Abstract
Recent findings suggest that maternal vitamin D insufficiency during pregnancy has consequences for the offspring’s bone health in later life. To investigate whether maternal vitamin D insufficiency affects fetal femur growth in ways similar to those seen in childhood rickets, and study the timing during gestation of any effect of maternal vitamin D status, we studied 424 pregnant women within a prospective longitudinal study of maternal nutrition and lifestyle before and during pregnancy (the Southampton Women’s Survey). Using high-resolution 3D ultrasound we measured fetal femur length and distal metaphyseal cross-sectional area, together with the ratio of femoral metaphyseal cross sectional area to femur length (“femoral splaying index”). Lower maternal 25-hydroxyvitamin vitamin D concentration was not related to fetal femur length, but was associated with greater femoral metaphyseal cross-sectional area and a higher femoral splaying index at 19 weeks gestation (r=−0.16, (95%CI:−0.25 to −0.06) and r=−0.17, (95%CI: −0.26 to −0.07), respectively) and at 34 weeks gestation (r=−0.10, 95%CI:−0.20 to 0.00) and r=−0.11, (95%CI: −0.21 to −0.01), respectively). Three groups of women were identified with 25-hydroxyvitamin vitamin D concentrations that were sufficient/borderline (>50 nmol/L, 63.4%), insufficient (25-50 nmol/L, 30.7%) and deficient (≤25 nmol/L, 5.9%). Across these groups, the geometric mean femoral splaying indices at 19 weeks gestation increased from 0.074 (sufficient/borderline) to 0.078 (insufficient) and 0.084 (deficient). Our observations suggest that maternal vitamin D insufficiency can influence fetal femoral development as early as 19 weeks gestation. This suggests that measures to improve maternal vitamin D status should be instituted in early pregnancy.
Keywords: vitamin D, fetus, developmental origins, osteoporosis, three-dimensional ultrasound
Introduction
Vitamin D insufficiency is common in pregnancy. In a study of pregnant women in Southampton UK during 1990-1991, the prevalence of vitamin D insufficiency was 31% among white Caucasian women(1); an even greater frequency has been reported among Asian women.(2) In the UK, Vitamin D supplementation is recommended in pregnancy(3-4) and, since 2007, women in receipt of certain benefits have been entitled to free vitamin D supplements through the Healthy Start scheme. In the United States, the Institute of Medicine (IOM) concluded in 1997 that there was no additional need to increase the vitamin D age-related adequate intake during pregnancy above that required for non-pregnant women;(5) however, during 2008 the IOM initiated a study to review Dietary Reference Intakes for vitamin D, with a report expected for release in 2010. The vitamin D axis is known to influence the acquisition of bone mineral in-utero and changes in women’s calcium homeostasis during pregnancy facilitate calcium supply for bone mineralisation in the rapidly growing fetal skeleton.(6) Mother-offspring cohort studies have shown that maternal vitamin D insufficiency has a detrimental effect on bone mineral accrual by the fetus, leading to reduced bone mass at birth and in childhood.(1,7)
There is, however, little information on the precise consequences of maternal vitamin D insufficiency on the morphology and growth of long bones, or whether its influence is apparent in early or late pregnancy. Answering these questions has been hindered by the limitations of ultrasound assessment of fetal bone and the need to avoid exposure to ionising radiation during pregnancy. The development of high-resolution three dimensional ultrasound (3DUS) techniques in recent years enables intra-uterine bone evaluation and we have examined the use of 3DUS to look for splaying of the distal femoral metaphyses, such as is commonly seen in childhood rickets. In this study, we address the relationship between low maternal vitamin D status and fetal femur development using this novel approach.
Materials and Methods
The Southampton Women’s Survey (SWS)(8) is a prospective cohort study which recruited non-pregnant women aged 20-34 years in Southampton. Its purpose is to assess how maternal nutrition and lifestyle before and during pregnancy influence development of the offspring. All women were interviewed at home about their lifestyle and social circumstances including receipt of social security benefits. Height, weight and skinfold thickness measurements were made. The women attended for ultrasound scans at 11, 19 and 34 weeks gestation. For this study we recruited all 525 SWS women with singleton pregnancies who had a 3DUS scan at 19 weeks gestation between October 2002 and June 2005. Four fetuses had congenital abnormalities and one was stillborn; one mother withdrew because of severe illness and two withdrew for other reasons. 491 of the remainder had a further 3DUS at 34 weeks gestation. At the time of the 34 week scan, a research nurse obtained a venous blood sample; an aliquot of maternal serum frozen at −80°C was available for measurement of maternal vitamin D status for 424 pregnancies (81% of the original 525 recruited). Serum 25-hydroxyvitamin D concentrations were analysed by radioimmunoassay (Diasorin, Stillwater, Minnesota, USA). This assay measures both vitamin D2 and D3. The assay met the requirements of the UK National External Quality Assurance Scheme (NEQAS) and intra-assay and inter-assay coefficients of variation were less than 10%. We categorised maternal vitamin D status according to 25-hydroxyvitamin D concentration as follows: ≤25 nmol/L ‘deficient’; 25-50 nmol/L ‘insufficient’; 50-70 nmol/L ‘borderline’; and > 70 nmol/L ‘sufficient’.(9)
At 19 and 34 weeks gestation, we performed 3DUS of the fetal thigh using a KretzGE Voluson® 730 3DUS system, fitted with a curved-array transducer with a frequency range between 4 and 8 MHz and a controllable scan angle between 15° and 75°. Thigh volumes were stored by a single operator (PM) who then used the multi-planar imaging mode to aid identification of structures and orientate anatomical slices. We took a standard linear measure of femur length from the stored scan(10) and derived a novel measurement, only possible using 3D ultrasound, of the cross-sectional area of the distal femoral metaphysis (Figure 1). The distal femur was chosen as it exhibits more evidence of splaying than the proximal end or the mid-shaft cross-sectional area. Each measurement was performed in triplicate and mean values were used in subsequent analyses. Precision of the measurements was assessed by replicate examinations in 50 pregnancies at both 19 and 34 weeks. The coefficient of variation for triplicate measurements of femur length was 0.6% at 19 weeks and 0.4% at 34 weeks. Corresponding coefficients of variation for measurements of femoral metaphyseal cross-sectional area were 4.4% at 19 weeks and 3.2% at 34 weeks; these values are similar to those for the reproducibility of measurements obtained in other studies using 3DUS(11). To assess femoral shape we derived a “femoral splaying index” as distal femoral metaphyseal cross-sectional area (cm2)/femur length (cm) (Figure 1). The index was devised for this study as a means of assessing the degree of metaphyseal splaying in relation to femur length. The duration of gestation was calculated from menstrual information or, when these were uncertain or discrepant with ultrasound assessments, fetal anthropometry in early pregnancy.
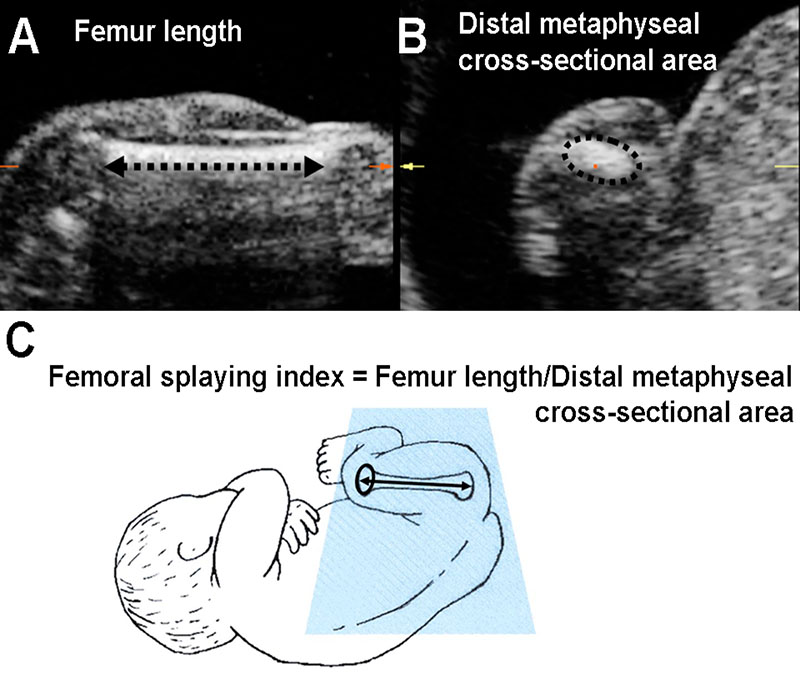
Figure 1.
Multi-planar display of fetal thigh volume using high resolution 3D ultrasound, illustrating measurements of: A. femur length measured on the longitudinal section; B. femoral distal metaphyseal cross-sectional area measured on the coronal section. C. diagrammatic representation of the “femoral splaying index”.
We used linear regression to relate maternal vitamin D status to femoral size and splaying index, together with Pearson’s correlation coefficients to show the strength of observed associations and their statistical significance. Where necessary we transformed the data to satisfy statistical assumptions of normality. Statistical analysis was performed using Stata Statistical Software Release 10, 2008 (Stata Corporation, College Station, Texas, USA).
The study had full approval from the Southampton and Southwest Hampshire Local Research Ethics Committee and all participants gave written, informed consent.
Results
424 mother-baby pairs were included in the study, of which 215 (50.7%) of the offspring were boys. The mean (SD) age of the mothers at delivery was 31.3 years (3.6); mean maternal height was 163.9 cm and 59.4% were in their first pregnancy. At pre-pregnancy recruitment, 13.7% were in receipt of benefits and 26.4% were smokers; 13.6% were still smoking in late pregnancy (Table 1). Mean (95% confidence interval (CI)) fetal femur length increased from 2.92 (2.90 to 2.94) cm to 6.36 (6.33 to 6.39) cm between 19 and 34 weeks gestation; corresponding geometric mean values for distal femoral cross-sectional area measurements were 0.222 (0.217 to 0.226) cm2 and 0.765 (0.748 to 0.783) cm2, a more than three-fold increase over this 15-week period. The geometric mean femoral splaying index rose from 0.076 (0.075 to 0.078) cm at 19 weeks gestation to 0.120 (0.118 to 0.123) cm2/cm at 34 weeks. Fetal femur length, distal cross-sectional area and splaying index were all similar in boys and girls at both 19 and 34 weeks gestation.
Table 1. Characteristics of the 424 mothers.
| Median | Inter-quartile range | n | |
|---|---|---|---|
| Age at delivery (years) | 31.7 | 28.7-33.9 | 424 |
| Height (cm) | 164 | 159-168 | 422 |
| Pre-pregnancy sum of skin-fold thickness (mm) | 63.9 | 48.7-82.8 | 419 |
| Pre-pregnancy body mass index (BMI) (kg/m2) | 23.9 | 21.5-26.4 | 422 |
| 34 week vitamin D concentration (nmol/L) | 61 | 41-85 | 424 |
| Percentage | |||
| Smoking status : smokers (pre-pregnancy) | 26.4 | ||
| smokers (early pregnancy) | 14.3 | ||
| smokers in late pregnancy | 13.6 | ||
| Parity : nulliparous (first child) | 59.4 | ||
| In receipt of benefits: | 13.7 | ||
| Ethnic origin: white | 97.4 | ||
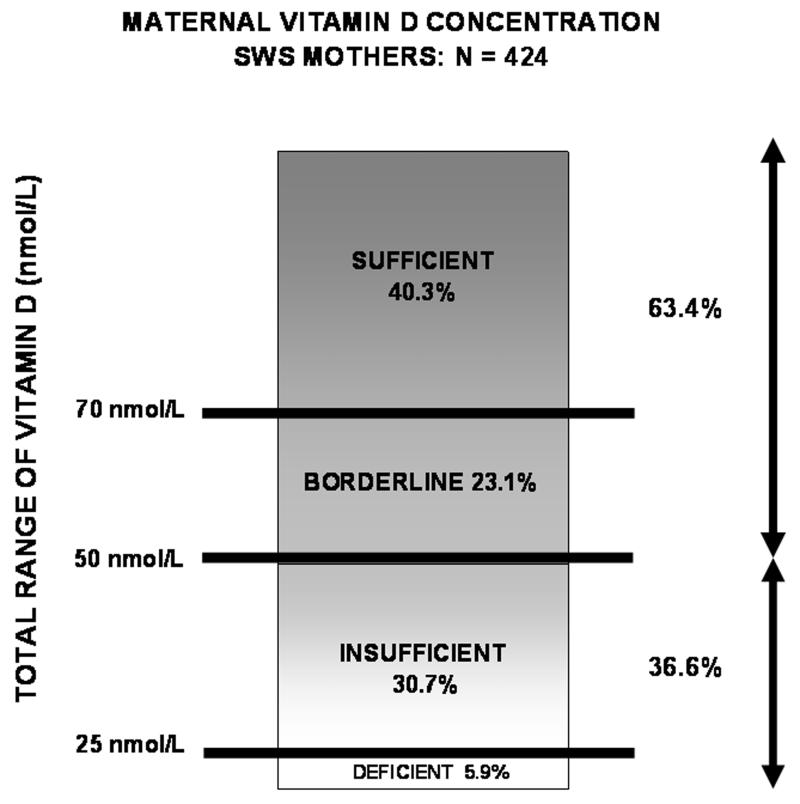
The median maternal serum 25-hydroxyvitamin D concentration at 34 weeks gestation was 61 nmol/L, (range 8-180 nmol/L, inter-quartile range 41-85 nmol/L). Vitamin D concentrations above 70 nmol/L were seen in 171 women (40.3%); a further 98 women (23.1%) had values between 50 and 70 nmol/L (borderline); 130 (30.7%) had values between 25 and 50 nmol/L (insufficient); the remaining 25 (5.9%) had values less than 25 nmol/L (deficient). 36.6% were either insufficient or deficient (Figure 2). Compared with other women, those in receipt of benefits were more likely to be vitamin D deficient (12.1% vs 4.9%, p=0.03) and marginally more likely to be vitamin D insufficient (32.8% vs 30.3). 72.0% of the vitamin D deficient women were not in receipt of benefits, as were 85.4% of the vitamin D insufficient women.
Figure 2.
Maternal vitamin D concentrations for the cohort (n = 424).
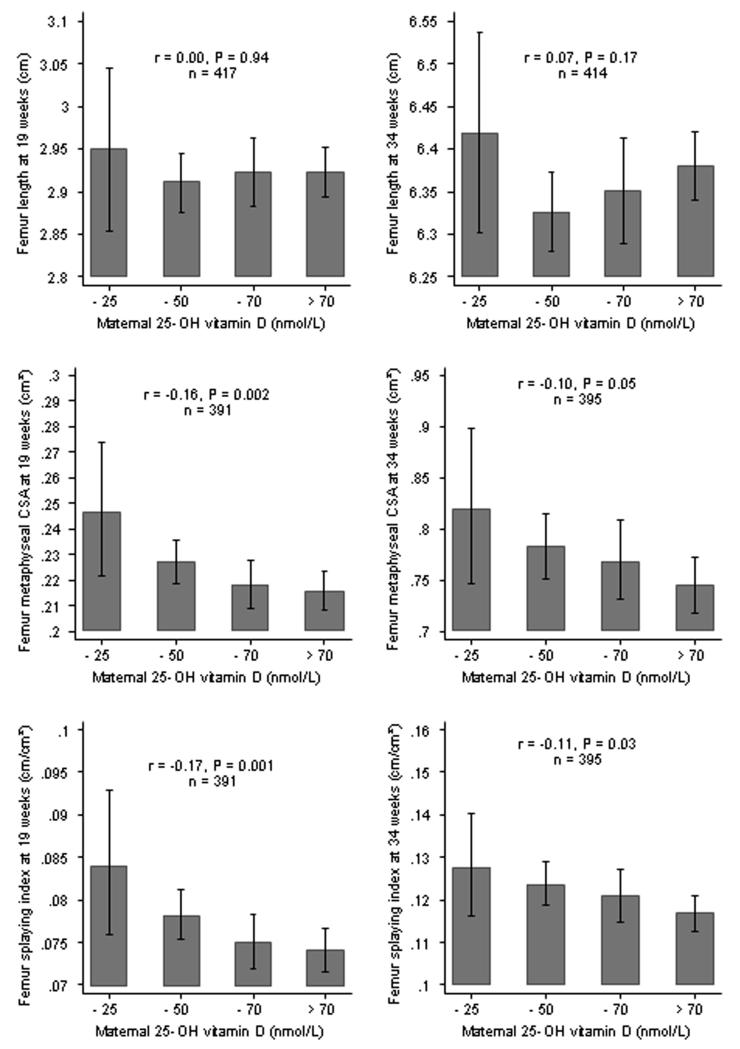
The panel of histograms on the left side of Figure 3 show the associations between maternal serum 25-hydroxyvitamin vitamin D concentration and fetal femur length, distal metaphyseal femoral cross-sectional area and splaying index at 19 weeks gestation. Maternal 25-hydroxyvitamin D concentration was not related to 19 week fetal femur length (r=0.00, 95%CI: −0.09 to 0.10), but a lower maternal 25-hydroxyvitamin D concentration was strongly associated with a greater distal metaphyseal cross-sectional area (r=−0.16, 95%CI: −0.25 to −0.06) and a higher femoral splaying index (r=−0.17, 95%CI: −0.26 to −0.07). Compared with fetuses whose mothers were vitamin D replete, metaphyseal cross-sectional area was 5% greater in those whose mothers were vitamin D insufficient and 14% greater in those whose mothers were vitamin D deficient. Likewise, there was a similar trend in femoral shape as assessed by the femoral splaying index across the categories of 25-hydroxyvitamin D concentration. When the data were grouped according to 25-hydroxyvitamin D status, the geometric mean femoral splaying index at 19 weeks gestation was 0.074 (95%CI 0.072 to 0.076) in fetuses whose mothers had 25-hydroxyvitamin vitamin D concentrations >50 nmol/L; in fetuses whose mothers who were vitamin D insufficient and deficient, splaying index values were 0.078 (0.075 to 0.081) and 0.084 (0.076 to 0.093), respectively. When analysed by gender, the associations with metaphyseal femoral cross-sectional area and splaying index at 19 weeks were similar in boys and girls. Taking account of the mother’s height, pre-pregnant weight, sum of skinfold thicknesses, smoking, parity and age had little effect on the association between the mother’s vitamin D status and metaphyseal femoral cross-sectional area and femoral splaying index (data not shown). Likewise season at measurement was not related to bone parameters and taking season into account had little effect on the associations described above.
Figure 3.
Mean fetal femur length, geometric mean distal femoral metaphyseal cross-sectional area (CSA) and geometric mean femoral splaying index according to maternal 25-hydroxyvitamin D concentration in 424 mother-offspring pairs at both 19 and 34 weeks gestation.
The panel of histograms on the right side of Figure 3 demonstrate the association between maternal 25-hydroxyvitamin D concentration and femur length, distal metaphyseal cross-sectional area and splaying index at 34 weeks gestation. There was no association between maternal vitamin D concentration and fetal femur length (r=0.07, 95%CI: −0.03 to 0.16); lower maternal vitamin D concentration was, however, associated with greater distal metaphyseal cross-sectional area (r=−0.10, 95%CI: −0.20 to 0.00) and a higher femoral splaying index (r=−0.11, 95%CI: −0.21 to −0.01). These relationships were similar to those observed at 19 weeks gestation but slightly weaker.
Discussion
Our data suggest that lower maternal 25-hydroxyvitamin D status during pregnancy is associated with splaying of the distal metaphysis of the fetal femur. The association we found was already apparent at 19 weeks gestation and still demonstrable at 34 weeks. We have utilised 3DUS to evaluate the size and shape of the fetal femur at 19 and 34 weeks gestation, and related this to maternal 25-hydroxyvitamin D concentrations in late pregnancy. Although previous studies of fetal femoral development have focused on femur length, we have derived a reproducible technique for measurement of the fetal femoral distal metaphyseal cross-sectional area. The distal femoral metaphyseal splaying we have demonstrated in fetuses of mothers with low vitamin D concentrations is directly analogous to that seen in childhood rickets and fetal femoral morphology could perhaps be a useful predictor of the risk of childhood rickets. This condition was once thought to have been almost eradicated in western populations, but has again become a public health problem.(12,13) Several reports have been published describing recent cases of infantile rickets.(14) The re-emergence of this metabolic bone disorder is thought to be due to an epidemic of vitamin D deficiency in mothers and children.(15) Neonatal vitamin D stores are completely reliant on maternal supply, thus poor maternal vitamin D status during pregnancy is a major risk factor for neonatal rickets(16). In-utero, or early life, vitamin D deficiency has also been linked to an increased risk of several disorders including neonatal craniotabes(17), pre-term birth(18), type I diabetes(19) and schizophrenia.(20)
Strengths and limitations
Strengths of the study were the strong biological basis for the association that we have demonstrated and the clear a priori hypothesis that we examined. Our study had several potential weaknesses. We solely studied a subset of SWS mothers; however, we have previously demonstrated that participants in this study were of similar age, socio-economic status, and nutritional status, to those who did not participate.(7,8) Second, we only measured total serum concentrations of 25-hydroxyvitamin D; pregnancy is associated with an increase in vitamin D binding protein, as well as higher 1,25-dihydroxyvitamin D concentrations.(21) Third, we only measured maternal vitamin D status in late pregnancy as we thought it more likely that maternal vitamin D insufficiency would influence femoral morphology in late gestation. The demonstration that low maternal vitamin D status is associated with altered femoral morphology as early as 19 weeks gestation is all the more surprising given the measurement of vitamin D in later gestation. However, in a previous study in Southampton(1) we found that, taking account of the month of sample collection, a 1 SD (standard deviation) difference in late pregnancy maternal 25-hydroxyvitamin D concentration was associated with a 0.33 SD difference in early pregnancy 25-hydroxyvitamin D concentration (unpublished). Fourth, our study relied on 3DUS to evaluate femoral length and metaphyseal cross-sectional area. Only the first of these measurements has been validated in-vitro, but it is difficult to see how systematic differences in the assessment of femoral distal cross-sectional area would have spuriously revealed an association with maternal vitamin D status. Despite adjustment for confounders in our analyses, we cannot exclude the possibility of residual confounding and causality cannot be assumed from these observational data.
Implications for practice
Evidence that lower prenatal vitamin D status alters fetal skeletal development with persisting effects on bone health has come from a previous Southampton cohort, in which a lower maternal concentration of 25-hydroxyvitamin D in late pregnancy was associated with lower whole body and lumbar spine bone mineral content in the children at aged nine years, with a suggestion of a threshold effect at 35 nmol/L.(1) Both the estimated exposure to ultraviolet B radiation during late pregnancy and the use of vitamin D supplements predicted maternal 25-hydroxyvitamin D concentrations and childhood bone mass. In the current study, we have demonstrated that changes in the distal femoral metaphysis were apparent as early as 19 weeks gestation. This has important implications for interventions to improve maternal vitamin D status as it supports existing guidelines for the recommendation of vitamin D supplements in pregnancy, but suggests that such measures should begin in early gestation. The test of these findings would be through a randomised controlled trial of vitamin D supplementation in early pregnancy, and we have embarked upon such a trial.
Our data reinforce guidance from the UK National Institute for Health and Clinical Excellence that health professionals should take particular care to check that women at greatest risk of vitamin D deficiency are following advice to take a vitamin D supplement during pregnancy and while breastfeeding.(22) The women we studied were pregnant during 2002-2005; more recently pregnant and lactating women in the UK in receipt of certain benefits have been entitled to free vitamin D supplements through the Healthy Start scheme. While women receiving benefits were more likely to be vitamin D deficient in our study, the majority of the deficient women would not have been eligible for free supplements. Our data support the provision of free vitamin D supplements within schemes such as Healthy Start but they also highlight a need for wider use of vitamin D supplements, as currently recommended in some countries.(22)
Later life implications
Much evidence now suggests that environmental influences that alter bone development during intrauterine and early postnatal life may increase the risk of osteoporotic fracture in adulthood.(23) Longitudinal studies in Finland have shown that poor early growth is associated with higher rates of hip fracture.(24) Studies in the US, UK, Sweden and Australia have shown that low birthweight and weight at one year are associated with low bone mass in adult life, as assessed by dual energy X-ray absorptiometry.(25-27) Preliminary data suggest important interactions between birthweight and genes that predict adult bone mass, including the vitamin D receptor gene.(28) The relationships between early development and later bone health are independent of known environmental risk factors for osteoporosis including smoking, alcohol consumption, lack of exercise and low calcium intakes. Later life consequences for metabolic conditions, infection risk and susceptibility to neoplastic diseases have been proposed,(13) but it is unknown whether early life metaphyseal splaying has any implications for later degenerative joint disease.
Conclusion and future studies
In summary, our study shows that the vitamin D status of mothers during pregnancy is correlated with the morphology of the developing fetal femur. Changes in the distal femoral metaphysis are already apparent at 19 weeks gestation. As ultrasound is likely to play an increasing part in antenatal fetal assessment, and the prevalence of vitamin D insufficiency among pregnant women is known to be high, there is a need for further evaluation of this methodology for the identification of fetuses for whom the skeletal consequences of maternal vitamin D insufficiency might be amenable to correction.
Acknowledgements
We thank the mothers who gave us their time, midwifery staff for their assistance and SWS research team for their assistance. PM conceived and undertook the ultrasound scans and measurements, supervised by CC and KMG; HMI, KMG and SMR initiated the SWS; SRC led the statistical analysis; NH, NKA and RS developed the vitamin D aspects of the study. All authors contributed to analysis and preparation of the manuscript. KMG is guarantor for the study.
This research was supported by grants from the Medical Research Council, Arthritis Research Campaign, Dunhill Medical Trust, International Osteoporosis Foundation and National Osteoporosis Society; a British Medical Ultrasound Society Pump Priming Grant was awarded in 2006. The funding agencies had no role in the conduct or reporting of this research.
Footnotes
Conflict of Interest Page
All authors have no conflicts of interest.
References
- 1.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 2.Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, Robinson VP, Winder SM. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J. 1980;280:751–754. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department of Health . Dietary reference values for food energy and nutrients for the United Kingdom. Report of the panel on dietary reference values of the Committee on Medical Aspects of Food Policy. HMSO; London: 1991. [PubMed] [Google Scholar]
- 4.Scientific Advisory Committee on Nutrition . Update on vitamin D. Position statement by the Scientific Advisory Committee on Nutrition. The Stationery Office; London: 2007. [Google Scholar]
- 5.Institute of Medicine . Dietary reference intakes for calcium, phosphorous, magnesium, vitamin D and fluoride. National Academy Press; Washington DC: 1997. [PubMed] [Google Scholar]
- 6.Specker B. Vitamin D requirements during pregnancy. Am J Clin Nutr. 2004;80:1740S–1747S. doi: 10.1093/ajcn/80.6.1740S. [DOI] [PubMed] [Google Scholar]
- 7.Harvey NC, Javaid MK, Poole JR, Taylor P, Robinson S, Inskip HM, Godfrey KM, Cooper C, Dennison EM, the Southampton Women’s Survey Study Group Paternal skeletal size predicts intrauterine bone mineral accrual. J Clin Endocrinol Metab. 2008;93:1676–81. doi: 10.1210/jc.2007-0279. [DOI] [PubMed] [Google Scholar]
- 8.Inskip H, Godfrey KM, Robinson SM, Law CM, Barker DJP, Cooper C, the SWS Study Group Cohort Profile: The Southampton Women’s Survey. Int J Epidemiol. 2006;35:42–48. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruston D, Hoare J, Henderson L, et al. The National Diet and Nutrition Survey: Adults Aged 19–64 Years. Vol. 4. Nutritional Status (Anthropometry and Blood Analytes), Blood Pressure and Physical Activity. The Stationery Office; London: 2004. [Google Scholar]
- 10.Chitty LS, Altman DG. Charts of fetal size: limb bones. British Journal of Obstetrics and Gynaecology. 2002;109:919–929. doi: 10.1111/j.1471-0528.2002.01022.x. [DOI] [PubMed] [Google Scholar]
- 11.Endres LK, Cohen L. Reliability and validity of three-dimensional fetal brain volumes. Journal of Ultrasound in Medicine. 2001;20:1265–1269. doi: 10.7863/jum.2001.20.12.1265. [DOI] [PubMed] [Google Scholar]
- 12.Prentice A, Schoenmakers I, Laskey MA, Debono S, Ginty F, Goldberg GR. Nutrition and bone growth and development. Proc Nutr Soc. 2006;65:348–360. doi: 10.1017/s0029665106005192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holick MF. Sunlight and Vitamin D for bone health and prevention of autoimmune diseases, cancers and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF. Resurrection of Vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cashman KD. Vitamin D in childhood and adolescence. Postgrad Med J. 2007;83:230–235. doi: 10.1136/pgmj.2006.052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reif S, Katzir Y, Eisenberg Z, Weisman Y. Serum 25-hydroxyvitamin D levels in congenital craniotabes. Acta Paediatr Scand. 1988;77:167–168. doi: 10.1111/j.1651-2227.1988.tb10620.x. [DOI] [PubMed] [Google Scholar]
- 17.Yorifuji J, Yorifuji T, Tachibana K, Nagai S, Kawai M, Momoi T, Nagasaka H, Hatayama H, Nakahata T. Craniotabes in normal newborns: the earliest sign of subclinical vitamin D deficiency. J Clin Endocrinol Metab. 2008;93:1784–8. doi: 10.1210/jc.2007-2254. [DOI] [PubMed] [Google Scholar]
- 18.Abrams S. In utero physiology: role in nutrient delivery and fetal development for calcium, phosphorus, and vitamin D. Am J Clin Nutr. 2007;85(suppl):604S–7S. doi: 10.1093/ajcn/85.2.604S. [DOI] [PubMed] [Google Scholar]
- 19.Hypponen E, Laara E, Reunanen A, Järvelin MR, Virtanen SM. Intake of Vitamin D and risk of Type 1 diabetes: a birth cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 20.McGrath J, Saari K, Hakko, Jokelainen J, Jones P, Järvelin MR, Chant D, Isohanni M. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophrenia Research. 2004;67:237–245. doi: 10.1016/j.schres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Hypponen E, Turner S, Cumberland P, Power C, Gibb I. Serum 25-hydroxyvitamin D measurement in a large population survey with statistical harmonization of assay variation to an international standard. J Clin Endocrin Metab. 2007;92:4615–22. doi: 10.1210/jc.2007-1279. [DOI] [PubMed] [Google Scholar]
- 22.National Institute for Health and Clinical Excellence PH11 Maternal and child nutrition: guidance. 2008 Mar 26; Available from: https://www.nice.org.uk/nicemedia/pdf/PH011guidance.pdf.
- 23.Harvey N, Cooper C. The developmental origins of osteoporotic fracture. J Br Menopause Soc. 2004;10:14–29. doi: 10.1258/136218004322986726. [DOI] [PubMed] [Google Scholar]
- 24.Cooper C, Eriksson JG, Forsen T, Osmond C, Tuomilehto J, Barker DJ. Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study. Osteoporos Int. 2001;12:623–9. doi: 10.1007/s001980170061. [DOI] [PubMed] [Google Scholar]
- 25.Cooper C, Fall C, Egger P, Hobbs R, Eastell R, Barker D. Growth in infancy and bone mass in later life. Ann Rheum Dis. 1997;56:17–21. doi: 10.1136/ard.56.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yarbrough DE, Barrett-Connor E, Morton DJ. Birth weight as a predictor of adult bone mass in postmenopausal women: the Rancho Bernardo Study. Osteoporos Int. 2000;11:626–630. doi: 10.1007/s001980070085. [DOI] [PubMed] [Google Scholar]
- 27.Javaid MK, Cooper C. Prenatal and childhood influences on osteoporosis. Best Practice & Research Clinical Endocrinology & Metabolism. 2002;16:349–367. doi: 10.1053/beem.2002.0199. [DOI] [PubMed] [Google Scholar]
- 28.Dennison EM, Arden NK, Keen RW, Syddall H, Day IN, Spector TD, Cooper C. Birthweight, vitamin D receptor genotype and the programming of osteoporosis. Paediatr Perinat Epidemiol. 2001;15:211–9. doi: 10.1046/j.1365-3016.2001.00350.x. [DOI] [PubMed] [Google Scholar]