Abstract
A scalable approach towards high-yielding and (stereo)selective glycosyl donors of the 2-ulosonic acid Kdo (3-deoxy-d-manno-oct-2-ulosonic acid) is a fundamental requirement for the development of vaccines against Gram-negative bacteria. Herein we disclose a short route to generate 3-iodo Kdo fluoride donors from Kdo glycal esters, which enable efficient α-specific glycosylations and significantly suppress the elimination side reaction. The potency of these donors is demonstrated in a straightforward, six-step access to a branched Chlamydia-related Kdo-trisaccharide ligand without the need of protecting groups at the Kdo glycosyl acceptor. The approach was further extended to include sequential iteration of the basic concept to produce the linear Chlamydia-specific α-Kdo-(2→8)-α-Kdo-(2→4)-α-Kdo trisaccharide in a good overall yield.
Keywords: glycosylation, lipopolysaccharide, Kdo, oligosaccharides, Chlamydia
Introduction
Lipopolysaccharides (LPS) are amphiphilic glycolipids located in the outer leaflet of the cell membrane of Gram-negative bacteria, which are of high biomedical relevance due to their interaction with components of the innate and adaptive immune system of their respective hosts.[1] In structural terms, enterobacterial LPS displays a tripartite architecture comprising the endotoxically active diglucosamine unit (Lipid A) followed by a core region and the O-antigenic polysaccharide chain.[2] 3-Deoxy-α-d-manno-oct-2-ulosonic acid (α-Kdo) serves as the common linkage sugar to Lipid A and constitutes the structurally conserved α-Kdo-(2→4)-α-Kdo disaccharide core domain. This basic Kdo unit is being extended by additional Kdo residues in the LPS of Chlamydiaceae, a biomedically important obligate intracellular parasite.[3] The genus Chlamydia trachomatis is a leading cause of sexually transmitted diseases, eventually resulting in infertility in women, but is also responsible for conjunctivitis and secondary blindness.[4] The second genus harboring the species Chlamydophila psittaci and Chlamydophila pneumoniae is involved in pulmonary infections and the latter species has additionally been linked to chronic infections such as arthritis and atherosclerosis.[5] All Chlamydiae share a truncated lipopolysaccharide wherein the Kdo part forms an immunodominant, family-specific trisaccharide epitope of the sequence α-Kdo-(2→8)-α-Kdo-(2→4)-α-Kdo.[6] In addition to this group-specific antigen, a second linear trisaccharide α-Kdo-(2→4)-α-Kdo-(2→4)-α-Kdo and the branched tetrasaccharide α-Kdo-(2→4)[α-Kdo-(2→8)]-α-Kdo-(2→4)-α-Kdo constitute further antigenic epitopes in the Chl. psittaci.[7] A similarly branched Kdo trisaccharide has also been identified in the core region of Acinetobacter lwoffii F78 LPS.[8] Recently it has been shown, that these Kdo residues are involved in important binding interactions with proteins, and insight into the recognition of Kdo saccharides by monoclonal antibodies and Toll-like receptor-4 (TLR-4) has been achieved at the molecular level.[9] Thus, the chemical synthesis of Kdo glycosides is an attractive goal in the field of (bio)organic chemistry in order to provide immunoreagents and antigens for the development of synthetic carbohydrate-based vaccines and diagnostics.[10]
Glycosylation reactions of 3-deoxy-2-ulosonic acid donors are challenging due to the lack of a stereodirecting neighboring group at C-3, the deactivation of the anomeric center by the adjacent carboxyl group, the high propensity of the glycosyl donors towards elimination reactions - leading to glycal ester derivatives (e.g. 3, Scheme 1) - and the acid sensitivity of the ketosidic linkage.[11] To enhance stereoselectivity and coupling yields of glycoside formation, different approaches have been reported which were mainly based on optimization of dedicated protecting and leaving groups. Thus, electron-donating (“arming”) protecting groups such as silyl ether, benzyl or isopropylidene groups have mostly been used in order to counteract the deactivating effect of the carboxyl group, while halides, thioalkyl, thioaryl, phosphites, and trihaloacetimidates served as leaving groups.[12] In particular, suitably protected Kdo-fluoride donors have been reported in the literature to afford good to moderate α/β-stereoselectivity in the outcome of glycosylation reactions.[13] Still, in most cases a large excess of donor is required to compensate for losses due to the elimination side reaction and the formation of anomeric mixtures eventually requires separation of the undesired anomer (and the glycal ester) by extensive chromatography.
Scheme 1.
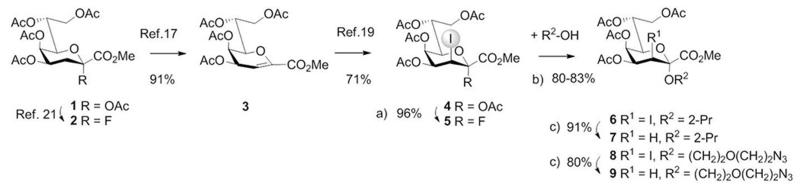
Synthesis of stereodirecting 3-iodo-Kdo fluoride donor 5 and Kdo glycosides. a) HF-pyridine, CH2Cl2; b) BF3.Et2O, CH2Cl2, mol. sieves 3 Å; c) lauroyl peroxide, cyclohexane.
In order to improve the selectivity in the glycosylation reaction in favor of the axial α-Kdo glycoside, temporary auxiliary groups such as 3-iodo, 3-thio- and 3-selenylphenyl groups have previously been introduced via addition reactions to Kdo glycal esters.[14] These groups provide anchimeric assistance and have to be removed after the coupling step. Recently, also direct iodonium-ion induced activation of Kdo glycal esters to give glycosides has been reported. Activation of a 4,5-O-isopropylidene-protected Kdo glycal ester by N-iodosuccinimide (NIS)/TMSOTf preferentially afforded β-linked Kdo oligosaccharides.[15] Activation towards α-configured Kdo spacer glycosides has been accomplished using the acetylated glycal ester 3 in the presence of an excess of triflic acid.[14a] Recently, iodoalkoxylation of a perbenzylated Kdo glycal ester was employed for sequential assembly of α-(2→8)-linked Kdo-oligosaccharides.[16]
However, highly activated primary hydroxy groups of perbenzylated open-chain glycosyl acceptors were necessary for this method and thus limited to the (2→8)-linkage, and stoichiometric amounts of triflic acid as promoter were needed to activate the benzylated glycal derivatives. Despite all these achievements, a versatile and α-selective Kdo donor suitable for efficient and scalable synthesis is still needed. Herein we disclose a short route to novel 3-iodo-Kdo fluoride donors and their efficient application in Chlamydia LPS ligand assembly.
Results and discussion
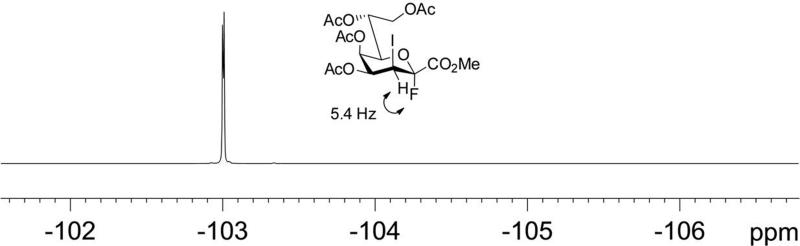
The acetyl-protected glycal methyl ester 3[17] - which can be prepared in three steps in multi-gram amounts from ammonium Kdo via the peracetylated Kdo methyl ester 1 [18] - was subjected to acetoxyiodination with N-iodosuccinimide (NIS) in acetic acid giving the known 2,3-trans-diaxial derivative 4.[19] In consideration of the stability of anomeric Kdo fluorides,[13,20] conversion of the 3-iodo derivative 4 into donor 5 was carried out. Previously, Kdo fluorides such as 2 have been mainly prepared by reaction of a Kdo 2-O-hemiketal (which is prone to 2,3-elimination) with DAST.[13,20] However, a direct replacement of the anomeric acetate using the easily scalable reaction of 4 with HF-pyridine complex afforded α-fluoride 5 (96%) neatly after flash-chromatography (Scheme 1).[21] Fluoride 5 is bench-top stable for several months and was obtained as a single anomer as seen from the 19F NMR spectrum (Figure 1). The α-anomeric configuration was in agreement with the 13C NMR chemical shift data for C-4 and C-6 (see Supporting Information) and the value of the vicinal 19F-1H coupling constant (3JFax,H3eq 5.4 Hz).[22]
Figure 1.
Proton-coupled 19F NMR spectrum of fluoride donor 5.
In order to evaluate its glycosyl donor properties, model glycosylation reactions of 5 with 2-propanol (2 eq.) in dichloromethane, promoted by BF3.Et2O were performed and compared to a similar glycosylation using the peracetylated Kdo-fluoride donor 2, lacking a stereodirecting group.[20d, 21] The latter glycosylation afforded a moderate yield (48%) of an α/β-glycoside mixture (4.8:1) and generated large amounts of glycal ester 3 (31%). By contrast, application of the new donor 5 revealed an α-specific outcome, giving the 2,3-trans-ketoside 6 in a high yield (83%) accompanied by only very minor formation of 3 (5%); for further details see Supporting Information.
For the subsequent dehalogenation of 6, free radical chain reduction using AIBN / tributyltin hydride gave moderate yields after extensive purification to remove the tin reagent. Hydrogenation over different Pd catalysts gave irreproducible yields due to concomitant epimerization of the 3-iodo substituent among other side reactions. Eventually, however, the 3-iodo-substituent was cleanly removed via hydrogen transfer from cyclohexane[23] induced by lauroyl peroxide to furnish the Kdo α-glycoside 7 (Scheme 1) in near-quantitative yield. The α-anomeric configuration was then confirmed on the basis of the chemical shift difference between the axial and equatorial protons at C-3, the low-field shift (> 5 ppm) observed for H-4 in 4-O-acetylated Kdo derivatives as well as 13C NMR chemical shifts for C-4 and C-6 which are shifted to higher field when compared to the β-anomers.[18]
We further evaluated the practicability of the dehalogenation method for Kdo-glycosides containing functionalized linkers, which are needed for conversion into neoglycoconjugates and for preparation of glycoarrays. Following the described procedure we obtained ω-azido-3-iodo-glycoside 8 as the α-anomer exclusively and in excellent yields (83% based on the amount of donor). Notably, during subsequent dehalogenation by hydrogen transfer the azide moiety remained unaffected giving the Kdo-spacer glycoside 9 in comparable yields.
In order to assess the glycosylation potential of the 3-iodo fluoride donor 5, the structurally conserved α-(2→4)-linkage of the enterobacterial Kdo region was then assembled in a highly efficient approach. Coupling of 10 with one equivalent only (!) of donor 5 proceeded smoothly and regioselectively and furnished the disaccharide 11 in 78% yield without formation of the β-anomeric product and only minor elimination (4% of 3) and donor hydrolysis (5%) (Scheme 2). Due to poor solubility of the 5-OH disaccharide derivative 11 in cyclohexane, the subsequent dehalogenation never reached completion and separation from unreacted starting material could only be achieved using HP-chromatography. In order to secure a smooth dehalogenation, disaccharide 11 was therefore acetylated (98%) - to give fully protected 12 - prior to dehalogenation towards 13 (97%). Global deprotection via sodium-methoxide catalyzed transesterification and alkaline hydrolysis of the methyl ester group afforded the known disaccharide methyl glycoside 14.[24]
Scheme 2.
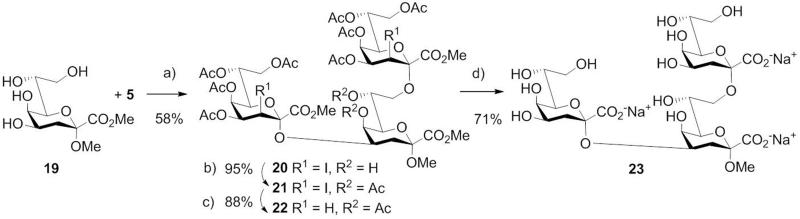
Synthesis of 2→4 and 2→8 linked Kdo disaccharides 14 and 18. a) BF3.Et2O, CH2Cl2, mol. sieves 3 Å; b) Ac2O, pyr., DMAP; c) lauroyl peroxide, cyclohexane; d) 0.1 M NaOMe, MeOH, then 0.1 M aq. NaOH.
To further extend the scope of Kdo-glycoside synthesis beyond the common enterobacterial α-(2→4)-linked Kdo disaccharide, the Chlamydia-specific[6] α-(2→8)-disaccharide 18 was then prepared along similar lines capitalizing on a glyco-desilylation approach.[25] Coupling of the 8-O-triethylsilyl (TES)-protected derivative 15 with 1.2 equivalents of donor 5 proceeded smoothly to give the α-(2→8)-linked disaccharide 16 in 62% yield. Again, no β-isomer was observed. The moderate yield is due to concomitant migration of the 7-O-acetyl protecting group towards the primary 8-OH group forming a regioisomer which was not glycosylated under these reaction conditions. The glycosylation of the corresponding 8-OH alcohol (derived from 15 by F− treatment) gave a similar yield (64%), while bulkier silyl protecting groups (8-O-TBDMS, 7,8-O-TIPDS) substantially lowered the isolated yield.
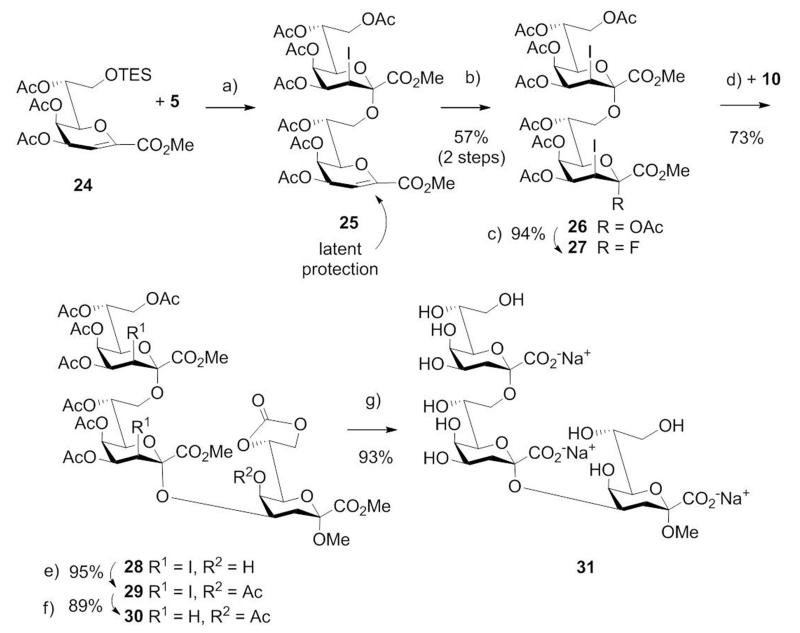
The extraordinary glycosylation properties of 5 prompted us to apply the donor for the one-pot synthesis of a branched Kdo trisaccharide, which corresponds to an LPS antigen of Chlamydophila psittaci and serves as a species-specific marker.[26] Indeed, glycosylation of unprotected tetraol 19 with 2.4 equivalents of donor 5 proceeded – although being heterogeneous - in a remarkably regioselective reaction, which allowed to isolate the target trisaccharide 20 in 58% yield (Scheme 3). As minor by-product the branched α-(2→4)[α-(2→7)]-linked Kdo trisaccharide was isolated in 7% yield. Processing of 20 as described for the disaccharides gave 21 and 22 which was then deblocked to afford the branched Kdo trisaccharide 23. This basic concept was further extended to iterative oligosaccharide-synthesis, demonstrated for the linear Kdo trisaccharide 31, representing the family-specific Chlamydia epitope (Scheme 4). After applying the 8-O-silyl protected glycal ester 24 as glycosyl acceptor, the resulting disaccharide 25 was treated likewise by iodoacetoxylation and reaction with HF-pyridine to afford the bis-iodo disaccharide donor 27. Thus, the glycal moiety as in 25 serves as a latent protecting group for the anomeric position, which may subsequently be activated. Interestingly, BF3.Et2O was not capable of promoting the subsequent coupling step with glycosyl acceptor 10, however, TfOH-catalyzed (0.15 eq.) coupling provided trisaccharide 28 stereospecifically in a good yield (73%). Acetylation and dehalogenation of 28 followed by global deprotection of 30 afforded the target trisaccharide 31. The 1H and 13C NMR data of the deprotected di- and trisaccharides 14, 18, 23 and 31 are in full agreement with published data of chlamydial oligosaccharides.[27]
Scheme 3.
One-pot glycosylation towards the branched Chl. psittaci specific Kdo trisaccharide 23. a) BF3.Et2O, CH2Cl2, mol. sieves 3 Å; b) Ac2O, pyr., DMAP; c) lauroyl peroxide, cyclohexane; d) 0.1 M NaOMe, MeOH, then 0.1 M aq. NaOH
Scheme 4.
Synthesis of the family-specific Chlamydia Kdo trisaccharide 31. a) BF3.Et2O, CH2Cl2, mol. sieves 5 Å; b) NIS, AcOH; c) HF-pyridine, CH2Cl2; d) TfOH, CH2Cl2, mol. sieves 3 Å; e) Ac2O, pyr., DMAP; f) lauroyl peroxide, cyclohexane; g) 0.1 M NaOMe, MeOH, then 0.1 M aq. NaOH.
Conclusions
In conclusion, the easily accessible 3-iodo-2-fluoride Kdo donors enable a very efficient and α-specific glycosylation of different Kdo glycosyl acceptors to generate biomedically important LPS fragments. In addition to the exquisite α-selectivity, the axial iodo-substituent substantially suppressed the elimination towards the glycal side-product (Table 1). Sequential iteration of this basic concept may be combined with variable protecting group patterns, thus considerably expanding the scope of the presented work towards other biomedically important Kdo-antigens.
Table 1.
Summary of glycosylation reactions using 3-iodo-Kdo fluoride donors
| Acceptor | Donor | Equiv | Product | Yield | α/β ratio | Glycal[a] |
|---|---|---|---|---|---|---|
| 2-PrOH[b] | 5 | 1.0 | 6 | 83% | α only | 7% |
|
|
5 | 1.0 | 8 | 80% | α only | traces |
| 10 | 5 | 1.0 | 11 | 78% | α only | 4% |
| 15 | 5 | 1.2 | 16 | 62% | α only | 7% |
| 19 | 5 | 2.4 | 20 | 58%[c] | α only | 11% |
| 24 | 5 | 1.2 | 25 | 85%[d] | α only | n.d. |
| 10 | 27 | 1.2 | 28 | 73%[e] | α only | n.d. |
relative to reacted donor.
2 equivalents of alcohol.
In addition, the 2→4/7 linked regioisomer was isolated in 7% yield.
As purification afforded a mixture of hydrolysed donor and 25, the yield was determined from 1H-NMR data.
promoted by TfOH
Experimental Section
General methods
All purchased chemicals were used without further purification unless stated otherwise. Solvents were dried over activated 4 Å (CH2Cl2, DMF, pyridine, cyclohexane) molecular sieves. 2-Propanol for glycosylation was dried over 5 Å molecular sieves for 24 h. THF was directly distilled on 4 Å molecular sieves shortly before use. Dry MeOH (secco solv) was purchased from Merck. Cation exchange resin DOWEX 50 H+ was regenerated by consecutive washing with HCl (3 M), water and dry MeOH. Aqueous solutions of salts were saturated unless stated otherwise. Concentration of organic solutions was performed under reduced pressure < 40 °C. Optical rotations were measured with a Perkin-Elmer 243 B Polarimeter. [α]D20 values are given in units of 10−1deg cm2g−1. Thin layer chromatography was performed on Merck precoated plates: generally on 5 × 10 cm, layer thickness 0.25 mm, Silica Gel 60F254; alternatively on HP-TLC plates with 2.5 cm concentration zone (Merck). Spots were detected by dipping reagent (anisaldehyde-H2SO4). For column chromatography silica gel (0.040 – 0.063 mm) was used. HP-column chromatography was performed on pre-packed columns (YMC-Pack SIL-06, 0.005 mm, 250x10 mm and 250x20 mm). Size exclusion chromatography was performed on Bio-Gel® P-2 Gel extra fine < 45 μm (wet) (1 × 30 cm) or on pre-packed PD-10 columns (GE Healthcare, SephadexTM G-25 M). NMR spectra were recorded with a Bruker Avance III 600 instrument (600.22 MHz for 1H, 150.93 MHz for 13C and 564.77 MHz for 19F) using standard Bruker NMR software. 1H NMR spectra were referenced to 7.26 (CDCl3) and 0.00 (D2O, external calibration to 2,2-dimethyl-2-silapentane-5-sulfonic acid) ppm unless stated otherwise. 13C NMR spectra were referenced to 77.00 (CDCl3) and 67.40 (D2O, external calibration to 1,4-dioxane) ppm. 19F NMR spectra were indirectly referenced according to IUPAC recommendations[28]. ESI-MS data were obtained on a Waters Micromass Q-TOF Ultima Global instrument.
General procedure for glycosylation
Under an atmosphere of argon a mixture of donor, glycosyl acceptor and ground 3 Å molecular sieves (~50 mg/mL CH2Cl2) in dry CH2Cl2 was stirred at ambient temperature for 2 h. At 0°C BF3.Et2O (2 eq./donor) was added dropwise and after complete addition the ice bath was removed immediately. Typically, the solution turned pink after a couple of minutes. When full conversion was determined by TLC, aq. NaHCO3 and CH2Cl2 were added. The aqueous phase was washed with CHCl3 and the combined organic phases were washed successively with aq. sodium thiosulfate (5 w%) and brine. The organic phase was dried (MgSO4), filtered and concentrated. The crude product was purified by column chromatography.
General procedure for dehalogenation
A solution/suspension of the iodo-compound in dry cyclohexane was degassed by bubbling argon through the mixture followed by heating to reflux under inert gas for 15 min. Lauroyl peroxide was added to the warm solution in one portion and refluxing was continued until complete conversion was detected by (HP-) TLC. The solvent was removed in vacuo and the residue was purified by column chromatography.
General procedure for global deprotection
The acetylated saccharide was treated with catalytic amounts of NaOMe (0.1 M in MeOH) in dry MeOH until cleavage of the majority of acetyl groups had been detected by TLC. Ion exchange resin DOWEX 50 ( H+) was added until the solution reacted neutral, the resin was filtered off and the filtrate was concentrated. The residue was dissolved in water and treated with aq NaOH (0.1 M) at ambient temperature. Neutralization of the solution with DOWEX 50 (H+) ion-exchange resin, filtration and lyophilisation of the filtrate afforded the product, which was purified by gel chromatography in order to remove residual sodium acetate.
For experimental data of compounds 6 - 18 and 32 - 33 and NMR spectra of compounds 5 - 18, 20 - 24 and 26 - 33 refer to Supporting Information.
Methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-3-iodo-d-glycero-α-d-talo-oct-2-ulopyranosyl)onate fluoride (5)
A solution of 4[19] (0.75 g, 1.275 mmol) in dry CH2Cl2 (13.0 mL) was treated with hydrogen fluoride pyridine (~70% HF, ~30% pyridine; 4.0 mL) at 0 °C for 2.5 h in a sealed Teflon flask. Ice-cold water (10 mL) was added, the phases were mixed thoroughly and separated. The aqueous phase was once again extracted with CH2Cl2 and the combined organic extracts were washed with aq. NaHCO3, dried (MgSO4), filtered and concentrated. The crude product was quickly purified by flash chromatography (n-hexane/EtOAc 1:1) affording 5 (0.67 g, 96%) as a colorless oil; [α]D20 + 18.8 (c 0.76, MeOH); Rf 0.51 (toluene/EtOAc 2:1); 1H NMR (CDCl3): δ 5.47 (ddd, 1H, J5,4 3.5, J5,6 1.9, J5,3 0.8 Hz, H-5), 5.37 - 5.34 (m, 1H, H-7), 4.97 (dd, 1H, J4,3 5.1 Hz, H-4), 4.64 (app dt, 1H, H-3), 4.52 (dd, 1H, J8a,8b 12.4, J8a,7 2.3 Hz, H-8a), 4.49 (dd, 1H, J6,7 9.8 Hz, H-6), 4.23 (dd, 1H, J8b,7 4.2 Hz, H-8b), 3.88 (s, 3H, CO2CH3), 2.12, 2.07, 2.05 and 1.98 (4 × s, each 3H, COCH3); 13C NMR (CDCl3): δ 170.42, 169.90, 169.36 and 169.17 (4 × s, COCH3), 163.29 (ds, J1,F 28.2 Hz, C-1), 108.92 (ds, J2,F 231.1 Hz, C-2), 70.15 (dd, J6,F 4.5 Hz, C-6), 67.11 (d, C-7), 64.56 (d, C-4), 62.76 (d, C-5), 61.84 (t, C-8), 53.49 (q, CO2CH3), 20.79, 20.66, 20.61 and 20.51 (4 × q, COCH3), 18.02 (dd, J3,F 31.3 Hz, C-3); 19F NMR (CDCl3): δ -103.00 (d, JF,H-3 5.4 Hz); ESI-TOF HRMS: m/z = 566.0533; calcd for C17H22FIO11NH4+: 566.0529.
Methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-3-iodo-d-glycero-α-d-talo-oct-2-ulopyranosyl)onate-(2→4)[methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-3-iodo-d-glycero-α-d-talo-oct-2-ulopyranosyl)onate-(2→8)]-methyl (methyl 3-deoxy-α-d-manno-oct-2-ulopyranosid)onate (20)
A mixture of predried 19[18] (16.3 mg, 0.061 mmol) and 5 (80.6 mg, 0.147 mmol) in dry CH2Cl2 (4.0 mL) was treated with BF3.Et2O (120.7 μL, 0.588 mmol) according to the general procedure for glycosylation. After 1 h the suspension was subjected to aqueous work-up and the crude product was purified by HP-column chromatography (toluene/EtOAc 2:1→1:1) yielding the glycal 3[17] (6.8 mg, 11%), the slower migrating (2→4/7)-regioisomer trisaccharide (5.4 mg, 7%) and finally the slowest migrating trisaccharide 20 (47.0 mg, 58%).
(2→4/7)-trisaccharide
colorless oil: Rf 0.39 (toluene/EtOAc 1:1, HP-TLC); 1H NMR (CDCl3): δ 5.46 - 5.44 (m, 2H, H-5′, H-5″), 5.38 – 5.34 (m, 2H, H-7′, H-7″), 5.04 (dd, 1H, J4″,3″ 4.6, J4″,5″ 3.7 Hz, H-4″), 4.97 (dd, 1H, J4′,3′ 4.8, J4′,5′ 3.6 Hz, H-4′), 4.74 (dd, 1H, J8″a,8″b 12.5, J8″a,7″ 2.9 Hz, H-8″a), 4.71 (dd, 1H, J8′a,7′ 12.5, J8′a,7′ 2.8 Hz, H-8′a), 4.69 (dd, 1H, J6″,7″ 9.2, J6″,5″ 2.2 Hz, H-6″), 4.55 (dd, 1H, J3′,5′ 0.8 Hz, H-3′), 4.47 (dd, 1H, J3″,4″ 0.9 Hz, H-3″), 4.26 - 4.22 (m, 2H, H-6′, H-8′b), 4.17 - 4.11 (m, 2H, H-4, H-8″b), 4.00 - 3.97 (m, 1H, H-8a), 3.94 - 3.88 (m, 8H, H-7, H-8b, 2 × CO2CH3), 3.80 (s, 3H, CO2CH3), 3.72 (dd, 1H, J6,7 5.2, J6,5 1.2 Hz, H-6), 3.63 - 3.61 (m, 1H, H-5), 3.24 (s, 3H, OCH3), 2.64 (b s, 1H, C8-OH), 2.49 (b s, 1H, C5-OH), 2.14 - 2.11 (m, 11H, H-3ax, H-3eq, 3 × COCH3), 2.10, 2.07, 2.06, 1.98 and 1.979 (5 × s, each 3H, COCH3).
20: colorless amorphous solid; [α]D20 + 63.1 (c 0.55, CHCl3); Rf 0.38 (toluene/EtOAc 1:1, HP-TLC); 1H NMR (CDCl3): δ 5.46 - 5.44 (m, 1H, H-5′), 5.39 - 5.32 (m, 3H, H-7′, H-5″, H-7″), 4.98 - 4.94 (m, 2H, H-4′, H-4″), 4.75 (dd, 1H, J8′a,8′b 12.5, J8′a,7′ 2.7 Hz, H-8′a), 4.60 (dd, 1H, J8″a,8″b 12.2, J8″a,7″ 2.2 Hz, H-8″a), 4.55 - 4.52 (m, 2H, H-3′, H-3″), 4.33 (dd, 1H, J6″,7″ 9.7, J6″,5″ 1.8 Hz, H-6″), 4.26 - 4.21 (m, 2H, H-6′, H-8″b), 4.17 (ddd, 1H, J4,3ax 11.2, J4,3eq 5.6, J4,5 2.9 Hz, H-4), 4.13 - 4.07 (m, 2H, H-7, H-8′b), 3.87 (s, 6H, 2 × CO2CH3), 3.82 (dd, 1H, J8a,8b 10.6, J8a,7 5.4 Hz, H-8a), 3.78 (s, 3H, CO2CH3), 3.76 - 3.74 (m, 1H, H-5), 3.53 (dd, 1H, J8b,7 3.0 Hz, H-8b), 3.49 (d, 1H, J6,7 8.2 Hz, H-6), 3.20 (s, 3H, OCH3), 2.87 (d, 1H, JOH,7 7.5 Hz, OH), 2.52 (b s, 1H, OH), 2.17 - 2.08 (m, 11H, H-3ax, H-3eq, 3 × COCH3), 2.07, 2.06, 2.05, 1.98 and 1.97 (5 × s, each 3H, COCH3); 13C NMR (CDCl3): δ 170.89, 170.67, 170.12, 170.00, 169.47, 169.43, 169.38 and 169.29 (8 × s, COCH3), 167.55, 166.96 and 166.78 (3 × s, C-1, C-1′, C-1″), 102.10 and 100.42 (2 × s, C-2′, C-2″), 98.90 (s, C-2), 70.33 (d, C-6), 70.13 (d, C-4), 69.23 (d, C-6′), 68.52 (d, C-6″), 68.34 (d, C-7), 67.73 (d, C-7′), 67.47 (d, C-7″), 67.12 (t, C-8), 65.19 and 65.09 (2 × d, H-4′, H-4″), 63.22 (d, C-5), 63.21 (d, C-5′), 63.15 (d, C-5″), 62.10 (t, C-8″), 61.33 (t, C-8′), 53.25, 53.24 and 52.52 (3 × q, CO2CH3), 50.81 (q, OCH3), 32.77 (t, C-3), 22.34 and 21.41 (2 × d, C-3′, C-3″), 20.91, 20.90, 20.78, 20.75, 20.71, 20.59 and 20.51 (7 × q, 8C, 8 × COCH3); ESI-TOF HRMS: m/z = 1340.1596; calcd for C44H60I2O30NH4+: 1340.1597.
Methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-3-iodo-d-glycero-α-d-talo-oct-2-ulopyranosyl)onate]-(2→4)[methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-3-iodo-d-glycero-α-d-talo-oct-2-ulopyranosyl)onate-(2→8)]-methyl (methyl 5,7-di-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosid)onate (21)
A solution of 20 (42.9 mg, 0.032 mmol) in dry pyridine (5.0 mL) was treated with acetic anhydride (0.25 mL) and 4-(N,N-dimethylamino)-pyridine (0.4 mg, 0.003 mmol) at 0 °C for 2.5 h. Excessive reagent was destroyed by slow addition of dry MeOH (0.5 mL). The mixture was stirred for 10 min followed by removal of the solvent by coevaporation with toluene. Column chromatography (toluene/EtOAc 1:1) of the residue provided 21 (43.2 mg, 95%) as a colorless oil; [α]D20 + 47.1 (c 0.74, CHCl3); Rf 0.37 (toluene/EtOAc 1:1); 1H NMR (CDCl3): δ 5.40 - 5.38 (m, 1H, H-5′), 5.36 - 5.31 (m, 3H, H-5″, H-7′, H-7″), 5.19 - 5.16 (m, 1H, H-5), 4.92 (dd, 1H, J4′,3′ 4.6, J4′,5′ 3.7 Hz, H-4′), 4.89 (dd, 1H, J4″,3″ 4.8, J4″,5″ 3.7 Hz, H-4″), 4.84 (ddd, 1H, J7,6 9.8, J7,8a 3.8, J7,8b 2.4 Hz, H-7), 4.75 (dd, 1H, J8′a,8′b 12.4, J8′a,7 2.8 Hz, H-8′a), 4.64 (dd, 1H, J8″a,8″b 12.3, J8″a,7 2.4 Hz, H-8″a), 4.54 - 4.53 (m, 1H, H-3″), 4.41 - 4.39 (m, 1H, H-3′), 4.27 - 4.23 (m, 2H, H-4, H-6′), 4.19 (dd, 1H, J8″b,7″ 3.8 Hz, H-8″b), 4.14 - 4.10 (m, 2H, H-6″, H-8′b), 3.99 (dd, 1H, J6,5 1.2 Hz, H-6), 3.89 - 3.86 (m, 4H, H-8a, CO2CH3), 3.83 (s, 3H, CO2CH3), 3.79 (s, 3H, CO2CH3), 3.68 (dd, 1H, J8b,8a 11.9 Hz, H-8b), 3.28 (s, 3H, OCH3), 2.27 - 2.23 (m, 1H, H-3eq), 2.15 - 2.09 (m, 10H, H-3ax, 3 × COCH3), 2.09, 2.07 and 2.066 (3 × s, each 3H, COCH3), 2.04 (s, 6H, 2 × COCH3), 1.97 and 1.96 (2 × s, each 3H, COCH3); 13C NMR (CDCl3): δ 170.58, 170.38, 170.09, 170.05, 169.80, 169.74, 169.43, 169.41, 169.39 and 169.25 (10 × s, COCH3), 167.10, 166.15 and 165.47 (3 × s, C-1, C-1′, C-1″), 102.10, 101.46 and 98.88 (3 × s, C-2, C-2′, C-2″), 69.84 and 69.29 (2 × d, C-4, C-6′), 68.61 (d, C-6″), 68.43 (d, C-7), 68.37 (d, C-6), 67.92 and 67.56 (2 × d, C-7′, C-7″), 65.14 and 65.12 (2 × d, C-5, C-4″), 64.99 (d, C-4′), 64.03 (t, C-8), 63.24 (d, C-5′), 63.01 (d, C-5″), 61.84 (t, C-8″), 61.46 (t, C-8′), 53.05, 52.90 and 52.60 (3 × q, CO2CH3), 51.38 (q, OCH3), 33.42 (t, C-3), 22.39 (d, C-3′), 21.36 (d, C-3″), 20.94, 20.88, 20.83, 20.76, 20.72, 20.65, 20.57 and 20.52 (8 × q, 10C, COCH3); ESI-TOF HRMS: m/z = 1429.1357; calcd for C48H64I2O32Na+: 1429.1362.
Methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate]-(2→4)[methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→8)]-methyl (methyl 5,7-di-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosid)onate (22)
According to the general procedure for dehalogenation 21 (38.2 mg, 0.027 mmol) was treated with lauroyl peroxide (6.5 mg, 0.016 mmol) in dry cyclohexane (4.0 mL) for 4 h. Removal of the solvent and subsequent column chromatography (toluene/EtOAc 1:1) afforded 22 (27.6 mg, 88%) as a colorless oil; [α]D20 + 79.4 (c 0.91, CHCl3); Rf 0.19 (toluene/EtOAc 1:1, HP-TLC); 1H NMR (CDCl3): δ 5.36 - 5.34 (m, 1H, H-5′), 5.30 - 5.28 (m, 1H, H-5″), 5.21 - 5.13 (m, 5H, H-4′, H-4″, H-5, H-7′, H-7″), 5.00 (ddd, 1H, J7,6 9.8, J7,8b 4.5, J7,8a 2.4 Hz, H-7), 4.66 (dd, 1H, J8′a,8′b 12.2, J8′a,7′ 2.7 Hz, H-8′a), 4.63 (ddd, 1H, J4,3ax 12.0, J4,3eq 5.0, J4,5 3.0 Hz, H-4), 4.58 (dd, 1H, J8″a,8″b 12.4, J8″a,7″ 2.2 Hz, H-8″a), 4.15 - 4.11 (m, 2H, H-6′, H-8″b), 4.09 (dd, 1H, J8′b,7′ 3.5 Hz, H-8′b), 4.01 (dd, 1H, J6″,7″ 9.6, J6″,5″ 1.4 Hz, H-6″), 3.98 (dd, 1H, J6,5 1.2 Hz, H-6), 3.87 - 3.83 (m, 4H, H-8a, CO2CH3), 3.80 (s, 3H, CO2CH3), 3.79 (s, 3H, CO2CH3), 3.73 (dd, 1H, J8b,8a 11.8, H-8b), 3.28 (s, 3H, OCH3), 2.24 - 2.20 (m, 1H, H-3″eq), 2.18 - 2.00 (m, 23H, H-3ax, H-3eq, H-3′ax, H-3′eq, H-3″ax, 6 × COCH3), 1.99, 1.98, 1.96 and 1.955 (4 × s, each 3H, COCH3); 13C NMR (CDCl3): δ 170.89, 170.49, 170.32, 170.28, 170.06, 169.83, 169.65, 169.63 and 169.62 (9 × s, 10C, COCH3), 167.47, 167.24 and 167.02 (3 × s, C-1, C-1′, C-1″), 99.08, 98.94 and 97.38 (3 × s, C-2, C-2′, C-2″), 69.32 (d, C-6′), 68.79 (d, C-6″), 68.48 and 68.43 (2 × d, C-6, C-7), 67.78 (d, C-7′), 67.65 (d, C-7″), 66.33 (d, C-4′), 66.11 (d, 2C, C-4, C-4″), 65.03 (d, C-5), 64.33 (d, C-5′), 64.13 (d, C-5″), 62.39 (t, C-8), 61.89 (t, C-8″), 61.64 (t, C-8′), 52.70, 52.67 and 52.47 (3 × q, CO2CH3), 51.19 (q, OCH3), 33.96 (t, C-3), 31.44 (t, 2C, C-3′, C-3″), 20.76, 20.73, 20.71, 20.66, 20.64, 20.59 and 20.53 (7 × s, 10C, COCH3); ESI-TOF HRMS: m/z = 1172.3875; calcd for C48H66O32NH4+: 1172.3875.
Sodium (3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate]-(2→4) [sodium (3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→8)]-sodium (methyl α-d-manno-oct-2-ulopyranosid)onate (23)
According to the general procedure for global deprotection a solution of 22 (20.5 mg, 0.018 mmol) in dry MeOH (2.0 mL) was treated with NaOMe (0.1 M in MeOH; 105 μL, 0.012 mmol), which was added in 3 portions within 24 h. After complete addition stirring was continued for 8 h. After work-up as described the residue was dissolved in water (2.0 mL) and treated with aq NaOH (0.1 M, 1.0 mL) for 3 h at rt. The solution was subjected to standard work-up und the crude product was desalted by successive purification on a PD10 (H2O) and BioGel-P2 (H2O/EtOH 19:1) column followed by lyophilisation of pooled fractions affording 23 (9.6 mg, 71%) as an amorphous colorless solid; [α]D20 + 80.1 (c 0.87, D2O); 1H NMR (D2O, pH ~ 6.5): δ 4.05 - 4.00 (m, 4H, H-4, H-4′, H-4″, H-5), 3.98 - 3.82 (m, 7H, H-5′, H-5″, H-7, H-7′, H-7″, H-8′a, H-8″a), 3.69 - 3.55 (m, 5H, H-6′, H-6″, H-8a, H-8′b, H-8″b), 3.52 - 3.46 (m, 2H, H-6, H-8b), 3.09 (s, 3H, OCH3), 2.08 (dd, 1H, J3″eq,3″ax 13.4, J3″eq,4″ 4.5 Hz, H-3″eq), 2.01 (dd, 1H, J3′eq,3′ax 13.2, J3′eq,4′ 4.8 Hz, H-3′eq), 1.93 - 1.89 (m, 1H, H-3eq), 1.84 (dd, 1H, J3ax,3eq 12.9, J3ax,4 11.6 Hz, H-3ax), 1.75 (dd, 1H, J 12.9, J 12.2 Hz, H-3′ax), 1.72 (dd, 1H, J3″ax,3″eq 13.2, J3″ax,4″ 12.3 Hz, H-3″ax); 13C NMR (D2O, pH ~ 6.5): δ 176.48, 176.44 and 175.92 (3 × s, C-1, C-1′, C-1″), 101.30, 101.22 and 100.43 (3 × s, C-2, C-2′, C-2″), 73.04 (d, C-6), 72.34 and 72.26 (2 × d, C-6′, C-6″), 70.77 and 70.10 (2 × d, C-7′, C-7″), 69.70 (d, C-7), 68.69 (d, C-4), 67.15 and 67.10 (2 × d, C-5′, C-5″), 66.85 and 66.68 (2 × d, C-4′, C-4″), 65.98 (t, C-8), 65.23 (d, C-5), 63.83 and 63.81 (2 × t, C-8′, C-8″), 51.40 (q, OCH3), 35.36 and 34.87 (2 × t, C-3′, C-3″), 34.03 (t, C-3); ESI-TOF HRMS: m/z = 715.1901; calcd for C25H40O22Na+: 715.1903.
Methyl (2,6-anhydro-4,5,7-tri-O-acetyl-3-deoxy-8-O-triethylsilyl-d-manno-oct-2-enos)onate (24)
Glycal 34 was prepared from 3 by standard transesterification catalyzed by sodium methoxide and was used without purification after neutralization and solvent evaporation. A suspension of glycal 34 (94 mg, 0.401 mmol) and 1,4-diazabicyclo[2.2.2]octane (77 mg, 0.682 mmol) in dry MeCN (10.0 mL) was treated with chlorotriethylsilane (74 μL, 0.442 mmol) at ambient temperature for 1 h. After addition of two more portions of chlorotriethylsilane (14.8 μL, 0.088 mmol) within 30 min, the mixture was concentrated. The residue was partitioned between EtOAc and half-saturated aq NaHCO3. The aqueous phase was extracted with EtOAc (3 times) and the combined organic phases were dried (MgSO4). After filtration the solvent was removed and the residual oil was dried under high vacuum. The residue was dissolved in dry pyridine (8.0 mL) and treated with acetic anhydride (0.4 mL) in the presence of 4-(N,N-dimethylamino)pyridine (5 mg) for 4 h at ambient temperature. Excessive reagent was destroyed at 0 °C by dropwise addition of dry MeOH (2.0 mL) and after stirring for 5 min the solvent was removed by repeated coevaporation with toluene. The crude product was purified by column chromatography (toluene/EtOAc 15:1) providing 24 (129 mg, 62%) as a colorless oil; [α]D20 - 18.1 (c 1.40, CHCl3); Rf 0.39 (toluene/EtOAc 9:1); 1H NMR (CDCl3): δ 5.87 (app t, 1H, H-3), 5.71 (ddd, 1H, J5,4 4.6, J5,3 2.2, J5,6 1.4 Hz, H-5), 5.47 (ddd, 1H, J4,3 2.0, J4,6 1.1 Hz, H-4), 5.09 (ddd, 1H, J7,6 9.6, J7,8a 3.1, J7,8b 2.1 Hz, H-7), 4.47 - 4.44 (m, 1H, H-6), 3.96 (dd, 1H, J8a,8b 11.6, H-8a), 3.86 (dd, 1H, H-8b), 3.81 (s, 3H, CO2CH3), 2.06, 2.05 and 2.02 (3 × s, each 3H, COCH3), 0.93 [t, 9H, J 8.0 Hz, Si(CH2CH3)3], 0.59 [q, 6H, J 8.0 Hz, Si(CH2CH3)3]; 13C NMR (CDCl3): δ 170.34, 170.01 and 169.83 (3 × s, COCH3), 161.68 (s, C-1), 144.85 (s, C-2), 107.42 (d, C-3), 72.69 (d, C-6), 69.97 (d, C-7), 64.93 (d, C-5), 61.44 (d, C-4), 60.73 (t, C-8), 52.35 (q, CO2CH3), 20.83, 20.62 and 20,55 (3 × q, COCH3), 6.60 [q, 3C, S[(CH2CH3)3], 4.22 [t, 3C, Si(CH2CH3)3]; ESI-TOF HRMS: m/z = 497.1821; calcd for C21H34O10SiNa+: 497.1813.
Methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-3-iodo-d-glycero-α-d-talo-oct-2-ulopyranosyl)onate-(2→8)-methyl (2,6-anhydro-4,5,7-tri-O-acetyl-3-deoxy-8-O-triethylsilyl-d-manno-oct-2-enos)onate (25) and methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-3-iodo-d-glycero-α-d-talo-oct-2-ulopyranosyl)onate-(2→8)-methyl (2,4,5,7-tetra-O-acetyl-3-deoxy-3-iodo-d-glycero-α-d-talo-oct-2-ulopyranosyl)onate (26)
A solution of 24 (200 mg, 0.421 mmol) and 5 (277 mg, 0.506 mmol) in dry CH2Cl2 (8.0 mL) was treated with BF3.Et2O (173 μL, 0.843 mmol) according to the general procedure for glycosylation. After 2 h complete conversion was determined by TLC and the mixture was subjected to aqueous work-up. Subsequent column chromatography (toluene/EtOAc 2:1) afforded a mixture of hydrolysed donor and 25 (yield determined by NMR ~320 mg, ~85%, see Supporting Info Figure S-2) as a yellow oil which was directly used in the next step. A solution of this mixture in glacial acetic acid (25 mL) was treated with N-iodosuccinimide at 65 °C for 46 h. The cold solution was slowly poured onto ice-cold saturated NaHCO3 solution and diluted with chloroform. The phases were separated, the aqueous phases was once again extracted with chloroform and the combined organic phases were successively washed with aq NaHCO3, aq NaS2SO3 (5 w%) and brine. After drying (MgSO4), filtration and concentration, the crude product was purified by HP-column chromatography (toluene/EtOAc 5:2) yielding 26 (256 mg, 57% over 2 steps) as a colorless oil; [α]D20 + 34.9 (c 0.80, CHCl3); Rf 0.34 (toluene/EtOAc 3:2); 1H NMR (CDCl3): δ 5.48 - 5.46 (m, 1H, H-5), 5.41 - 5.37 (m, 2H, H-5′, H-7′), 5.07 (app td, 1H, J7,6 9.8 Hz, H-7), 5.05 (dd, 1H, J4,3 4.9, J4,5 3.7 Hz, H-4), 4.76 (dd, 1H, J4′,3′ 4.9, J4′,5′ 3.7 Hz, H-4′), 4.63 - 4.60 (m, 2H, H-3′, H-8′a), 4.51 (dd, 1H, J3,5 0.9 Hz, H-3), 4.30 (dd, 1H, J6,5 2.1 Hz, H-6), 4.22 (dd, 1H, J8′b,8′a 12.4, J8′b,7′ 4.9 Hz, H-8′b), 4.20 (dd, 1H, J6′,7′ 9.6, J6′,5′ 2.0 Hz, H-6′), 3.96 (dd, 1H, J8a,8b 11.9, J8a,7 2.3 Hz, H-8a), 3.83 (s, 3H, CO2CH3), 3.79 (s, 3H, CO2CH3), 3.48 (dd, 1H, J8b,7 2.7 Hz, H-8b), 2.19, 2.13, 2.13, 2.12, 2.09, 2.06, 2.03 and 2.00 (8 × s, each 3H, COCH3); 13C NMR (CDCl3): δ 170.88, 170.11, 169.87, 169.66, 169.64, 169.28, 169.22 and 168.21 (8 × s, COCH3), 166.60 and 165.31 (2 × s, C-1, C-1′), 102.49 and 98.89 (2 × s, C-2, C-2′), 68.74 (d, C-6), 68.67 (d, C-6′), 67.92 (d, C-7), 67.60 (d, C-7′), 65.47 (d, C-4′), 64.98 (d, C-4), 63.54 (t, C-8), 63.26 (d, C-5′), 63.03 (d, C-5), 62.36 (t, C-8′), 53.11 and 53.07 (2 × q, CO2CH3), 22.10 (d, C-3′), 20.95, 20.85, 20.81, 20.80, 20.77, 20.68, 20.65 and 20.52 (9C, C-3, 8 × COCH3); ESI-TOF HRMS: m/z = 1092.0708; calcd for C34H44I2O23NH4+: 1092.0701.
Methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-3-iodo-d-glycero-α-d-talo-oct-2-ulopyranosyl)onate-(2→8)-methyl (4,5,7-tri-O-acetyl-3-deoxy-3-iodo-d-glycero-α-d-talo-oct-2-ulopyranosyl)onate fluoride (27)
A solution of 26 (125 mg, 0.116 mmol) in dry CH2Cl2 (3.1 mL) was treated with hydrogen fluoride/pyridine (~70% HF, ~30% pyridine; 0.8 mL) at 0 °C for 1 h in a sealed Teflon flask. Ice-cold water was added, the phases were mixed thoroughly and separated. The aqueous phase was once again extracted with CH2Cl2 and the combined organic extracts were washed with aq NaHCO3, dried (MgSO4), filtered and concentrated. The crude product was quickly purified by flash chromatography (n-hexane/EtOAc 1:1) affording 27 (113 mg, 94%) as a colorless oil; [α]D20 + 34.5 (c 0.58, CHCl3); Rf 0.38 (n-hexane/EtOAc 1:1); 1H NMR (CDCl3): δ 5.50 - 5.48 (m, 1H, H-5), 5.39 (ddd, 1H, J7′,6′ 9.8, J7′,8′b 4.8, J7′,8′a 2.3 Hz, H-7′), 5.31 - 5.30 (m, 1H, H-5′), 5.22 - 5.19 (m, 1H, H-7), 4.99 (dd, 1H, J4,3 5.0, J4,5 3.6 Hz, H-4), 4.90 (dd, 1H, J4′,3′ 4.8, J4′,5′ 3.7 Hz, H-4′), 4.74 - 4.69 (m, 2H, H-3, H-8′a), 4.66 (dd, 1H, J6,7 9.9, J6,5 1.8 Hz, H-6), 4.58 (dd, 1H, J3′,5′ 0.8 Hz, H-3′), 4.23 (dd, 1H, J6′,5′ 1.9 Hz, H-6′), 4.21 (dd, 1H, J8′b,8′a 12.4 Hz, H-8′b), 4.01 (dd, 1H, J8a,8b 11.2, J8a,7 3.5 Hz, H-8a), 3.89 (s, 3H, CO2CH3), 3.83 (s, 3H, CO2CH3), 3.43 (dd, 1H, J8b,7 1.6 Hz, H-8b), 2.14, 2.12, 2.11, 2.09, 2.05, 2.05 and 1.99 (7 × s, each 3H, COCH3); 13C NMR (CDCl3): δ 170.61, 170.15, 169.77, 169.51, 169.49, 169.12 and 169.02 (7 × s, COCH3), 165.93 (s, C-1′), 162.84 (ds, J1,F 27.8 Hz, C-1), 109.03 (ds, J2,F 230.2 Hz, C-2), 101.66 (s, C-2′), 69.74 (dd, J6,F 4.9 Hz, C-6), 68.60 (d, C-6′), 67.62 (d, C-7′), 67.47 (d, C-7), 65.30 (d, C-4′), 64.56 (d, C-4), 64.01 (t, C-8), 63.08 (d, C-5′), 62.77 (d, C-5), 62.13 (t, C-8′), 53.41 (q, CO2CH3), 53.03 (q, CO2CH3), 21.37 (d, C-3′), 20.90, 20.78, 20.74, 20.67, 20.58 and 20.51 (6 × q, 7C, COCH3), 18.32 (dd, J3,F 30.6 Hz, C-3); 19F NMR (CDCl3): δ - 103.32; ESI-TOF HRMS: m/z = 1052.0570; calcd for C32H41FI2O21NH4+: 1052.0552.
Methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-3-iodo-d-glycero-α-d-talo-oct-2-ulopyranosyl)onate-(2→8)-methyl (4,5,7-tri-O-acetyl-3-deoxy-3-iodo-d-glycero-α-d-talo-oct-2-ulopyranosyl)onate-(2→4)-methyl (methyl 7,8-O-carbonyl-3-deoxy-α-d-manno-oct-2-ulopyranosid) onate (28)
A suspension of 10 (11.4 mg, 0.039 mmol) and 27 (48.4 mg, 0.047 mmol) in dry CH2Cl2 (2.0 mL) containing 5 Å grounded molecular sieves (~50 mg) was stirred at ambient temperature for 2 h under an atmosphere of argon. The mixture was then cooled to 0 °C and trifluoromethanesulfonic acid (1.1 μL, 0.006 mmol) in dry CH2Cl2 (0.2 mL) was added dropwise. After stirring at ambient temperature for 1 h aq NaHCO3 and CH2Cl2 were added, the phases were separated and the aqueous phase was once again extracted with CH2Cl2. The combined organic phases were washed consecutively with aq NaHCO3, aq NaS2O3 (5 w%) and brine, dried (MgSO4), filtered and concentrated. The crude mixture was purified by column chromatography (n-hexane/EtOAc 1:1 → 1:2) providing 28 (37.2 mg, 73%) as a colorless amorphous solid; [α]D20 + 53.8 (c 0.67, CHCl3); Rf 0.22 (toluene/EtOAc 1:1, HP-TLC); 1H NMR (CDCl3): δ 5.43 - 5.36 (m, 4H, H-5′, H-5″, H-7′, H-7″), 4.92 (ddd, 1H, J7,8b 8.3, J7,8a 6.9, J7,6 4.0 Hz, H-7), 4.89 (dd, 1H, J4″,3″ 4.7, J4″,5″ 3.5 Hz, H-4″), 4.86 (dd, 1H, J4′,3′ 4.9, J4′,5′ 3.4 Hz, H-4′), 4.74 - 4.70 (m, 2H, H-8a, H-8″a), 4.55 (app t, 1H, J8b,8a 9.0 Hz, H-8b), 4.52 - 4.49 (m, 2H, H-3′, H-3″), 4.24 - 4.20 (m, 2H, H-6″, H-8″b), 4.18 (ddd, 1H, J4,3ax 11.8, J4,3eq 5.2, J4,5 3.1 Hz, H-4), 4.06 (dd, 1H, J6′,7′ 7.6, J6′,5′ 2.0 Hz, H-6′), 3.88 - 3.85 (m, 7H, H-6, 2 × CO2CH3), 3.80 (s, 3H, CO2CH3), 3.79 - 3.74 (m, 2H, H-8′a, H-8′b), 3.68 - 3.66 (m, 1H, H-5), 3.27 (s, 3H, OCH3), 2.45 - 2.43 (m, 1H, OH), 2.14 (s, 3H, COCH3), 2.12 (s, 3H, COCH3), 2.10 (app t, 1H, H-3ax), 2.10, 2.09, 2.06, 2.05 and 1.98 (5 × s, each 3H, COCH3), 1.95 (dd, 1H, J3eq,3ax 12.5 Hz, H-3eq); 13C NMR (CDCl3): δ 170.39, 170.10, 169.91, 169.48, 169.36, 169.31 and 169.27 (7 × s, COCH3), 167.14, 166.94 and 165.44 (3 × s, C-1, C-1′, C-1″), 154.60 [s, O(C=O)O], 101.25 and 100.54 (2 × s, C-2′, C-2″), 98.87 (s, C-2), 75.69 (d, C-7), 71.03 (d, C-6′), 70.46 (d, C-6), 69.34 (d, C-4), 68.56 (d, C-7′), 68.41 (d, C-6″), 67.62 (d, C-7″), 66.11 (t, C-8), 65.27 (d, C-4″), 64.99 (d, C-4′), 64.36 (d, C-5), 64.31 (t, C-8′), 63.12 (d, C-5′), 63.03 (d, C-5″), 62.06 (t, C-8″), 53.43, 53.01 and 52.86 (3 × q, CO2CH3), 51.31 (q, OCH3), 32.77 (t, C-3), 21.56 and 21.24 (2 × d, C-3′, C-3″), 20.92, 20.86, 20.77, 20.73, 20.72, 20.51 and 20.39 (7 × q, COCH3); ESI-TOF HRMS: m/z = 1324.1275; calcd for C43H56I2O30NH4+: 1324.1284.
Methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-3-iodo-d-glycero-α-d-talo-oct-2-ulopyranosyl)onate-(2→8)-methyl (4,5,7-tri-O-acetyl-3-deoxy-3-iodo-d-glycero-α-d-talo-oct-2-ulopyranosyl)onate-(2→4)-methyl (methyl 5-O-acetyl-7,8-O-carbonyl-3-deoxy-α-d-manno-oct-2-ulopyr-anosid)onate (29)
A solution of 28 (36.4 mg, 0.028 mmol) in dry pyridine (2.0 mL) was treated with acetic anhydride (0.1 mL) and 4-(N,N-dimethylamino)-pyridine (0.5 mg, 0.004 mmol) at ambient temperature for 12 h. Excess of reagent was destroyed by slow addition of dry MeOH (1 mL) at 0 °C and after 10 min the solvent was removed by coevaporation with toluene (3 x). The residue was purified by column chromatography (n-hexane/EtOAc 2:3) yielding 29 (35.5 mg, 95%) as a colorless amorphous solid; [α]D20 + 61.9 (c 0.62, CHCl3); Rf 0.44 (n-hexane/EtOAc 1:2); 1H NMR (CDCl3): δ 5.41 - 5.35 (m, 4H, H-5′, H-5″, H-7′, H-7″), 5.05 - 5.02 (m, 1H, H-5), 4.90 (dd, 1H, J4″,3″ 4.7, J4″,5″ 3.6 Hz, H-4″), 4.86 (dd, 1H, J4′,3′ 4.6, J4′,5′ 3.7 Hz, H-4′), 4.80 (ddd, 1H, J7,8b 8.3, J7,8a 6.1, J7,6 3.6 Hz, H-7), 4.72 (dd, 1H, J8″a,8″b 12.4, J8″a,7″ 2.4 Hz, H-8″a), 4.57 (dd, 1H, J8a,8b 8.4 Hz, H-8a), 4.52 - 4.50 (m, 1H, H-3″), 4.37 - 4.32 (m, 2H, H-3′, H-8b), 4.27 - 4.21 (m, 3H, H-4, H-6″, H-8″b), 4.05 (dd, 1H, J6′,7′ 7.7, J6′,5′ 2.1 Hz, H-6′), 3.98 (dd, 1H, J6,5 1.0 Hz, H-6), 3.87, 3.83 and 3.81 (3 × s, each 3H, CO2CH3), 3.79 - 3.76 (m, 2H, H-8′a, H-8′b), 3.27 (s, 3H, OCH3), 2.16 – 2.11 (m, 1H, H-3ax), 2.15, 2.13, 2.11, 2.09 and 2.089 (5 × s, each 3H, COCH3), 2.06 – 2.01 (m, 1H, H-3eq), 2.04, 2.036 and 1.97 (3 × s, each 3H, COCH3); 13C NMR (CDCl3): δ 170.35, 170.14, 170.06, 170.02, 169.41, 169.35, 169.33 and 169.26 (8 × s, COCH3), 166.95, 165.47 and 165.44 (3 × s, C-1, C-1′, C-1″), 154.09 [s, O(C=O)O], 101.28 and 101.12 (2 × s, C-2′, C-2″), 98.85 (s, C-2), 74.97 (d, C-7), 71.19 (d, C-6′), 70.37 (d, C-6), 68.86 (d, C-7′), 68.44 and 68.31 (2 × d, C-4, C-6″), 67.75 (d, C-7″), 66.40 (d, C-5), 65.32 (d, C-4″), 64.90 (d, C-4′), 64.66 and 64.54 (2 × t, C-8, C-8′), 63.30 (d, C-5′), 63.18 (d, C-5″), 61.92 (t, C-8″), 53.29, 53.05 and 52.94 (3 × q, CO2CH3), 51.56 (q, OCH3), 34.10 (t, C-3), 21.75 (d, C-3′), 21.48 (d, C-3″), 20.97, 20.89, 20.84, 20.74, 20.54 and 20.41 (6 × q, 8C, COCH3); ESI-TOF HRMS: m/z = 1366.1338; calcd for C45H58I2O31NH4+: 1366.1390.
Methyl (4,5,7,8-tetra-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→8)-methyl (4,5,7-tri-O-acetyl-3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→4)-methyl (methyl 5-O-acetyl-7,8-O-carbon-yl-3-deoxy-α-d-manno-oct-2-ulopyranosid)onate (30)
Following the general procedure for dehalogenation 29 (26.6 mg, 0.020 mmol) was treated with lauroyl peroxide (4.7 mg, 0.012 mmol) in dry cyclohexane (4.0 mL) for 24 h. Evaporation of the solvent and purification of the residue by column chromatography (n-hexane/EtOAc 1:2) afforded 30 (19.2 mg, 89%) as an amorphous white solid; [α]D20 + 82.3 (c 0.43, CHCl3); Rf 0.24 (toluene/EtOAc 1:2, HP-TLC); 1H NMR (CDCl3): δ 5.35 - 5.30 (m, 3H, H-5′, H-5″, H-7′), 5.26 - 5.21 (m, 2H, H-5, H-7″), 5.14 - 5.09 (m, 2H, H-4′, H-4″), 4.76 (ddd, 1H, J7,8b 8.3, J7,8a 6.4, J7,6 4.3 Hz, H-7), 4.64 (dd, 1H, J8″a,8″b 12.3, J8″a,7″ 2.4 Hz, H-8″a), 4.62 (dd, 1H, J8a,8b 8,5 Hz, H-8a), 4.55 (ddd, 1H, J4,3ax 11.8, J4,3eq 5.0, J4,5 2.9 Hz, H-4), 4.39 (app t, 1H, H-8b), 4.21 (dd, 1H, J8″b,7″ 4.9 Hz, H-8″b), 4.08 (dd, 1H, J6″,7″ 9.8, J6″,5″ 1.4 Hz, H-6″), 4.05 (dd, 1H, J6,5 1.4 Hz, H-6), 3.94 (dd, 1H, J8′a,8′b 10.6, J8′a,7′ 2.9 Hz, H-8′a), 3.89 (dd, 1H, J6′,7′ 8.1, J6′,5′ 1.4 Hz, H-6′), 3.84, 3.83 and 3.80 (3 × s, each 3H, CO2CH3), 3.66 (dd, 1H, J8′b,7′ 9.5 Hz, H-8′b), 3.27 (s, 3H, OCH3), 2.14 - 1.94 (m, 30H, 8 × COCH3, H-3ax, H-3eq, H-3′ax, H-3′eq, H-3″ax, H-3″eq); 13C NMR (CDCl3): δ 170.39, 170.31, 169.99, 169.96, 169.88, 169.64 and 169.54 (7 × s, 8C, COCH3), 167.14, 167.03 and 166.94 (3 × s, C-1, C-1′, C-1″), 154.16 [s, O(C=O)O], 99.12, 98.31 and 97.75 (3 × s, C-2, C-2′, C-2″), 74.87 (d, C-7), 71.37 (d, C-6′), 70.38 (d, C-6), 68.84 (d, C-7′), 68.47 (d, C-6″), 67.86 (d, C-7″), 66.63 (d, C-5), 66.34 and 66.20 (2 × d, C-4′, C-4″), 65.93 (d, C-4), 65.21 (t, C-8), 64.24 (d, C-5′), 64.21 (d, C-5″), 62.79 (t, C-8′), 62.03 (t, C-8″), 53.05, 52.83 and 52.69 (3 × q, CO2CH3), 51.35 (q, OCH3), 34.16 (t, C-3), 31.54 and 31.35 (2 × t, C-3′, C-3″), 20.72, 20.67, 20.61 and 20.54 (4 × s, 8C, COCH3); ESI-TOF HRMS: m/z = 1114.3440; calcd for C45H60O31NH4+: 1114.3457.
Sodium (3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→8)- sodium (3-deoxy-α-d-manno-oct-2-ulopyranosyl)onate-(2→4)-sodium (methyl 3-deoxy-α-d-manno-oct-2-ulopyranosid)onate (31)
According to the general procedure for global deprotection a solution of 30 (10.2 mg, 0.009 mmol) in dry MeOH (2.0 mL) was treated with NaOMe (0.1 M in MeOH; 157 μL, 0.015 mmol), which was added in 3 portions within 24 h. After complete addition stirring was continued for 4 h. After the described work-up the residue was dissolved in water (2.0 mL) and treated with aq NaOH (0.1 M, 1.0 mL). After 3 h it was subjected to standard work-up und the crude product was desalted by purification on a PD10 column (H2O) followed by lyophilisation of pooled fractions affording 31 (6.5 mg, 93%) as an amorphous colorless solid; [α]D20 + 82.6 (c 0.64, D2O); 1H NMR (D2O, pH ~ 6.5): δ 4.12 - 4.06 (m, 2H, H-7′, H-4″), 4.03 - 3.99 (m, 4H, H-4′, H-5, H-5′, H-5″), 3.97 - 3.84 (m, 5H, H-4, H-7, H-7″, H-8a, H-8″a), 3.70 -3.64 (m, 3H, H-6″, H-8′a, H-8″b), 3.62 - 3.55 (m, 2H, H-6′, H-8b), 3.47 (dd, 1H, J8′b,8′a 10.2, J8′b,7′ 8.3 Hz, H-8′b), 3.44 (dd, 1H, J6,7 8.8, J6,5 1.0 Hz, H-6), 3.09 (s, 3H, OCH3), 2.06 (ddd, 1H, J3′eq,3′ax 13.3, J3′eq,4′ 4.8, J3′eq,5′ 0.8 Hz, H-3′eq), 2.03 - 1.99 (m, 1H, H-3″eq), 1.90 - 1.71 (m, 4H, H-3eq, H-3ax, H-3′ax, H-3″ax); 13C NMR (D2O, pH ~ 6.5): δ 177.00, 176.14 and 175.91 (3 × s, C-1, C-1′, C-1″), 101.32, 100.76 and 100.42 (3 × s, C-2, C-2′, C-2″), 73.10 (d, C-6′), 72.09 and 72.01 (2 × d, C-6, C-6″), 70.47, 70.45 and 70.26 (3 × d, C-7, C-7′, C-7″), 69.76 (d, C-4), 67.55 and 67.27 (2 × d, C-5′, C-5″), 66.85 and 66.68 (2 × d, C-4′, C-4″), 65.05 (d, C-5), 65.00 (t, C-8′), 64.02 (2t, C-8, C-8″), 51.40 (q, OCH3), 35.31 and 35.09 (2 × t, C-3′, C-3″), 34.02 (t, C-3); ESI-TOF HRMS: m/z = 715.1938; calcd for C25H40O22Na+: 715.1903.
Supplementary Material
Acknowledgements
This work was supported by the Austrian Science Fund, FWF (grant P 24921).
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].Raetz CR, Whitfield C. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].a) Holst O. In: Endotoxin in Health and Disease. Brade H, Opal SM, Vogel SN, Morrison DC, editors. Marcel Dekker; New York: 1999. pp. 115–154. [Google Scholar]; b) Holst O. FEMS Microbiol. Lett. 2007;271:3–11. doi: 10.1111/j.1574-6968.2007.00708.x. [DOI] [PubMed] [Google Scholar]; c) Holst O. In: Bacterial Lipopolysaccharides. Knirel YA, Valvano MA, editors. Springer; Wien-New York: 2011. pp. 21–39. [Google Scholar]
- [3].Schachter J. In: Chlamydia: Intracellular biology, pathogenesis & immunity. Stephens RS, editor. Amer. Society for Microbiology; Washington DC: 1999. pp. 150–196. [Google Scholar]
- [4].a) Morrison RP. J. Clin. Invest. 2003;111:1647–1649. doi: 10.1172/JCI18770. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bebear C, de Barbeyrac B. Clin. Microbiol. Infect. 2009;15:4–10. doi: 10.1111/j.1469-0691.2008.02647.x. [DOI] [PubMed] [Google Scholar]; c) Carey AJ, Beagley KW. Amer. J. Repr. Immunol. 2010;63:576–586. doi: 10.1111/j.1600-0897.2010.00819.x. [DOI] [PubMed] [Google Scholar]
- [5].a) Beatty W, Morrison RP, Byrne GJ. Microb. Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Corsaro D, Valassina M, Venditti D. Crit. Rev. Microbiol. 2003;29:37–78. doi: 10.1080/713610404. [DOI] [PubMed] [Google Scholar]; c) Campbell LA, Kuo CC. Nature Rev. Microbiol. 2004;2:23–32. doi: 10.1038/nrmicro796. [DOI] [PubMed] [Google Scholar]
- [6].a) Brade H, Brade L, Nano FE. Proc. Natl. Acad. Sci. USA. 1987;84:2508–2512. doi: 10.1073/pnas.84.8.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Holst O, Brade L, Kosma P, Brade H. J. Bacteriol. 1991;173:1862–1866. doi: 10.1128/jb.173.6.1862-1866.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Holst O, Bock K, Brade H. Eur. J. Biochem. 1995;229:194–200. [PubMed] [Google Scholar]
- [8].Hanuszkiewicz A, Hübner G, Vinogradov E, Lindner B, Brade L, Brade H, Debarry H, Heine H, Holst O. Chem. Eur. J. 2008;14:10251–10258. doi: 10.1002/chem.200800958. [DOI] [PubMed] [Google Scholar]
- [9].a) Gomery K, Müller-Loennies S, Brooks CL, Brade L, Kosma P, di Padova F, Brade H, Evans SV. Proc. Natl. Acad. Sci. USA. 2012;109:20877–20882. doi: 10.1073/pnas.1209253109. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]; c) Nguyen HP, Seto NOL, MacKenzie CR, Brade L, Kosma P, Brade H, Evans SV. Nature Struct. Biol. 2003;10:1019–1025. doi: 10.1038/nsb1014. [DOI] [PubMed] [Google Scholar]; Parker MJ, Gomery K, Richard G, MacKenzie CR, Cox AD, Richards JC, Evans SV. Glycobiol. 2014;24:442–449. doi: 10.1093/glycob/cwu009. [DOI] [PubMed] [Google Scholar]
- [10].Astronomo RD, Burton DR. Nature Rev. Drug Discov. 2010;9:308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) Unger FM. Adv. Carbohydr. Chem Biochem. 1981;38:323–388. [Google Scholar]; b) Oscarson S, Hansson J. Curr. Org. Chem. 2000;4:535–564. [Google Scholar]; c) Kosma P. In: Microbial Glycobiology. Moran AP, Holst O, Brennan PJ, von Itzstein M, editors. Elsevier; Amsterdam: 2009. pp. 429–454. [Google Scholar]
- [12].For examples see:; a) Paulsen H, Krogmann C. Carbohydr. Res. 1990;205:31–44. doi: 10.1016/0008-6215(90)80125-m. [DOI] [PubMed] [Google Scholar]; b) Kiso M, Fujita M, Ogawa Y, Ishida H, Hasegawa A. Carbohydr. Res. 1990;196:59–73. doi: 10.1016/0008-6215(90)84106-5. [DOI] [PubMed] [Google Scholar]; c) Boons GJPH, van Delft FL, van der Klein PAM, van der Marel GA, van Boom JH. Tetrahedron. 1992;48:885–904. [Google Scholar]; d) Shimoyama A, Saeki A, Tanimura N, Tsutsui H, Miyake K, Suda Y, Fujimoto Y, Fukase K. Chem. Eur. J. 2011;17:1464–14474. doi: 10.1002/chem.201003581. [DOI] [PubMed] [Google Scholar]; e) Shimoyama A, Fujimoto Y, Fukase K. Synlett. 2011:2359–2362. [Google Scholar]; f) Boltje TJ, Zhong W, Park J, Wolfert MA, Chen W, Boons G-J. J. Am. Chem. Soc. 2012;134:14255–4262. doi: 10.1021/ja306274v. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Yi R, Ogaki A, Fukunaga M, Nakajima H, Ichiyanagi T. Tetrahedron. 2014;70:3675–3682. [Google Scholar]
- [13].For examples see:; a) Yoshizaki H, Fukuda N, Sato K, Oikawa M, Fukase K, Suda Y, Kusumoto S. Angew. Chem. Int. Ed. 2001;40:1475–1480. doi: 10.1002/1521-3773(20010417)40:8<1475::AID-ANIE1475>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]; b) Kusumoto S, Kusunose N, Kamikawa T, Shiba T. Tetrahedron Lett. 1988;29:6325–6326. [Google Scholar]; c) Fujimoto Y, Iwata M, Imakita N, Shimoyama A, Suda Y, Kusumoto S, Fukase K. Tetrahedron Lett. 2007;48:6577–6581. [Google Scholar]
- [14].a) Mannerstedt K, Ekelöf K, Oscarson S. Carbohydr. Res. 2007;342:631–637. doi: 10.1016/j.carres.2006.08.021. [DOI] [PubMed] [Google Scholar]; b) Ikeda K, Akamatsu S, Achiwa K. Chem. Pharm. Bull. 1990;38:279–281. doi: 10.1248/cpb.38.1766. [DOI] [PubMed] [Google Scholar]; c) Ikeda K, Akamatsu S, Achiwa K. Carbohydr. Res. 1989;189:C1–C4. [Google Scholar]; d) Yang Y, Martin CE, Seeberger PH. Chem. Sci. 2011;3:896–899. [Google Scholar]; e) Ekelöf K, Oscarson S. Carbohydr. Res. 1995;278:289–300. doi: 10.1016/0008-6215(95)00269-3. [DOI] [PubMed] [Google Scholar]
- [15].Pradhan TK, Lin CC, Mong K-KT. Org. Lett. 2014;16:1474–1477. doi: 10.1021/ol500275j. [DOI] [PubMed] [Google Scholar]
- [16].Tanaka H, Takahashi D, Takahashi T. Angew. Chem. Int. Ed. 2006;45:770–773. doi: 10.1002/anie.200503299. [DOI] [PubMed] [Google Scholar]
- [17].Claesson A, Luthman K. Acta Chem. Scand. B. 1982;36:719–720. [Google Scholar]
- [18].Unger FM, Stix D, Schulz G. Carbohydr. Res. 1980;80:191–195. [Google Scholar]
- [19].Kosma P, Sekljic H, Balint G. J. Carbohydr. Chem. 1996;15:701–714. [Google Scholar]; The yield of 4 could be improved to 78%.
- [20].For examples see:; a) Imoto M, Kusunose N, Kusumoto S, Shiba T. Tetrahedron Lett. 1988;29:2227–2230. [Google Scholar]; b) Imoto M, Kusunose N, Matsuura Y, Kusumoto S, Shiba T. Tetrahedron Lett. 1987;28:6277–6280. [Google Scholar]; c) Ichiyanagi T, Fukunaga M, Tagashira R, Hayashi S, Nanjo M, Yamasaki R. Tetrahedron. 2011;67:5964–5971. [Google Scholar]; d) Rosenbrook W, Jr., Riley DA, Lartey PA. Tetrahedron Lett. 1985;26:3–4. [Google Scholar]
- [21].Solomon D, Fridman M, Zhang J, Baasov T. Org. Lett. 2001;3:4311–4314. doi: 10.1021/ol010257h. [DOI] [PubMed] [Google Scholar]
- [22].a) Williamson KL, Hsu Y-FL, Hall FH, Swager S, Coulter MS. J. Am. Chem. Soc. 1968;90:6717–6722. [Google Scholar]; b) Hall LD, Manville JF. Can. J. Chem. 1967;45:1299–1303. [Google Scholar]
- [23].Boivin J, Quiclet-Sire B, Ramos L, Zard SZ. Chem. Commun. 1997:353–354. [Google Scholar]
- [24].Brade H, Zähringer U, Rietschel ET, Christian R, Schulz G, Unger FM. Carbohydr. Res. 1984;134:157–166. [Google Scholar]
- [25].a) Ziegler T, Eckhardt E, Pantkowski G. J. Carbohydr. Chem. 1994;13:81–109. [Google Scholar]; b) D’Souza FW, Kosma P, Brade H. Carbohydr. Res. 1994;262:223–244. doi: 10.1016/0008-6215(94)84181-0. [DOI] [PubMed] [Google Scholar]
- [26].a) Kosma P, Hofinger A, Mueller-Loennies S, Brade H. Carbohydr. Res. 2010;345:704–708. doi: 10.1016/j.carres.2009.12.015. [DOI] [PubMed] [Google Scholar]; b) Gerstenbruch S, Brooks CL, Kosma P, Brade L, MacKenzie CR, Evans SV, Brade H, Müller-Loennies S. Glycobiol. 2010;20:461–472. doi: 10.1093/glycob/cwp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].a) Bock K, Thomsen JU, Kosma P, Christian R, Holst O, Brade H. Carbohydr. Res. 1992;229:213–224. doi: 10.1016/s0008-6215(00)90571-8. [DOI] [PubMed] [Google Scholar]; b) Rund S, Lindner B, Brade H, Holst O. Eur. J. Biochem. 2000;267:5717–5726. doi: 10.1046/j.1432-1327.2000.01635.x. [DOI] [PubMed] [Google Scholar]
- [28].Harris RK, Becker ED, de Menezes SMC, Goodfellow R, Granger P. Pure Appl. Chem. 2001;73:1795–1818. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.