Abstract
The heptose-deficient inner core of the lipopolysaccharide of several pathogenic strains of the Moraxellaceae family (Moraxella, Acinetobacter) and of Bartonella henselae, respectively, comprises an α-D-glucopyranose attached to position 5 of Kdo. In continuation of the synthesis of fragments of Acinetobacter haemolyticus LPS, the branched α-Glcp-(1→5)[α-Kdo-(2→4)]-α-Kdo trisaccharide motif was elaborated. The glycosylation of a suitably protected, α-(2→4)-interlinked Kdo-disaccharide was achieved in high yield and fair anomeric selectivity using a 4,6-O-benzylidene N-phenyltrifluoroacetimidate glucosyl donor. Subsequent regioselective reductive benzylidene opening afforded a trisaccharide acceptor, which was extended with β-D-glucopyranosyl and isomaltosyl residues. Global deprotection provided tri- to pentasaccharide structures corresponding to the inner core region of A. haemolyticus lipopolysaccharide.
Keywords: oligosaccharide synthesis, Acinetobacter, glycosylation, Kdo, lipopolysaccharide
1. Introduction
The Gram-negative bacterial cell wall is covered by densely packed lipopolysaccharide (LPS) molecules which trigger major immune responses of the respective host during pathogenesis.1,2 In structural terms, these glycolipids comprise the endotoxically active lipid anchor (lipid A) and an extended oligosaccharide fraction, which harbors a core region of limited structural variability and the strain-specific, highly diverse O-antigenic polysaccharides.3,4 The core domain may be further divided into an outer and an inner core region, wherein a 3-deoxy-d-manno-2-octulosonic acid (Kdo) usually forms the link to the lipid A part. In many bacterial genera, this Kdo unit is extended by a lateral α-(2→4)-linked Kdo moiety.5 In a few cases - such as in some Acinetobacter strains - Kdo is non-stoichiometrically replaced by its 3-oxy analogue d-glycero-d-talo-2-octulosonic acid (Ko).6 The α-(2→4)-Kdo disaccharide is frequently substituted at position 5 by a l-glycero-d-manno-heptose residue, and d-mannose, d-galactosamine and Kdo itself were also occasionally identified as the branching sugar.7 Specifically, an α-(1→5)-linked d-glucopyranosyl residue has been found in LPS of the Moraxellaceae family and was shown to elicit cross-reactive antibodies recognizing different serotypes.8 In addition, Bartonella henselae – the causative agent of the cat-scratch disease - expresses a truncated core region being composed of α-Glcp-(1→5)[α-Kdo-(2→4)]-α-Kdo linked to lipid A.9 Furthermore, in A. haemolyticus this basic trisaccharide pattern is elongated at the glucose unit by a β-(1→4)-linked isomaltotriose.10 The observation of a high binding affinity of isolated inner core fragments from A. haemolyticus towards different mannose binding lectins11 prompted us to synthesize part structures thereof in order to identify the relevant binding epitopes.
Previously, stereoselective α-(1→5)-coupling of glucosyl donors to Kdo monosaccharide acceptors was successfully performed by Oscarson using a thioglycoside donor in the presence of DMTST.12 In a previous contribution we have capitalized on the use of an NPTFA-glucosyl donor and an orthogonal blocking group at position-4 of Kdo to allow for the introduction of the lateral Kdo at a later stage.13 A limited number of papers has been published addressing the synthesis of 5-O-glycosylated α-Kdo-(2→4)-α-Kdo disaccharides.14 Ichiyanagi et al. recently reported on the convenient preparation of 5-O-substituted Kdo disaccharides applying a Kdo disaccharide acceptor for coupling with heptose, d-mannose and 2-azido-2-deoxy-d-galactose (as a precursor for d-galactosamine) donors.15 Extending these studies, we present herein a high-yielding method to access the branched α-Glcp-(1(5)[α-Kdo-(2→4)]-α-Kdo trisaccharide with fair stereoselectivity and its subsequent elongation into tetra- and pentasaccharide derivatives.
2. Results and discussion
2.1. Approach A: Using an α-Glc-(1→5)-Kdo acceptor
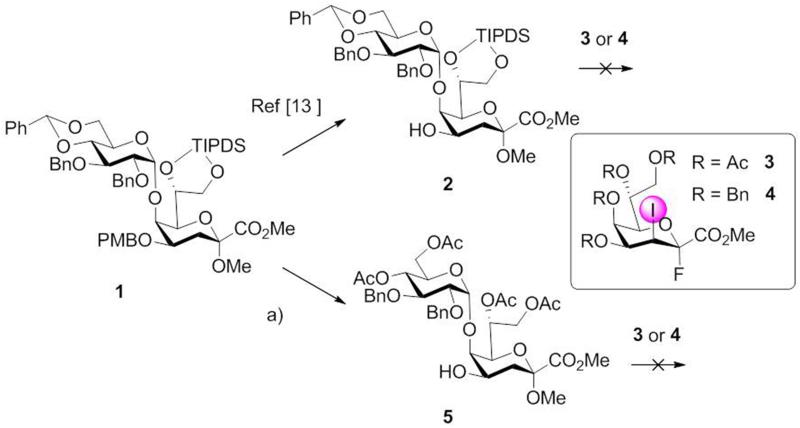
Previously, α-Glc-(1→5)-Kdo disaccharide 1 has been prepared with excellent stereoselectivity and in a high yield.13 The orthogonal protecting group pattern allowed for selective cleavage of the p-methoxybenzyl group (PMB) affording the 4-OH Kdo acceptor 2. Starting from 2 we envisaged to introduce the lateral Kdo moiety using the Kdo fluoride donors 3 and 4, which were recently reported to yield only α-anomeric products without significant elimination side reaction.16 Specifically, the per-O-acetyl protected Kdo donor 3 proved to be an efficient glycosyl donor for regioselective coupling to a 4,5-diol Kdo acceptor yielding the α-(2→4)-interconnected Kdo disaccharide as a single isomer in high yield.16a The donor, however, was unreactive in the presence of base - such as triethylamine or sym-collidine - which were added to capture hydrogen fluoride. Since the 7,8-O-disiloxane-1,3-diyl (TIPDS) and the 4,6-O-acetal group in acceptor 2 were not compatible with glycosylation conditions lacking a HF-scavenger, these protecting groups were replaced by acetyl groups. Accordingly, acid hydrolysis of the benzylidene group, followed by cleavage of the silyl ether, O-acetylation and DDQ-oxidation of the PMB group furnished the disaccharide acceptor 5 in 40% overall yield (Scheme 1). Attempted coupling of 5 with acetyl-protected donor 3 under BF3·Et2O promotion (2 eq.) in dichloromethane in the presence of 3 Å molecular sieves at room temperature, however, failed and trisaccharide formation was not detected.
Scheme 1.
Reagents and conditions: a) pTosOH, MeOH, 40 °C, 2 h; then TBAF, THF, 0 °C, 20 min; then Ac2O, DMAP, pyridine, r.t., 14 h; then DDQ, CH2Cl2/MeOH (3:1), r.t., 3.5 h, 40% (4 steps).
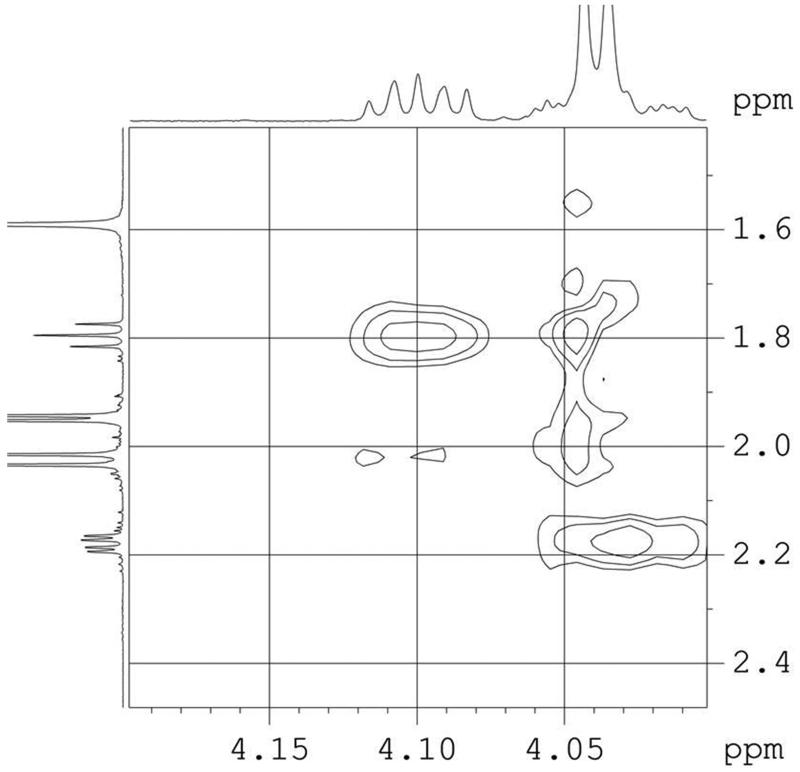
Next, the armed benzylated donor 4 was tried, which had been shown to react with 2-propanol under BF3·Et2O promotion also in the presence of triethylamine, although higher temperatures were necessary.16b First, when using donor 4 in the absence of base and under conditions as used for donor 3, degradation of acceptor 2 was observed. In the presence of triethylamine, acceptor 2 was unaffected, but glycosylation did not take place. In a last attempt we intended to exploit the high reactivity of 4 and the compatibility of glycosyl acceptor 5 towards base-free conditions. Again, however, glycoside formation could not be achieved. NMR analysis of 5 revealed a through-space interaction between the axial hydrogen at position-3 of Kdo with H-5′ of the glucosyl residue (Fig. 1). This would indicate that the glucose unit is in close proximity to the Kdo ring and thus sterically shields the 4-OH group towards the incoming electrophile.
Fig. 1.
Section of the NOESY spectrum of compound 5 in CDCl3 showing a correlation between H-3ax and H-5′.
In addition to the Kdo-fluoride based glycosylation attempts, coupling of acceptor 2 with peracetylated Kdo bromide under Helferich conditions was also tested but did not lead to significant product formation. Previously, this approach had successfully been performed for the introduction of a lateral Kdo-unit onto a L-glycero-d-manno-heptosyl-(1→5)-Kdo glycosyl acceptor in 65% yield.17
2.2. Approach B: Using an α-Kdo-(2→4)-Kdo acceptor
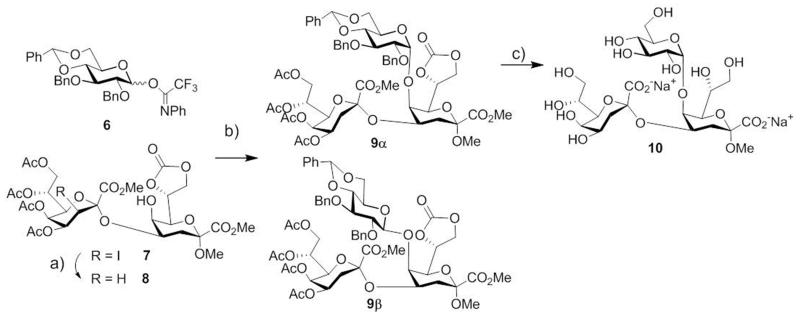
As an alternative approach, the sequence of glycosylation steps was inverted by attaching a suitably protected glucose donor to a preassembled α-Kdo-(2→4)-Kdo disaccharide acceptor. Thus, we could capitalize on the previously elaborated route towards Kdo2 7, which was obtained in high yields and without any formation of β-products.16a Recently, Ichiyanagi et al. successfully coupled a Kdo2 5-OH acceptor with heptose and mannose trichloroacetimidate donors exploiting the trans-directing effect of C-2 ester groups.15 First, the 5-OH acceptor 8 was obtained from iodo-precursor 7 by hydrogen atom transfer18 in cyclohexane catalyzed by lauroyl peroxide. Previously, dehalogenation depended on fully blocked compounds to guarantee sufficient solubility in cyclohexane for smooth conversion.16a Notably, using a mixture of cyclohexane and 1,2-dichloroethane18 allowed for direct and neat dehalogenation of disaccharide 7 which provided acceptor 8 in high yield (92%).
In previous experiments, N-phenyltrifluoroacetimidate glucose donor 613 afforded excellent α-selectivity in the glycosylation of a 5-OH Kdo monosaccharide due to the directing effect of the 4,6-O-benzylidene group.19 Coupling of 5-OH acceptor 8 with donor 6 in CH2Cl2 under TMSOTf catalysis in the presence of ground 4 Å molecular sieves provided traces of both α- and β-trisaccharides 9α and 9β. By exploiting the known20 1,2-cis directing effect of ether solvents, the α/β-ratio could be increased significantly (from α/β = 1:1.2 in CH2Cl2 to 2.7:1 in Et2O in preliminary experiments). The observation that glycoside formation ceased after a short initial phase led to the assumption that TfOH formed by partial hydrolysis of TMSOTf was the active promotor, which would then be rapidly scavenged by 4 Å molecular sieves. Indeed, applying TfOH as activator in the presence of 5 Å molecular sieves improved the total yield to 31%. This still disappointing outcome was attributed to donor degradation that proceeded faster than glycoside formation. Eventually, using inverse glycosylation conditions21 in diethyl ether containing molecular sieves (5 Å) afforded a mixture of anomers 9α and 9β in a total yield of 91% (α/β = 4:1) which could be conveniently separated on an HPLC column (isolated yield of α-product 9α: 67%). The α-anomeric configuration was readily assigned on the basis of the coupling constant J1″,2″ (3.8 Hz). Lactone formation between C-1′ of the lateral Kdo unit and the free 5-hydroxyl group was also observed but to a low degree only. Sequential removal of benzyl (Pd/C, H2) and acetyl (NaOMe) groups from trisaccharide 9α and ensuing methyl ester saponification under alkaline conditions (NaOH) gave trisaccharide 10 in 94% yield (Scheme 2).
Scheme 2.
Reagents and conditions: a) Lauroyl peroxide, cyclohexane/ClCH2CH2Cl (7.2:1), reflux, 2 h, 92%; b) TfOH, 5 Å molecular sieves, CH2Cl2/Et2O (2:1), r.t., 3 h, 91% (α/β 4:1, isolated 9α: 67%, 9β: 16%); c) Pd-C (10%), H2 (1 bar), MeOH, r.t., 4 h; then 0.1 M NaOMe, MeOH, r.t., 17 h; then 0.1 M aq. NaOH, 0 °C → r.t., 4 h, 94%.
2.3. Synthesis of oligoglucosyl fragments
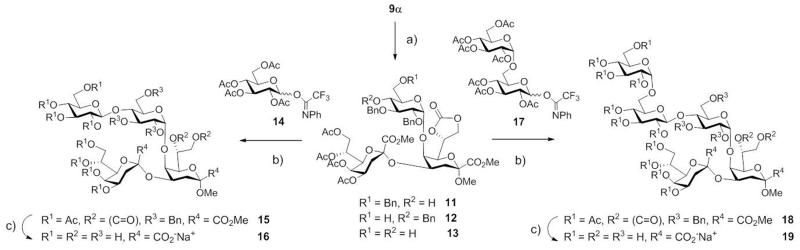
Proceeding towards the oligoglucosyl core structures, regioselective opening of the benzylidene group in compound 9α to provide the 6-OBn protected derivative 11 was foreseen, to serve as an acceptor for the extended glucosyl core structure of A. haemolyticus.
Hence, treatment of trisaccharide 9α with triethylsilane/BF3·Et2O22 in dry dichloromethane afforded the desired 4-OH acceptor derivative 11 as the major product (72%) together with its 6-OH regioisomer 12 (5%) and traces of diol 13 (2%). By using the peracetylated glucopyranosyl NPTFA donor 1423 under TfOH promotion (5 mol% for 15, 12.5 mol% for 18), tetrasaccharide 15 was obtained (Scheme 3). The isolated yield for the β-tetrasaccharide 14, however, did not exceed 46%, since several purification steps by HPLC were needed to produce a pure tetrasaccharide. Using the same strategy with the isomaltosyl donor 17, the pentasaccharide 18 was readily accessible in a 3+2 approach in a good yield (71%). Global deprotection afforded the oligosaccharide ligands 16 (99%) and 19 (90%) in excellent yields. The 13C NMR data of the oligosaccharide (Table 1) are in good agreement with the LPS-oligosaccharides isolated from B. henselae ATCC 49882T* and A. haemolyticus respectively.9,10 Deviating assignments were only noted for C-7 of both Kdo units.9
Scheme 3.
Reagents and conditions: a) Triethylsilane, BF3·Et2O, CH2Cl2, 0 °C, 1 h, 11: 72%, 12: 5%, 13: 2% ; b) TfOH, 5 Å molecular sieves, CH2Cl2, 0 °C, 15 min (for →15)/45 min (for →18), 15: 46%, 18: 71%; c) Pd-C (10%), H2 (1 atm), MeOH, r.t., 3 h (for →16)/4 h (for →19); then 0.1 M NaOMe, MeOH, r.t., 14 h (for →16)/20 h (for →19) ; then 0.1 M aq. NaOH, 0 °C → r.t., 3 h, 16: 99%, 19: 90%.
Table 1.
13C NMR chemical shifts (δ) of compounds 10, 16 and 19
| Residue | Carbon | 10 | 16 | 19 |
|---|---|---|---|---|
| →4,5)-α-Kdop | ||||
| 1 | 176.05 | 175.94 | 176.05b | |
| 2 | 101.35 | 101.35 | 101.34 | |
| 3 | 35.24 | 35.21 | 35.19 | |
| 4 | 72.12 | 72.04a | 72.04c | |
| 5 | 74.47 | 73.94 | 73.78 | |
| 6 | 73.22 | 73.11 | 73.10 | |
| 7 | 69.26 | 69.35 | 69.29 | |
| 8 | 63.98 | 64.00 | 64.08 | |
| α-Kdop-(2→4)- | ||||
| 1 | 176.05 | 176.03 | 175.92b | |
| 2 | 102.87 | 102.82 | 102.84 | |
| 3 | 35.42 | 35.48 | 35.50 | |
| 4 | 66.67 | 66.73 | 66.75 | |
| 5 | 67.68 | 67.66 | 67.67 | |
| 6 | 72.50 | 72.55 | 72.55d | |
| 7 | 71.52 | 71.50 | 71.52 | |
| 8 | 63.58 | 63.82 | 63.63 | |
| α-Glcp-(1→5)- | ||||
| 1 | 100.31 | 99.72 | 99.61 | |
| 2 | 72.88 | 72.60 | 72.45 | |
| 3 | 73.29 | 71.90a | 72.03c | |
| 4 | 69.76 | 79.07 | 79.98 | |
| 5 | 72.64 | 71.25 | 71.06 | |
| 6 | 60.69 | 60.29 | 60.30 | |
| β-Glcp-(1→4)- | ||||
| 1 | 103.36 | 103.69 | ||
| 2 | 73.94 | 73.81 | ||
| 3 | 76.33 | 76.40 | ||
| 4 | 70.33 | 70.49 | ||
| 5 | 76.78 | 74.97 | ||
| 6 | 61.46 | 66.74 | ||
| α-Glcp-(1→6)- | ||||
| 1 | 98.79 | |||
| 2 | 72.32 | |||
| 3 | 73.62 | |||
| 4 | 70.49 | |||
| 5 | 72.71d | |||
| 6 | 61.39 | |||
| OCH3 | 51.37 | 51.38 | 51.37 |
Assigments within a column may be reversed
2.4. Conclusions and outlook
In summary, a straightforward method to prepare the α-Glcp-(1→5)[α-Kdo-(2→4)]-α-Kdo trisaccharide capitalizing on a Kdo disaccharide acceptor was established. Good α-selectivity relied on diethyl ether as a solvent and high yields were obtained by slowly adding the donor to a pre-stirred mixture of the Kdo2 acceptor and TfOH as a promotor. The 4,6-O-benzylidene blocking group of the donor eventually provided access to extended oligosaccharides after regioselective reductive opening. Subsequent elongation with 2-O-acetyl protected N-phenyl trifluoroacetimidate glucosyl donors allowed for the introduction of β-Glcp-(1→4)- as well as α-Glcp-(1→6)-β-Glcp-(1→4)- units. Thus, three fragments of the A. haemolyticus inner core were obtained which serve as ligands in binding studies with C-type collectins.
3. Experimental
3.1. General
All purchased chemicals were used without further purification unless stated otherwise. The promotor BF3·Et2O was used as a solution in diethyl ether (≥46% according to the manufacturer). Solvents were dried over activated 3 Å (acetone, Et2O) or 4 Å (CH2Cl2, ClCH2CH2Cl, cyclohexane, N,N-dimethylformamide, pyridine) molecular sieves. Dry MeOH (Merck) and dry THF (Sigma-Aldrich) were purchased. Cation exchange resin DOWEX 50 H+ was regenerated by consecutive washing with HCl (3 M), water and dry MeOH. Aqueous solutions of salts were saturated unless stated otherwise. Concentration of organic solutions was performed under reduced pressure < 40 °C. Optical rotations were measured with a Perkin-Elmer 243 B Polarimeter. [α]D20 values are given in units of 10−1deg cm2g−1. Thin layer chromatography was performed on Merck precoated plates: generally on 5 × 10 cm, layer thickness 0.25 mm, Silica Gel 60F254; alternatively on HPTLC plates with 2.5 cm concentration zone (Merck). Spots were detected by dipping reagent (anisaldehyde-H2SO4). For column chromatography silica gel (0.040 – 0.063 mm) was used. HP-column chromatography was performed on pre-packed columns (YMC-Pack SIL-06, 0.005 mm, 250×10 mm and 250×20 mm). Desalting after ester saponification was performed on pre-packed PD-10 columns (GE Healthcare, Sephadex™ G-25 M). NMR spectra were recorded with a Bruker Avance III 600 instrument (600.22 MHz for 1H, 150.93 MHz for 13C) using standard Bruker NMR software. 1H spectra were referenced to 7.26 (CDCl3) and 0.00 (D2O, external calibration to 2,2-dimethyl-2-silapentane-5-sulfonic acid) ppm unless stated otherwise. 13C spectra were referenced to 77.00 (CDCl3) and 67.40 (D2O, external calibration to 1,4-dioxane) ppm. ESI-MS data were obtained on a Waters Micromass Q-TOF Ultima Global instrument.
3.2. 4,6-Di-O-acetyl-2,3-di-O-benzyl-α-d-glucopyranosyl-(1→5)-methyl [methyl 7,8-di-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosid]onate (5)
A solution of disaccharide 113 (100 mg, 0.094 mmol) in dry MeOH (4.0 mL) containing p-toluenesulfonic acid-monohydrate (1.6 mg, 0.008 mmol) was kept at 40 °C for 2 h under an atmosphere of argon. The cooled solution was treated with solid NaHCO3 (ca. 50 mg) for 5 min, the solvent was removed under reduced pressure and the residue was partitioned between EtOAc and aq. NaHCO3. The organic phase was washed with brine, dried (MgSO4), filtered and concentrated. The residue was dissolved in dry THF (6.0 mL) and treated with tetrabutylammonium fluoride (1 M in THF, 0.14 mL, 0.142 mmol) at 0 °C for 20 min. After addition of dry MeOH (1.0 mL) the solvent was removed and EtOAc was added. The residue was filtered over a short pad of silica and rinsed with EtOAc. Concentration of the filtrate afforded a crude product, which was acetylated using acetic anhydride (0.8 mL), 4-(N,N-dimethylamino)pyridine (5 mg) and dry pyridine (4.0 mL). After 14 h at ambient temperature excessive reagent was destroyed with dry MeOH (1.0 mL) at 0 °C (10 min) followed by removal of solvent by codistillation with toluene (3×). Chromatography of the crude product provided the PMB-protected intermediate (52 mg, 61%) as a colorless oil.
This intermediate (41 mg, 0.046 mmol) was dissolved in dry CH2Cl2 (2.1 mL) and dry MeOH (0.7 mL) under Ar and treated with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (164 mg, 0.672 mmol) in eight portions within 2.5 h. After complete addition the mixture was stirred for 1 h, diluted with CHCl3 and washed with aq. NaHCO3. The aqueous phase was once more extracted with CHCl3 and the combined organic phases were washed with aq. NaHCO3 (2×), dried (MgSO4) and filtered. Contentration of the filtrate and purification of the residue by chromatography (toluene/EtOAc 9:1 to remove excessive reagent, then 2:1 to elute product) afforded 4-OH acceptor 5 (23 mg, 65%, total yield: 40%) as a colorless oil: [α]D20 + 41.9 (c 0.56, CHCl3); Rf 0.18 (n-hexane/EtOAc 1:1); 1H NMR (CDCl3): δ 7.36 - 7.27 (m, 10H, Ar), 5.43 (app dt, 1H, J7,6 ~ 5.7, J7,8a 2.1 Hz, H-7), 4.87 (app t, 1H, J4′,3′ ~ J4′,5′ 9.5 Hz, H-4′), 4.86 (d, 1H, J1′,2′ 3.7 Hz, H-1′), 4.85 (d, 1H, J 11.1 Hz, CHHPh), 4.82 (dd, 1H, J8a,8b 12.5 Hz, H-8a), 4.74 (d, 1H, J 12.2 Hz, CHHPh), 4.70 (d, 1H, J 12.4 Hz, CHHPh), 4.68 (d, 1H, J 11.6 Hz, CHHPh), 4.29 (dd, 1H, J8b,7 5.3 Hz, H-8b), 4.10 (dt, 1H, J5′,6′ 4.6 Hz, H5′), 4.07 - 4.00 (m, 3H, H-4, H-6′a, H-6′b), 3.98 - 3.96 (br s, 1H, H-5), 3.95 (dd, J6,5 1.0 Hz, H-6), 3.93 (app t, 1H, H-3′), 3.83 (s, 3H, CO2CH3), 3.56 (dd, 1H, J2′,3′ 9.5, J2′,1′ 3.5 Hz, H-2′), 3.37 (d, 1H, JOH,4 11.5 Hz, OH), 3.27 (s, 3H, OCH3), 2.20 - 2.16 (m, 1H, H-3eq), 2.03, 2.01, 1.95 and 1.94 (4 s, each 3H, COCH3), 1.80 (app t, 1H, J3ax,3eq ~ J3ax,4 12.5 Hz, H-3ax); 13C NMR (CDCl3): δ 170.53, 170.43, 169.95 and 169.76 (4 s, 4C, COCH3), 168.02 (s, C-1), 138.39 and 137.85 (2 s, 2C, Ar), 128.56, 128.39, 128.00, 127.95, 127.67 and 127.63 (6 d, 10C, Ar), 99.73 (d, C-1′), 99.04 (s, C-2), 79.42 (d, C-5), 78.79 (d, C-3′), 78.30 (d, C-2′), 75.17 (t, CH2Ph), 73.73 (t, CH2Ph), 71.54 (d, C-6), 71.13 (d, C-7), 69.90 and 69.51 (2 d, 2C, C-4′, C-5′), 66.19 (d, C-4), 62.84 (t, C-6′), 62.25 (t, C-8), 52.62 (q, CO2CH3), 51.04 (q, OCH3), 36.08 (t, C-3′), 21.02, 20.76, 20.70 and 20.63 (4 q, 4C, COCH3); ESI-TOF HRMS: m/z = 799.2784; calcd for C38H48O17Na+: 799.2784.
3.3. Methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→4)-methyl (methyl 7,8-O-carbonyl-3-deoxy-α-d-manno-oct-2-ulopyranosid)onate (8)
A suspension of disaccharide 716a (0.71 g, 0.87 mmol) in a mixture of dry ClCH2CH2Cl (9.0 mL) and dry cyclohexane (65.0 mL) was degassed with argon and heated to reflux for 15 min. Lauroyl peroxide (0.12 g, 0.30 mmol) was added to the warm solution and the refluxed for 2 h. The solvent was removed under reduced pressure and the residue was subjected to chromatography (n-hexane/EtOAc 1:2) to provide dehalogenated compound 8 (0.56 g, 92%) as a colorless amorphous solid: [α]D20 + 73.1 (c 0.63, CHCl3); Rf 0.31 (toluene/EtOAc 2:3); 1H NMR (CDCl3): δ 5.40 - 5.38 (m, 1H, H-5′), 5.26 - 5.19 (m, 2H, H-4′, H-7′), 4.91 (ddd, 1H, J7,8b 8.2, J7,8a 6.9 Hz, J7,6 3.7, H-7), 4.75 (dd, 1H, J8a,8b 8.9, H-8a), 4.65 (dd, 1H, J8′a,8′b 12.5, J8′a,7′ 2.7 Hz, H-8′a), 4.54 (app t, 1H, H-8b), 4.25 (ddd, 1H, J4,3ax 11.2, J4,3eq 5.7, J4,5 3.2 Hz, H-4), 4.11 (dd, 1H, J6′,7′ 9.6, J6′,5′ 1.3 Hz, H-6’), 4.08 (dd, 1H, J8′b,7′ 3.5 Hz, H-8′b), 3.89 - 3.87 (m, 1H, H-6), 3.84 (s, 3H, CO2CH3), 3.81 (s, 3H, CO2CH3), 3.69 - 3.67 (m, 1H, H-5), 3.22 (s, 3H, OCH3), 2.46 (bs, 1H, OH), 2.20 (dd, 1H, J3′eq,3′ax ~ J3′eq,4′ 12.7, H-3′eq), 2.13 (s, 3H, COCH3), 2.12 - 2.03 (m, 6H, H-3ax, H-3′ax, H-3eq, COCH3), 2.00 and 1.98 (2 s, each 3H, COCH3); 13C NMR (CDCl3): δ 170.98, 170.20, 169.88 and 169.61 (4 s, 4C, COCH3), 168.87 and 167.58 (2 s, 2C, C-1, C-1′), 154.66 [s, OC(=O)O], 99.10 and 98.08 (2 s, 2C, C-2, C-2′), 76.03 (d, C-7), 70.38 (d, C-6), 69.61 (d, C-6′), 67.64 and 67.45 (2 d, 2C, C-4, C-7′), 66.10 (t, C-8), 65.88 (d, C-4′), 64.73 (d, C-5), 64.18 (d, C-5′), 61.58 (t, C-8′), 53.43 and 52.71 (2 q, 2C, CO2CH3), 51.14 (q, OCH3), 33.00 and 32.29 (2 t, 2C, C-3, C-3′), 20.70, 20.60, 20.58 and 20.56 (4 q, 4C, COCH3); ESI-TOF HRMS: m/z = 712.2298; calcd for C28H38O20NH4+: 712.2295.
3.4. Methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→4)-[2,3-di-O-benzyl-4,6-O-benzylidene-α-d-glucopyranosyl-(1→5)]-methyl (methyl 7,8-O-carbonyl-3-deoxy-α-d-manno-oct-2-ulopyranosid)onate (9α) and methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→4)-[2,3-di-O-benzyl-4,6-O-benzylidene-β-d-glucopyranosyl-(1→5)]-methyl (methyl 7,8-O-carbonyl-3-deoxy-α-d-manno-oct-2-ulopyranosid)onate (9β)
A solution of disaccharide acceptor 8 (77 mg, 0.111 mmol) in dry Et2O (1.5 mL) containing ground 5 Å molecular sieves (75 mg) was stirred at ambient temperature for 2 h under Ar. Diluted triflic acid (0.49 μL, 5.55 μmol) in dry CH2Cl2 (0.1 mL) was added and after 5 min a separately prepared solution of donor 613 (344 mg, 0.555 mmol) in dry CH2Cl2 (3.0 mL) was added to this mixture using a syringe pump (1 mL/h). After complete addition the promotor was neutralized by adding triethylamine (32 μL, 0.222 mmol) and the suspension was filtered over Celite. The filtrate was concentrated and the residual solid was purified by chromatography (n-hexane/EtOAc 1:1) affording a mixture of the anomers 9α and 9β (114 mg, 91%, α:β = 4:1 according to 1H NMR). The compounds were separated on an HPLC column (toluene/EtOAc 3:1 → 2:1) providing the desired α-anomer 9α (83 mg, 67%) and the slower migrating β-isomer 9β (20 mg, 16%) as colorless oils. Data for 9α: [α]D20 + 111.4 (c 0.82, CHCl3); Rf 0.44 (toluene/EtOAc 2:1, HP-TLC); 1H NMR (CDCl3): δ 7.51 - 7.28 (m, 15H, Ar), 5.58 (s, 1H, PhCH), 5.35 - 5.33 (m, 1H, H-5′), 5.27 (ddd, 1H, J4′,3′ax 12.5, J4′,3′eq 4.8, J4′,5′ 3.0 Hz, H-4′), 5.19 (ddd, 1H, J7′,6′ 9.6, J7′,8′b 3.9, J7′,8′a 2.8 Hz, H-7′), 5.07 (d, 1H, J1″,2″ 3.8 Hz, H-1″), 5.00 (d, 1H, J 11.5 Hz, CHHPh), 4.92 (d, 1H, J 10.6 Hz, CHHPh), 4.85 (d, 1H, J 11.5 Hz, CHHPh), 4.67 (dd, 1H, J8′a,8′b 12.4 Hz, H-8′a), 4.66 - 4.62 (m, 1H, H-7), 4.59 (d, 1H, J 10.9 Hz, CHHPh), 4.44 (dd, 1H, J8a,8b 8.6, J8a,7 5.8 Hz, H-8a), 4.30 (dd, 1H, J6″a,6″b 10.4, J6″a,5″ 4.8 Hz, H-6″a), 4.20 (dd, 1H, J6′,5′ 1.5 Hz, H-6′), 4.16 (ddd, 1H, J4,3ax 11.8, J4,3eq 4.7, J4,5 2.4 Hz, H-4), 4.06 (app t, 1H, J8b,7 ~ 8.4 Hz, H-8b), 4.01 (dd, 1H, H-8′b), 3.93 - 3.88 (m, 2H, H-3″, H-5″), 3.84 (s, 3H, CO2CH3), 3.83 - 3.82 (m, 1H, H-5), 3.79 (s, 3H, CO2CH3), 3.75 - 3.69 (m, 2H, H-6, H-6″b), 3.66 (app t, 1H, J4″,3″ ~ J4″,5″ 9.3 Hz, H-4″), 3.59 (dd, 1H, H-2″), 3.16 (s, 3H, OCH3), 2.21 - 2.15 (m, 2H, H-3eq, H-3′eq), 2.10 (app. t, 1H, J3ax,3eq ~ 12.1 Hz, H-3ax), 2.09 and 2.07 (2 s, each 3H, COCH3), 2.07 (app t, 1H, J3′ax,3′eq 12.7 Hz, H-3′ax), 1.99 and 1.95 (2 s, each 3H, COCH3); 13C NMR (CDCl3): δ 170.85, 170.27, 169.69 and 169.60 (4 s, 4C, COCH3), 168.06 and 167.35 (2 s, 2C, C-1, C-1′), 154.57 [s, OC(=O)O], 138.24, 137.70 and 137.28 (3 s, 3C, Ar), 128.95, 128.64, 128.46, 128.33, 128.25, 128.23, 128.20, 127.93 and 125.99 (9 d, 15C, Ar), 101.28 (d, PhCH), 100.56 (d, C-1″), 99.34 and 98.92 (2 s, C-2, C-2′), 82.63 (d, C-4″), 79.86 (d, C-2″), 77.86 (d, C-3″), 76.00 (d, C-5), 75.16 (d, C-7), 75.01 (t, CH2Ph), 74.81 (t, CH2Ph), 72.03 (d, C-6), 69.50 (d, C-4), 69.44 (d, C-6′), 68.95 (t, C-6″), 67.72 (d, C-7′), 65.95 (t, C-8), 65.92 (d, C-4′), 64.37 (d, C-5′), 63.59 (d, C-5″), 61.58 (t, C-8′), 53.11 and 52.69 (2 q, 2C, CO2CH3), 51.23 (q, OCH3), 33.85 (t, C-3), 32.40 (t, C-3′), 20.70, 20.63 and 20.58 (3 q, 4C, 4 COCH3); ESI-TOF HRMS: m/z = 1147.3625; calcd for C55H64O25Na+: 1147.3629.
9β: [α]D20 + 49.8 (c 0.82, CHCl3); Rf 0.23 (toluene/EtOAc 2:1, HP-TLC); 1H NMR (CDCl3): δ 7.49 - 7.24 (m, 15H, Ar), 5.56 (s, 1H, PhCH), 5.35 - 5.33 (m, 1H, H-5′), 5.23 (ddd, 1H, J4′,3′ax 12.3, J4′,3′eq 4.9, J4′,5′ 3.0 Hz, H-4′), 5.18 (ddd, 1H, J7′,6′ 9.2, J7′,8′b 4.3, J7′,8′a 2.8 Hz, H-7′), 4.94 (d, 1H, J1″,2″ 7.7 Hz, H-1″), 4.90 (d, 1H, J 11.5 Hz, CHHPh), 4.89 (d, 1H, J 11.3 Hz, CHHPh), 4.83 - 4.74 (m, 4H, 2 × CHHPh, H-7, H-8a), 4.62 (dd, 1H, J8′a,8′b 12.5 Hz, H-8′a), 4.44 (app t, 1H, J8b,8a ~ J8b,7 8.1 Hz, H-8b), 4.37 (dd, 1H, J6″a,6″b 10.4, J6″a,5″ 5.0 Hz, H-6″a), 4.29 (ddd, 1H, J4,3ax 11.7, J4,3eq 5.2, J4,5 2.7 Hz, H-4), 4.11 - 4.09 (m, 1H, H-5), 4.04 - 4.00 (m, 2H, H-6′, H-8′b), 3.96 - 3.94 (m, 1H, H-6), 3.85 (s, 3H, CO2CH3), 3.81 (app t, 1H, J3″,2″ ~ J3″,4″ 8.9 Hz, H-3″), 3.74 (s, 3H, CO2CH3), 3.74 (app t, 1H, J6″b,5″ 10.4 Hz, H-6″b), 3.60 (app t, 1H, J4″,5″ 9.2 Hz, H-4″), 3.46 (app dt, 1H, H-5″), 3.31 (app t, 1H, H-2″), 3.24 (s, 3H, OCH3), 2.23 - 2.19 (m, 1H, H-3′eq), 2.16 (app t, 1H, J3ax,3eq ~ 12.2 Hz, H-3ax), 2.10 - 2.04 (m, 8H, 2 × COCH3, H-3eq, H-3′ax), 2.00 and 1.98 (2 s, each 3H, COCH3); 13C NMR (CDCl3): δ 170.93, 170.23, 169.64 and 169.57 (4 s, 4C, COCH3), 168.18 and 167.52 (2 s, 2C, C-1, C-1′), 154.64 [s, OC(=O)O], 138.47, 138.03 and 137.13 (3 s, 3C, Ar), 129.02, 128.36, 128.25, 127.91, 127.76, 127.69, 127.58 and 125.96 (8 d, 15C, Ar), 102.92 (d, C-1″), 101.22 (d, PhCH), 99.16 and 99.04 (2 s, 2C, C-2, C-2′), 81.67 (d, C-4″), 81.32 (d, C-2″), 80.72 (d, C-3″), 76.72 (d, C-7), 75.14 (t, CH2Ph), 74.50 (t, CH2Ph), 71.06 (d, C-6), 69.97 (d, C-5), 69.78 (d, C-6′), 68.88 (d, C-4), 68.67 (t, C-6″), 67.76 (d, C-7′), 66.11 (d, C-4′), 65.95 (d, C-5″), 65.51 (t, C-8), 64.23 (d, C-5′), 61.86 (t, C-8′), 53.16 and 52.68 (2 q, 2C, CO2CH3), 51.32 (q, OCH3), 34.12 (t, C-3), 32.40 (t, C-3′), 20.78, 20.64 and 20.62 (3 q, 4C, COCH3); ESI-TOF HRMS: m/z = 1142.4088; calcd for C55H64O25NH4+: 1142.4075.
3.5. Sodium (3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→4)-[α-d-glucopyranosyl-(1→5)]-sodium (methyl α-d-manno-oct-2-ulopyranosid)onate (10)
Trisaccharide 9α (11.3 mg, 0.010 mmol) was dissolved in dry MeOH (1.0 mL), the atmosphere was exchanged to argon and 10% Pd-C (1 mg) was added. The atmosphere was exchanged successively to argon and hydrogen (1 atm). After 4 h the catalyst was filtered off, rinsed with MeOH and the filtrate was concentrated. The residue was dried and then dissolved in anhydrous MeOH (1.0 mL) and treated with sodium methoxide (0.1 M in MeOH, 60 μL, 0.006 mmol) for 17 h at rt. Ion exchange resin DOWEX 50 (H+ form) was added until the solution reacted neutral. The resin was filtered off immediately, rinsed with MeOH and the filtrate was concentrated. A solution of the residue in H2O (1.0 mL) was treated with aq NaOH (0.1 M, 1.0 mL) at 0 °C for 4 h at ambient temperature. The mixture was made neutral by adding DOWEX 50 resin (H+-form). Filtration of the resin and freeze-drying of the filtrate afforded a salt-containing product, which was desalted on a PD10 SEC column (H2O). Lyophilisation of the pooled fractions yielded trisaccharide 10 (6.4 mg, 94%) as a colorless amorphous solid: [α]D20 + 114.9 (c 0.59, H2O); 1H NMR (D2O, pD ~ 7.4): δ 5.24 (d, 1H, J1″,2″ 3.6 Hz, H-1″), 4.19 - 4.17 (m, 1H, H-5), 4.08 - 4.03 (m, 2H, H-5″, H-7), 3.97 - 3.91 (m, 3H, H-4, H-5′, H-7′), 3.91 (ddd, 1H, J4′,5′ 3.0 Hz, H-4′), 3.89 - 3.86 (m, 2H, H-8a, H-8′a), 3.85 (dd, 1H, J6″a,6″b 12.2, J6″a,5″ 2.6 Hz, H-6″), 3.78 (app t, 1H, J3″,4″ 9.2 Hz, H-3″), 3.665 (br d, 1H, J6′,7′ 7.5 Hz, H-6′), 3.66 (dd, 1H, J8′a,8′b 11.8, J7′,8′b 7.2 Hz, H-8′b), 3.58 (dd, 1H, J8b,8a 11.8, J8b,7 6.5 Hz, H-8b), 3.51 (app t, 1H, J5″,4″ 9.7 Hz, H-4″), 3.50 (br d, 1H, J6,7 9.5 Hz, H-6), 3.46 (dd, 1H, J2″,3″ 9.9 Hz, H-2″), 3.07 (s, 3H, OCH3), 2.05 (dd, 1H, J3′eq,3′ax 13.2, J3′eq,4′ 4.7 Hz, H-3′eq), 2.01 (dd, 1H, J3eq,3ax 12.6, J3eq,4 4.8 Hz, H-3eq), 1.94 (app t, 1H, J3ax,4 12.3 Hz, H-3ax), 1.75 (app t, 1H, J3′ax,4′ 12.7 Hz, H-3′ax); 13C NMR (D2O, pD ~ 7.4): see Table 1; ESI-TOF HRMS: m/z = 657.1838; calcd for C23H38O20Na+: 657.1849.
3.6. Methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→4)-[2,3,6-tri-O-benzyl-α-d-glucopyranosyl-(1→5)]-methyl (methyl 7,8-O-carbonyl-3-deoxy-α-d-manno-oct-2-ulopyranosid)onate (11), methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→4)-[2,3,4-tri-O-benzyl-α-d-glucopyranosyl-(1→5)]-methyl (methyl 7,8-O-carbonyl-3-deoxy-α-d-manno-oct-2-ulopyranosid)onate (12) and methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→4)-[2,3-di-O-benzyl-α-d-glucopyranosyl-(1→5)]-methyl (methyl 7,8-O-carbonyl-3-deoxy-α-d-manno-oct-2-ulopyranosid)onate (13)
A cooled (0 °C) solution of compound 9α (188 mg, 0.167 mmol) in dry CH2Cl2 (6.0 mL) was treated with triethylsilane (400 μL, 2.506 mmol) and BF3·Et2O (41 μL, 0.334 mmol) successively. After 1 h at 0 °C the mixture was diluted with CH2Cl2 and washed with aq. NaHCO3. The aqueous phase was once again extracted with CH2Cl2 and the combined organic phases were washed with water, dried (MgSO4) and concentrated. The residue was purified on an HPLC column (n-hexane/EtOAc 3:2 → 1:2) providing some recovered starting material 9α (7 mg, 3%), the desired 6-OBn compound 11 (135 mg, 72%), the 4-OBn isomer 12 (10 mg, 5%) and the diol 13 (3 mg, 2%) successively. Data for 11: Colorless oil; [α]D20 + 102.6 (c 1.12, CHCl3); Rf 0.38 (n-hexane/EtOAc 1:1, HP-TLC); 1H NMR (CDCl3): δ 7.41 - 7.27 (m, 15H, Ar), 5.34 - 5.33 (m, 1H, H-5′), 5.23 (ddd, 1H, J4′,3′ax 12.6, J4′,3′eq 5.0, J4′,5′ 2.8 Hz, H-4′), 5.20 (ddd, 1H, J7′,6′ 9.4, J7′,8′b 4.4, J7′,8′a 2.8 Hz, H-7′), 5.10 (d, 1H, J1″,2″ 3.6 Hz, H-1″), 4.97 (d, 1H, J 11.4 Hz, CHHPh), 4.87 (d, 1H, J 11.4 Hz, CHHPh), 4.82 (d, 1H, J 10.8 Hz, CHHPh), 4.77 – 4.73 (m, 1H, H-7), 4.62 (dd, 1H, J8′a,8′b 12.1, J8′a,7′ 2.6 Hz, H-8′a), 4.59 (d, 1H, J 10.8 Hz, CHHPh), 4.55 (d, 1H, J 11.5 Hz, CHHPh), 4.48 (d, 1H, J 11.5 Hz, CHHPh), 4.49 - 4.46 (m, 1H, H-8a), 4.22 - 4.18 (m, 2H, H-4, H-8b), 4.10 (dd, 1H, J6′,5′ 1.7 Hz, H-6′), 4.06 (dd, 1H, H-8′b), 3.86 - 3.85 (m, 1H, H-5), 3.84 - 3.81 (m, 4H, H-5″, CO2CH3), 3.80 (s, 3H, CO2CH3), 3.76 (dd, 1H, J6″a,6″b 10.1, J6″a,5″ 3.8 Hz, H-6″a), 3.74 - 3.67 (m, 3H, H-3″, H-4″, H-6), 3.58 (dd, 1H, J6″b,5″ 4.5 Hz, H-6″b), 3.53 (dd, 1H, J2″,3″ 9.2 Hz, H-2″), 3.18 (s, 3H, OCH3), 2.62 (d, 1H, JOH,4″ 2.2 Hz, OH), 2.24 - 2.20 (m, 1H, H-3′eq), 2.12 (app t, 1H, J3ax,3eq ~ J3ax,4 12.2 Hz, H-3ax), 2.09 and 2.08 (2 s, each 3H, COCH3), 2.09 – 2.01 (m, 2H, H-3′ax, H-3eq), 1.99 and 1.91 (2 s, each 3H, COCH3); 13C NMR (CDCl3): δ 170.93, 170.30, 169.71 and 169.63 (4 s, 4C, COCH3), 168.36 and 167.46 (2 s, 2C, C-1, C-1′), 154.49 [s, O(C=O)O], 138.50, 137.90 and 137.74 (3 s, 3C, Ar), 128.65, 128.58, 128.41, 128.24, 128.00, 127.95, 127.92, 127.76 and 127.62 (9 d, 15C, Ar), 99.76 (d, C-1″), 99.12 and 99.04 (2 s, 2C, C-2, C-2′), 80.95 (d, C-3″), 80.12 (d, C-2″), 75.79 (d, C-5), 75.14 (t, CH2Ph), 74.97 (d, C-7), 74.15 (t, CH2Ph), 73.45 (t, CH2Ph), 72.22 (d, 2C, C-4″, C-6), 70.70 (d, C-5″), 70.10 (t, C-6″), 69.62 (d, C-6′), 68.70 (d, C-4), 67.82 (d, C-7′), 66.21 (t, C-8), 66.06 (d, C-4′), 64.29 (d, C-5′), 61.94 (t, C-8′), 53.12 (q, CO2CH3), 52.64 (q, CO2CH3), 51.20 (q, OCH3), 34.18 (t, C-3), 31.96 (t, C-3′), 20.69, 20.62 and 20.59 (3 q, 4C, COCH3); ESI-TOF HRMS: m/z = 1144.4239; calcd for C55H66O25NH4+: 1144.4231.
12: Colorless oil; [α]D20 + 110.8 (c 0.89, CHCl3); Rf 0.30 (n-hexane/EtOAc 1:1, HP-TLC); 1H NMR (CDCl3): δ 7.41 - 7.27 (m, 15H, Ar), 5.36 - 5.32 (m, 2H, H-4′, H-5′), 5.19 (ddd, 1H, J7′,6′ 9.4, J7′,8′b 4.1, J7′,8′a 2.8 Hz, H-7′), 5.09 (d, 1H, J1″,2″ 3.6 Hz, H-1″), 4.97 (d, 1H, J 11.2 Hz, CHHPh), 4.94 (d, 1H, J 11.2 Hz, CHHPh), 4.88 (d, 1H, J 10.7 Hz, CHHPh), 4.83 (d, 1H, J 10.8 Hz, CHHPh), 4.72 (d, 1H, J 10.6 Hz, CHHPh), 4.66 (dd, 1H, J8′a,8′b 12.3 Hz, H-8′a), 4.61 - 4.54 (m, 3H, H-7, H-8a, CHHPh), 4.32 (app t, 1H, J8b, 8a ~ J8b,7 7.6 Hz, H-8b), 4.18 (dd, 1H, J6′,5′ 1.3 Hz, H-6′), 4.12 (ddd, 1H, J4,3ax 12.0, J4,3eq 4.6, J4,5 2.5 Hz, H-4), 4.02 (dd, 1H, H-8′b), 3.86 (s, 3H, CO2CH3), 3.86 - 3.84 (m, 1H, H-5), 3.83 - 3.79 (m, 3H, H-3″, H-6″a, H-6″b), 3.78 (s, 3H, CO2CH3), 3.75 - 3.72 (m, 2H, H-5″, H-6), 3.67 (dd, 1H, J 9.8, J 9.1 Hz, H-4″), 3.54 (dd, 1H, J2″,3″ 9.6 Hz, H-2″), 3.16 (s, 3H, OCH3), 2.90 (app t, 1H, J 6.6 Hz, OH), 2.28 - 2.24 (m, 1H, H-3′eq), 2.16 -2.12 (m, 1H, H-3eq), 2.08 and 2.06 (2 s, each 3H, COCH3), 2.07 - 2.01 (m, 2H, H-3ax, H-3′ax), 2.00 and 1.99 (2 s, each 3H, COCH3); 13C NMR (CDCl3): δ 170.79, 170.52, 170.16 and 169.68 (4 s, 4C, COCH3), 168.27 and 167.44 (2 s, 2C, C-1, C-1′), 154.65 [s, O(C=O)O], 138.40, 137.91 and 137.70 (3 s, 3C, Ar), 128.70, 128.56, 128.51, 128.38, 128.19, 128.13, 127.99 and 127.83 (8 d, 15C, Ar), 99.89 (d, C-1″), 99.67 and 98.93 (2 s, 2C, C-2, C-2′), 81.50 (d, C-3″), 80.56 (d, C-2″), 77.72 (d, C-4″), 76.15 (d, C-5), 75.93 (d, C-7), 75.38 (t, CH2Ph), 75.35 (t, CH2Ph), 74.47 (t, CH2Ph), 72.57 (d, C-5″), 71.84 (d, C-6), 70.05 (d, C-4), 69.65 (d, C-6′), 67.80 (d, C-7′), 66.41 (d, C-4′), 65.88 (t, C-8), 64.36 (d, C-5′), 61.63 (t, C-8′), 61.07 (t, C-6″), 53.17 (q, CO2CH3), 52.67 (q, CO2CH3), 51.20 (q, OCH3), 33.81 and 32.53 (2 t, 2C, C-3, C-3′), 20.86, 20.63 and 20.59 (3 q, 4C, COCH3); ESI-TOF HRMS: m/z = 1149.3771; calcd for C55H66O25Na+: 1149.3785.
13: Colorless oil; Rf 0.06 (n-hexane/EtOAc 1:1); 1H NMR (CDCl3): δ 7.40 - 7.31 (m, 10H, Ar), 5.35 - 5.33 (m, 1H, H-5′), 5.31 (ddd, 1H, J4′,3′ax 12.1, J4′,3′eq 4.7, J4′,5′ 3.0 Hz, H-4′), 5.20 (ddd, 1H, J7′,6′ 9.4, J7′,8′b 4.2, J7′,8′a 2.8 Hz, H-7′), 5.13 (d, 1H, J1″,2″ 3.5 Hz, H-1″), 5.02 (d, 1H, J 11.5 Hz, CHHPh), 4.83 (d, 2H, J 11.3 Hz, CHHPh, CHHPh), 4.68 (ddd, 1H, J7,8b 8.3, J7,8a 5.7, J7,6 3.9 Hz, H-7), 4.65 (dd, 1H, J8′a,8′b 12.4 Hz, H-8′a), 4.60 (d, 1H, J 10.9 Hz, CHHPh), 4.57 (dd, 1H, J8a,8b 8.4 Hz, H-8a), 4.32 (app t, 1H, H-8b), 4.18 - 4.14 (m, 2H, H-4, H-6′), 4.04 (dd, 1H, H-8′b), 3.88 - 3.87 (m, 1H, H-5), 3.86 (s, 3H, CO2CH3), 3.80 (s, 3H, CO2CH3), 3.79 - 3.76 (m, 2H, H-6″a, H-6″b), 3.75 - 3.66 (m, 4H, H-3″, H-4″, H-5″, H-6), 3.56 - 3.51 (m, 1H, H-2″), 3.18 (s, 3H, OCH3), 2.85 (b s, 1H, OH), 2.44 (b s, 1H, OH), 2.27 (dd, 1H, J3′eq,3′ax 12.7 Hz, H-3′eq), 2.14 - 2.10 (m, 2H, H-3ax, H-3eq), 2.083 and 2.077 (2 s, each 3H, COCH3), 2.05 (app t, 1H, H-3′ax), 2.00 and 1.98 (2 s, each 3H, COCH3); 13C NMR (CDCl3): δ 170.83, 170.53, 170.15 and 169.68 (4 s, 4C, COCH3), 168.30 and 167.46 (2s, 2C, C-1, C-1′), 154.54 [s, O(C=O)O], 138.41 and 137.62 (2s, 2C, Ar), 128.73, 128.69, 128.39, 128.06, 128.03 and 127.81 (6 d, 10C, Ar), 99.71 (d, C-1″), 99.54 and 99.02 (2 s, 2C, C-2, C-2′), 81.21 (d, C-3″), 80.43 (d, C-2″), 75.85 (d, C-5), 75.41 (d, C-7), 75.12 (t, CH2Ph), 74.17 (t, CH2Ph), 72.41 (d, C-5″), 72.03 (d, C-6), 70.50 (d, C-4″), 69.76 and 69.66 (2 d, 2C, C-4, C-6′), 67.77 (d, C-7′), 66.40 (d, C-4′), 66.09 (t, C-8), 64.34 (d, C-5′), 61.88 (t, C-6″), 61.74 (t, C-8′), 53.20 (q, CO2CH3), 52.69 (q, CO2CH3), 51.24 (q, OCH3), 33.94 (t, C-3), 32.40 (t, C-3′), 20.86, 20.64 and 20.59 (3 q, 4C, COCH3); ESI-TOF HRMS: m/z = 1059.3303; calcd for C48H60O25Na+: 1059.3316.
3.7. Methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→4)-[2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl-(1→4)-2,3,6-tri-O-benzyl-α-d-glucopyranosyl-(1→5)]-methyl (methyl 7,8-O-carbonyl-3-deoxy-α-d-manno-oct-2-ulopyranosid)onate (15)
A solution of acceptor 11 (24.8 mg, 0.022 mmol) and donor 1421 (22.9 mg, 0.044 mmol) in dry CH2Cl2 (1.0 mL) containing 5 Å molecular sieves (50 mg) was stirred at ambient temperature for 1 h. The cooled (0 °C) mixture was treated with TfOH (0.10 μL, 1.1 μmol) in dry CH2Cl2 (20 μL) and stirred for 15 min at 0°C. The promotor was neutralized with triethylamine (15 μL, 0.110 mmol), the suspension was filtered over Celite®, rinsed with CH2Cl2 and the filtrate was concentrated. The residue was subjected to HP-chromatography (toluene/EtOAc 2:1 → 1:2) affording impure product fractions which were further purified by HPLC (n-hexane/EtOAc 2:1 → 1:2) to obtain pure 15 (14.7 mg, 46%) as a colorless oil: [α]D20 + 93.5 (c 0.50, CHCl3); Rf 0.30 (toluene/EtOAc 1:1, HP-TLC); 1H NMR (CDCl3): δ 7.44 - 7.18 (m, 15H, Ar), 5.36 - 5.34 (m, 1H, H-5′), 5.26 (ddd, 1H, J4′,3′ax 12.5, J4′,3′eq 5.0, J4′,5′ 2.9 Hz, H-4′), 5.21 (ddd, 1H, J7′,6′ 9.2, J7′,8′b 4.1, J7′,8′a 2.7 Hz, H-7′), 5.07 (d, 1H, J1″,2″ 3.8 Hz, H-1″), 5.05 (d, 1H, J 11.5 Hz, CHHPh), 5.01 (app t, 1H, J4‴,3‴ ~ J4‴,5‴ 9.5 Hz, H-4‴), 4.94 (app. t, 1H, J3‴,2‴ 9.2 Hz, H-3‴), 4.92 - 4.89 (m, 1H, H-2‴), 4.75 - 4.71 (m, 3H, 2 × CHHPh, CHHPh), 4.68 (app td, 1H, J7,8b 8.1, J7,6 ~ J7,8a 5.0 Hz, H-7), 4.61 (dd, 1H, J8′a,8′b 12.4 Hz, H-8′a), 4.50 (d, 1H, J 10.9 Hz, CHHPh), 4.48 (d, 1H, J1‴,2‴ 7.7 Hz, H-1‴), 4.45 (dd, 1H, J8a,8b 8.5, J8a,7 5.5 Hz, H-8a), 4.31 (d, 1H, J 11.6 Hz, CHHPh), 4.20 (ddd, 1H, J 9.7, J 7.0, J4,5 2.5 Hz, H-4), 4.14 (dd, 1H, J6″a,5″ 4.3, J6″a,6″b 12.5 Hz, H-6″a), 4.12 (br, d, 1H, J6′,7′ 9.2 Hz, H-6′), 4.11 (app t, 1H, H-8b), 4.07 (dd, 1H, H-8′b), 3.94 (app t, 1H, J4″,3″ ~ J4″,5″ 9.5 Hz, H-4″), 3.90 (dd, 1H, J6‴b,6‴a 12.2, J6‴,5‴ 2.3 Hz, H-6‴b), 3.85 - 3.84 (m, 1H, H-5), 3.82 (s, 3H, CO2CH3), 3.81 (dd, 1H, J6″a,6″b 10.5, J6″a,5″ 2.5 Hz, H-6″a), 3.81 (s, 3H, CO2CH3), 3.68 (app t, 1H, H-3″), 3.67 - 3.65 (m, 2H, H-5″, H-6), 3.53 (dd, 1H, J6″b,5″ 1.1 Hz, H-6″b), 3.48 (dd, 1H, J2″,3″ 9.8 Hz, H-2″), 3.29 (ddd, 1H, J5‴,6‴a 4.2 Hz, H-5‴), 3.19 (s, 3H, OCH3), 2.25 (dd, 1H, J3′eq,3′ax 13.0 Hz, H-3′eq), 2.10 (app t, 1H, H-3′ax), 2.095 and 2.08 (2 s, each 3H, COCH3), 2.04 – 2.01 (m, 2 H, H-3ax, H-3eq), 2.01, 1.99, 1.98, 1.97, 1.94 and 1.90 (6 s, each 3H, COCH3); 13C NMR (CDCl3): δ 170.92, 170.59, 170.33, 170.16, 169.91, 169.62, 169.34 and 169.10 (8 s, 8C, COCH3), 168.36 and 167.56 (2 s, 2C, C-1, C-1′), 154.57 [s, O(C=O)O], 139.00, 137.81 and 137.76 (3 s, 3C, Ar), 128.73, 128.54, 128.31, 128.23, 128.18, 127.56 and 127.48 (7 d, 15C, Ar), 100.10 (d, C-1″), 99.97 (d, C-1‴), 99.07 and 98.97 (2 s, 2C, C-2, C-2′), 79.68 (d, C-2″), 79.62 (d, C-3″), 77.0 (d, C-4″, overlapped by solvent peak), 76.16 (d, C-5), 75.06 (d, C-7), 75.03 (t, CH2Ph), 74.52 (t, CH2Ph), 73.49 (t, CH2Ph), 73.19 (d, C-3‴), 72.26 (d, C-6), 71.90 (d, C-2‴), 71.46 (d, C-5‴), 71.35 (d, C-5″), 69.66 (d, C-6′), 68.55 (d, C-4), 68.21 (d, C-4‴), 67.85 (d, C-7′), 67.75 (t, C-6″), 66.06 (t, C-8), 66.03 (d, C-4′), 64.25 (d, C-5′), 61.92 (t, C-8′), 61.66 (t, C-6‴), 53.17 and 52.65 (2 q, 2C, CO2CH3), 51.22 (q, OCH3), 34.28 (t, C-3), 31.91 (t, C-3′), 20.72, 20.63, 20.59 and 20.56 (4 q, 8C, COCH3); ESI-TOF HRMS: m/z = 1479.4740; calcd for C69H84O34Na+: 1479.4736.
3.8. Sodium (3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→4)-[β-d-glucopyranosyl-(1→4)-α-d-glucopyranosyl-(1→5)]-sodium (methyl α-d-manno-oct-2-ulopyranosid)onate (16)
According to the procedure for compound 10, tetrasaccharide 15 (14.0 mg, 0.010 mmol) was debenzylated in dry MeOH (1.0 mL) over 10% Pd-C (1 mg) under hydrogen at atmospheric pressure for 3 h at rt, and deacetylated by treatment with sodium methoxide (0.1 M in MeOH, 24 μL, 2.4 μmol) in dry MeOH (1.0 mL) for 14 h at rt. A solution of the deblocked intermediate in H2O (2 mL) and aq NaOH (0.1 M, 0.5 mL) was stirred for 2 h at 0 °C and for 1 h at ambient temperature followed by neutralization with Dowex resin and processing as described for 10. The filtrate was lyophilized and the residue was desalted on a PD10 SEC column (H2O) to give 16 (8.0 mg, 99%) as a colorless amorphous solid: [α]D20 + 91.4 (c 0.56, H2O); 1H NMR (D2O, pD ~ 7.4): δ 5.25 (d, 1H, J1″,2″ 3.5 Hz, H-1″), 4.49 (d, 1H, J1‴,2‴ 7.7 Hz, H-1‴), 4.25 - 4.20 (m, 2H, H-5, H-5″), 4.03 (ddd, 1H, J7,6 9.4, J7,8b 6.4, J7,8a 2.9 Hz, H-7), 3.97 - 3.84 (m, 10H, H-3″, H-4, H-4′, H-5′, H-6″a, H-6″b, H-6‴a, H-7′, H-8a, H-8′a), 3.71 (app t, 1H, J4″,3″ ~ J4″,5″ 9.7 Hz, H-4″), 3.70 (dd, 1H, J6‴b,6‴a 12.1, J6‴b,5‴ 6.1 Hz, H-6‴b), 3.67 (d, 1H, J6′,7′ 8.2 Hz, H-6′), 3.66 (dd, 1H, J8′b,8′a 11.6, J8′b,7′ 7.3 Hz, H-8′b), 3.59 (dd, 1H, J8b,8a 11.9, H-8b), 3.52 (dd, 1H, J2″,3″ 10.3 Hz, H-2″), 3.51 (d, J6,7 10.3 Hz, H-6), 3.49 - 3.44 (m, 2H, H-3‴, H-5‴), 3.38 (app t, 1H, J4‴,3‴ ~ J4‴,5‴ 9.5 Hz, H-4‴), 3.28 (dd, 1H, J2‴,3‴ 9.3 Hz, H-2‴), 3.08 (s, 3H, OCH3), 2.07 - 1.99 (m, 2H, H-3eq, H-3′eq), 1.95 (app t, 1H, J3ax,3eq ~ J3ax,4 12.3 Hz, H-3ax), 1.76 (app t, J3′ax,3′eq ~ J3′ax,4′ 12.5 Hz, H-3′ax); 13C NMR (D2O, pD ~ 7.4): see Table 1; ESI-TOF HRMS: m/z = 819.2375; calcd for C29H48O25Na+: 819.2377.
3.9. 2,3,4,6-Tetra-O-acetyl-α-d-glucopyranosyl-(1→6)-2,3,4-tri-O-acetyl-α,β-d-glucopyranosyl (N-phenyl)trifluoroacetimidate (17)
A solution of isomaltose (100 mg, 0.292 mmol) in dry pyridine (2.0 mL) was treated with acetic anhydride (0.8 mL) and 4-(N,N-dimethylamino)pyridine (5 mg) at 0 °C and after 20 h at ambient temperature, excessive reagent was destroyed by adding dry MeOH (2.0 mL) at 0 °C. After 10 min the solvent was coevaporated with toluene (3 ×) and the residual oil was purified by chromatography (n-hexane/EtOAc 1:1 → 1:2) affording an α/β-mixture (1:1) of peracetylated isomaltose (198 mg, quantitative yield). The residue (101 mg, 0.149 mmol) was dissolved in dry DMF (2.0 mL) and treated with hydrazine acetate (27 mg, 0.298 mmol) at 40 °C for 2 h under Ar. The mixture was diluted with EtOAc, washed with cold brine and the organic layer was dried (MgSO4) and filtered. Concentration of the filtrate provided a crude product which was purified by chromatography (n-hexane/EtOAc 1:2) to obtain an α/β-mixture (1:0.3) of the hemiacetal (85 mg, 90%): 1H NMR (CDCl3): δ 5.53 (dd, 1H, J3α,2α 10.1, J3α,4α 9.4 Hz, H-3α), 5.46 (app t, 1H, J3′β,2′β ~ J3′β,4′β 9.8 Hz, H-3′β), 5.45 (app t, 1H, J3′α,2′α ~ J3′α,4′α 9.8 Hz, H-3′α), 5.40 (app t, 1H, J1α,2α ~ J1α,OH 3.5 Hz, H-1α), 5.23 (app t, 1H, J3β,2β ~ J3β,4β Hz, H-3β), 5.14 (d, 1H, J1′β,2′β 3.8 Hz, H-1′β), 5.13 (d, 1H, J1′α,2′α 3.8 Hz, H-1′α), 5.02 - 4.97 (m, 3H, H-4′α, H-4β, H-4α), 4.94 (dd, 1H, J4α,5α 10.3 Hz, H-4α), 4.85 - 4.78 (m, 4H, H-2α, H-2′α, H-2β, H-2′β), 4.73 (app t, 1H, J 8.0 Hz, H-1β), 4.24 (ddd, 1H, J5α,6aα 6.5, J5α,6bα 2.5 Hz, H-5α), 4.21 - 4.05 (m, 7H, H-5′α, H-5′β, H-6′aα, H-6′aβ, H-6′bα, H-6′bβ, OHβ), 4.02 - 3.99 (m, 1H, OHα), 3.74 - 3.57 (m, 5H, H-5β, H-6aα, H-6aβ, H-6bα, H-6bβ), 2.10 to 2.00 (10 s, 14 × COCH3); ESI-TOF HRMS: m/z = 659.1794; calcd for C26H36O18Na+: 659.1798.
The residue (70 mg, 0.110 mmol) was dissolved in dry CH2Cl2 (1.8 mL) and dry acetone (1.0 mL). Potassium carbonate (30 mg, 0.220 mmol) and 2,2,2-trifluoro-N-phenylacetimidoyl chloride (35 μL, 0.220 mmol) were added successively under Ar. The suspension was stirred for 12 h at rt, then filtered through a pad of Celite, rinsed with CH2Cl2 and the filtrate was concentrated. Swift chromatographic purification (n-hexane/EtOAc 1:1) provided 17 (80 mg, 90%) which was directly used for the subsequent glycosylation reaction. 1H NMR spectra of 17 (CDCl3) displayed broad signals corresponding to a 2.5:1 mixture; 13C NMR (125 MHz, CDCl3, selected data): δ 169.8-168.9 (C=O, Ac), 142.8, 128.9, 128.8 124.7 and 119.4 (arom. C), 96.0 (C-1, C-1′), 66.4 and 66.2 (C-6), 61.7 (C-6′), 20.6-20.4 (CH3CO).
3.10. Methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→4)-[2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl-(1→6)-2,3,4-tri-O-acetyl-β-d-glucopyranosyl-(1→4)-2,3,6-tri-O-benzyl-α-d-glucopyranosyl-(1→5)]-methyl (methyl 7,8-O-carbonyl-3-deoxy-α-d-manno-oct-2-ulopyranosid)onate (18)
A suspension of acceptor 11 (20.0 mg, 0.018 mmol) and donor 17 (28.7 mg, 0.035 mmol) in dry CH2Cl2 (1.0 mL) containing 5 Å molecular sieves (50 mg) was stirred at ambient temperature for 1 h. The cooled (0 °C) mixture was treated with TfOH (0.04 μL, 0.44 μmol) in dry CH2Cl2 (10 μL) and stirred for 30 min at 0°C. An additional portion of TfOH (0.16 μL, 1.76 μmol) was added (0 °C) and after 15 min triethylamine (12.3 μL, 0.089 mmol) was added. The mixture was filtered over Celite, rinsed with CH2Cl2 and the filtrate was concentrated. The residue was subjected to HP-chromatography (toluene/EtOAc 2:1 → 1:2) affording impure product fractions which were further purified by HPLC (n-hexane/EtOAc 1:1 → 1:2) to give pure 18 (22.0 mg, 71%) as a colorless oil: [α]D20 + 105.8 (c 1.10, CHCl3); Rf 0.16 (n-hexane/EtOAc 1:1, HP-TLC); 1H NMR (CDCl3): δ 7.44 - 7.25 (m, 15H, Ar), 5.38 (app t, 1H, J3″″,2″″ ~ J3″″,4″″ 9.7 Hz, H-3″″), 5.34 - 5.33 (m, 1H, H-5′), 5.24 (ddd, 1H, J4′,3′ax 12.6, J4′,3′eq 4.9, J4′,5′ 2.9 Hz, H-4′), 5.20 (ddd, 1H, J7′,6′ 9.3, J7′,8′b 4.3, J7′,8′a 2.8 Hz, H-7′), 5.03 (d, 1H, J1″,2″ 3.9 Hz, H-1″), 5.02 (app t, 1H, J4″″,5″″ 9.7 Hz, H-4″″), 5.00 (app t, 1H, J3‴,2‴ ~ J3‴,4‴ 9.3 Hz, H-3‴), 4.98 (d, 1H, J1″″,2″″ 3.4 Hz, H-1″″), 4.92 (app t, 1H, J4‴,5‴ 9.5 Hz, H-4‴), 4.90 - 4.87 (m, 2H, H-2‴, CHHPh), 4.83 - 4.80 (m, 2H, H-2″″, CHHPh), 4.79 (d, 1H, J 11.7 Hz, CHHPh), 4.69 (d, 1H, J 11.7 Hz, CHHPh), 4.67 (ddd, 1H, J7,8b 8.3, J7,8a 5.4, J7,6 4.6 Hz, H-7), 4.60 (dd, 1H, J8′a,8′b 12.3 Hz, H-8′a), 4.55 (d, 1H, J 11.0 Hz, CHHPh), 4.51 (d, 1H, J1‴,2‴ 8.0 Hz, H-1‴), 4.42 (dd, 1H, J8a,8b 8.6 Hz, H-8a), 4.29 (d, 1H, J 11.8 Hz, CHHPh), 4.21 (dd, 1H, J6″″a,6″″b 12.3, J6″″a,5″″ 4.0 Hz, H-6″″a), 4.19 (ddd, 1H, J4,3ax 11.3, J4,3eq 5.5, J4,5 2.5 Hz, H-4), 4.11 (dd, 1H, J6′,5′ 1.4 Hz, H-6′), 4.08 - 4.04 (m, 2H, H-8b, H-8′b), 3.96 (dd, 1H, J6″″b,5″″ 2.3 Hz, H-6″″b), 3.94 (app t, 1H, J4″,3″ ~ J4″,5″ 9.5 Hz, H-4″), 3.88 (ddd, 1H, H-5″″), 3.84 - 3.83 (m, 1H, H-5), 3.82 (s, 3H, CO2CH3), 3.80 (s, 3H, CO2CH3), 3.78 (dd, 1H, J6″a,6″b 10.8, J6″a,5″ 2.8 Hz, H-6″a), 3.67 - 3.63 (m, 3H, H-3″, H-5″, H-6), 3.49 (dd, 1H, J6″b,5″ 1.5 Hz, H-6″b), 3.46 (dd, 1H, J2″,3″ 9.7, H-2″), 3.42 (dd, 1H, J6‴a,6‴b 11.6, J6‴a,5‴ 5.4 Hz, H-6‴a), 3.35 (dd, 1H, J6‴b,5‴ 4.2 Hz, H-6‴b), 3.30 - 3.26 (m, 1H, H-5‴), 3.18 (s, 3H, OCH3), 2.22 - 2.18 (m, 1H, H-3′eq), 2.11 (app t, 1H, J3′ax,3′eq 13.1 Hz, H-3′ax), 2.07 - 1.99 (m, 2H, H-3eq, H-3ax), 2.09, 2.08, 2.06, 2.02, 2.01, 2.00, 1.99, 1.98, 1.96 and 1.89 (10 s, 33H, COCH3); 13C NMR (CDCl3): δ 170.89, 170.48, 170.31, 170.14, 169.94, 169.91, 169.90, 169.60, 169.50, 169.45 and 169.16 (11 s, 11C, COCH3), 168.38 and 167.55 (2 s, 2C, C-1, C-1′), 154.58 [s, O(C=O)O], 138.81, 137.86 and 137.83 (3 s, 3C, Ar), 128.71, 128.60, 128.33, 128.24, 128.19, 128.14, 127.99 and 127.53 (8 d, 15C, Ar), 100.15 (d, C-1″), 99.76 (d, C-1‴), 99.09 and 98.95 (2 s, 2C, C-2, C-2′), 96.12 (d, C-1″″), 79.71 (d, C-2″), 79.33 (d, C-3″), 76.88 (d, C-4″), 76.07 (d, C-5), 75.09 (d, C-7), 75.01 (t, CH2Ph), 74.58 (t, CH2Ph), 73.53 (t, CH2Ph), 73.24 (d, C-3‴), 72.51 (d, C-5‴), 72.27 (d, C-6), 71.89 (d, C-2‴), 71.36 (d, C-5″), 70.45 (d, C-2″″), 70.01 (d, C-3″″), 69.80 (d, C-4‴), 69.64 (d, C-6′), 68.60 (d, C-4), 68.28 (d, C-4″″), 68.12 (t, C-6‴), 68.04 (t, C-6″), 67.84 (d, C-7′), 67.64 (d, C-5″″), 66.03 (t, C-8), 66.01 (d, C-4′), 64.23 (d, C-5′), 61.92 (t, C-8′), 61.67 (t, C-6″″), 53.14 (q, CO2CH3), 52.60 (q, CO2CH3), 51.19 (q, OCH3), 34.23 (t, C-3), 31.90 (t, C-3′), 20.68, 20.64, 20.62, 20.59, 20.56 and 20.55 (6 q, 11C, COCH3); ESI-TOF HRMS: m/z = 1762.6029; calcd for C81H100O42NH4+: 1762.6027.
3.11. Sodium (3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→4)-[α-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl-(1→4)-α-d-glucopyranosyl-(1→5)]-sodium (methyl α-d-manno-oct-2-ulopyranosid)onate (19)
According to the procedure for compound 10, pentasaccharide 18 (12.0 mg, 0.007 mmol) was debenzylated in dry MeOH (1.0 mL) over 10% Pd-C (1 mg) under an atmosphere of hydrogen (1 atm) for 4 h at rt, and deacetylated by treatment with sodium methoxide (0.1 M in MeOH, 100 μL, 10.0 μmol; 3 portions) in dry MeOH (1.0 mL) for 20 h at rt. Workup of the solution as described for 10 afforded a residue which was dissolved in H2O (2 mL) and treated with aq NaOH (0.1 M, 1.0 mL) for 3 h at 0 °C followed by neutralization and freeze-drying. The residue was desalted on a PD10 column (H2O) and lyophilisation of the pooled fractions provided compound 19 (6.2 mg, 90%) as a colorless amorphous solid: [α]D20 + 100.8 (c 0.43, H2O); 1H NMR (D2O, pD ~ 7.4): δ 5.25 (d, 1H, J1″,2″ 3.7 Hz, H-1″), 4.94 (d, 1H, J1″″,2″″ 3.8 Hz, H-1″″), 4.52 (d, 1H, J1‴,2‴ 8.0 Hz, H-1‴), 4.24 (app td, 1H, J5″,4″ 10.1, J5″,6″a ~ J5″,6″b 2.4 Hz, H-5″), 4.21 (d, 1H, J5,6 2.2 Hz, H-5), 4.03 (ddd, 1H, J7,6 9.4, J7,8b 6.3, J7,8a 3.0 Hz, H-7), 3.97 - 3.75 (m, 13H, H-3″, H-3″″, H-4, H-4′, H-5′, H-6″a, H-6″b, H-6‴a, H-6‴b, H-6″″a, H-7′, H-8a, H-8′a), 3.73 - 3.63 (m, 6H, H-4″, H-5‴, H-5″″, H-6′, H-6″″b, H-8′b), 3.58 (dd, 1H, J8′b,8′a 11.9 Hz, H-8b), 3.54 - 3.44 (m, 5H, H-2″, H-2″″, H-3‴, H-4‴, H-6), 3.37 (app t, 1H, J4″″,3″″ ~ J4″″,5″″ 9.5 Hz, H-4″″), 3.29 (app t, J2‴,3‴ ~ 8.7 Hz, H-2‴), 3.07 (s, 3H, OCH3), 2.06 - 1.99 (m, 2H, H-3eq, H-3′eq), 1.94 (app t, 1H, J3ax,3eq ~ J3ax,4 12.1 Hz, H-3ax), 1.76 (app t, 1H, J3′ax,3′eq ~ J3′ax,4′ 12.5 Hz, H-3′ax); 13C NMR (D2O, pD ~ 7.4): see Table 1; ESI-TOF HRMS: m/z = 981.2906; calcd for C35H58O30Na+: 981.2095.
Acknowledgments
The authors thank the Austrian Science Fund FWF for financial support (grant P 24921) and Dr. Andreas Hofinger for recording the NOESY spectrum.
References
- 1.Beutler B, Rietschel ETH. Nature Rev. Immun. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 2.Raetz CRH, Whitfield C. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Holst O. In: Bacterial Lipopolysaccharides. Knirel Y, Valvano MA, editors. Springer-Verlag; Vienna: 2011. pp. 21–39. [Google Scholar]; b) Holst O. FEMS Microbiol. Lett. 2007;271:3–11. doi: 10.1111/j.1574-6968.2007.00708.x. [DOI] [PubMed] [Google Scholar]
- 4.Knirel YA. In: Bacterial Lipopolysaccharides. Knirel YA, Valvano MA, editors. Springer-Verlag; Vienna: 2011. pp. 41–115. [Google Scholar]
- 5.(a) Unger FM. Adv. Carbohydr. Chem. Biochem. 1981;38:323–388. [Google Scholar]; (b) Brade H, Rietschel ET. Eur J. Biochem. 1984;145:231–236. doi: 10.1111/j.1432-1033.1984.tb08543.x. [DOI] [PubMed] [Google Scholar]; (c) Paulsen H, Stiem M, Unger FM. Tetrahedron Lett. 1986;27:1135–1138. [Google Scholar]
- 6.(a) Kawahara K, Brade H, Rietschel ET, Zähringer U. Eur. J. Biochem. 1987;163:489–495. doi: 10.1111/j.1432-1033.1987.tb10895.x. [DOI] [PubMed] [Google Scholar]; (b) Zähringer U, Kawahara K, Kosma P. Carbohydr. Res. 2013;378:63–70. doi: 10.1016/j.carres.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Holst O, Molinaro A. In: Microbial Glycobiology. Moran AP, Holst O, Brennan PJ, von Itzstein M, editors. Elsevier; Amsterdam: 2009. pp. 29–55. [Google Scholar]
- 8.(a) Masoud H, Perry MB, Brisson J-R, Uhrin D, Richards JC. Can. J. Chem. 1994;72:1466–1477. [Google Scholar]; (b) Cox AD, Michael F. St., Cairns CM, Lacelle S, Filion AL, Neelamegan D, Wenzel CQ, Horan H, Richards JC. Glycoconjugate J. 2011;28:165–182. doi: 10.1007/s10719-011-9332-7. [DOI] [PubMed] [Google Scholar]
- 9.Zähringer U, Lindner B, Knirel YA, Van der Akker WM, Hiestand R, Heine H, Dehio C. J. Biol. Chem. 2004;279:21046–21054. doi: 10.1074/jbc.M313370200. [DOI] [PubMed] [Google Scholar]
- 10.(a) Vinogradov EV, Müller-Loennies S, Petersen BO, Meshkov S, Thomas-Oates JE, Holst O, Brade H. Eur. J. Biochem. 1997;247:82–90. doi: 10.1111/j.1432-1033.1997.00082.x. [DOI] [PubMed] [Google Scholar]; (b) Vinogradov EV, Bock K, Petersen BO, Holst O, Brade H. Eur. J. Biochem. 1997;243:122–127. doi: 10.1111/j.1432-1033.1997.0122a.x. [DOI] [PubMed] [Google Scholar]
- 11.Müller-Loennies S. personal communication. [Google Scholar]
- 12.Ekelöf K, Oscarson S. Carbohydr. Res. 1995;278:289–300. doi: 10.1016/0008-6215(95)00269-3. [DOI] [PubMed] [Google Scholar]
- 13.Pokorny B, Müller-Loennies S, Kosma P. Carbohydr. Res. 2014;391:66–81. doi: 10.1016/j.carres.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulsen H, Heitmann AC. Liebigs Ann. Chem. 1989:655–663. [Google Scholar]
- 15.Yi R, Ogaki A, Fukunaga M, Nakajima H, Ichiyanagi T. Tetrahedron. 2014;70:3675–3682. [Google Scholar]
- 16.(a) Pokorny B, Kosma P. Chem. Eur. J. 2015;21:305–313. doi: 10.1002/chem.201405424. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pokorny B, Kosma P. Org. Lett. 2015;17:110–113. doi: 10.1021/ol5033128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulsen H, Brenken M. Liebigs Ann. Chem. 1991:1113–1126. [Google Scholar]
- 18.(a) Boivin J, Quiclet-Sire B, Ramos L, Zard SZ. Chem. Commun. 1997;4:353–354. [Google Scholar]; (b) Quiclet-Sire B, Zard SZ. J. Am. Chem. Soc. 1996;118:9190–9191. [Google Scholar]
- 19.Crich D, Cai W. J. Org. Chem. 1999;64:4926–4930. doi: 10.1021/jo990243d. [DOI] [PubMed] [Google Scholar]
- 20.For examples see; a) Eby R, Schuerch C. Carbohydr. Res. 1974;34:79–90. [Google Scholar]; b) Wulff G, Rohle G. Angew. Chem. Int. Ed. 1974;13:157–170. doi: 10.1002/anie.197401571. [DOI] [PubMed] [Google Scholar]; c) Manabe S, Ito Y, Ogawa T. Synlett. 1988:628–630. [Google Scholar]; d) Demchenko A, Stauch T, Boons GJ. Synlett. 1977:818–820. [Google Scholar]; e) Satoh H, Hansen HS, Manabe S, van Gunsteren WF, Hünenberger PH. J. Chem. Theory Comp. 2010;6:1783–1797. doi: 10.1021/ct1001347. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt RR, Töpfer A. Tetrahedron Lett. 1991;32:3353–3356. [Google Scholar]
- 22.Debenham SD, Toone EJ. Tetrahedron: Asymmetry. 2000;11:385–387. [Google Scholar]
- 23.Thomas M, Gesson J-P, Papot S. J. Org. Chem. 2007;72:4262–4264. doi: 10.1021/jo0701839. [DOI] [PubMed] [Google Scholar]