rev Cell Dev Biol. 2002;18:379–420. doi: 10.1146/annurev.cellbio.18.030602.133733. [DOI] [PubMed] [Google Scholar]
- Suda Y, Kurokawa K, Hirata R, Nakano A. Rab GAP cascade regulates dynamics of Ypt6 in the Golgi traffic. Proc Natl Acad Sci U S A. 2013;110:18976–18981. doi: 10.1073/pnas.1308627110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda Y, Nakano A. The yeast Golgi apparatus. Traffic. 2012;13:505–510. doi: 10.1111/j.1600-0854.2011.01316.x. [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.