Abstract
Objectives
Manual and physical therapists incorporate neurodynamic mobilisation (NDM) to improve function and decrease pain. Little is known about the mechanisms by which these interventions affect neural tissue. The objective of this research was to assess the effects of repetitive straight leg raise (SLR) NDM on the fluid dynamics within the fourth lumbar nerve root in unembalmed cadavers.
Methods
A biomimetic solution (Toluidine Blue Stock 1% and Plasma) was injected intraneurally, deep to the epineurium, into the L4 nerve roots of seven unembalmed cadavers. The initial dye spread was allowed to stabilise and measured with a digital calliper. Once the initial longitudinal dye spread stabilised, an intervention strategy (repetitive SLR) was applied incorporating NDMs (stretch/relax cycles) at a rate of 30 repetitions per minute for 5 minutes. Post-intervention calliper measurements of the longitudinal dye spread were measured.
Results
The mean experimental posttest longitudinal dye spread measurement (1.1 ± 0.9 mm) was significantly greater (P = 0.02) than the initial stabilised pretest longitudinal dye spread measurement. Increases ranged from 0.0 to 2.6 mm and represented an average of 7.9% and up to an 18.1% increase in longitudinal dye spread.
Discussion
Passive NDM in the form of repetitive SLR induced a significant increase in longitudinal fluid dispersion in the L4 nerve root of human cadaveric specimen. Lower limb NDM may be beneficial in promoting nerve function by limiting or altering intraneural fluid accumulation within the nerve root, thus preventing the adverse effects of intraneural oedema.
Keywords: Lumbar, Nerve root, Neuropathic pain, Nerve injury, Intraneural oedema
Background
Low back pain (LBP) and nerve root compression syndromes are common conditions that are multifactorial. Neural injuries that accompany LBP demonstrate intraneural oedema as a common response that can contribute to neural ischaemia, permeability changes, nerve structural damage, and functional alterations.1–3 The absence of lymphatic vessels in the endoneurium lends to limited oedema drainage,3,4 thereby creating the potential for a ‘minicompartment syndrome’ within the neural tube.5,6 This ‘minicompartment syndrome’ is a result of increased endoneurial pressure and may cascade to fibrosis and adhesions impairing neural interfascicular gliding.5
Neurodynamic mobilisation (NDM) is often used in clinical practice for managing patients with LBP and nerve root compromise. Although the techniques appear beneficial,7–11 their mechanisms of action remain unclear. The NDM techniques include passive or active movements that focus on restoring the ability of the nervous system to tolerate the normal compressive, frictional and tensile forces associated with daily activities.12 The hypothesised benefits from these techniques include pain reduction, nerve glide facilitation, nerve adherence diminution, dispersion of noxious fluids, increased neural vascularity and improvements in axoplasmic flow.7,8,10,12–14 Selected textbooks15,16 describe NDM techniques but echo a lack in both the quantity and quality of available research that explains and supports such a therapeutic approach. Moreover, most clinical trials have evaluated cervicobrachial nerves,17–22 while a qualitative analysis revealed limited evidence to support the use of NDM.10
For potentially persistent and debilitating conditions, such as LBP and lower limb peripheral neuropathic pain, selected neural mobility tests commonly used in practice include the straight leg raise (SLR), the passive neck flexion (PNF) test, the slump test and the prone knee bend test.11,15,16 Other manifestations of the SLR are emerging with bias to other terminal branches such as the sural nerve.23 The same test positions and movements can be used as NDM treatment techniques with or without modifications.15,16 However, a contemporary clinical evidence-supported practice model for the use of these manoeuvres in patients suffering from LBP or after spinal surgery is sparse and conflicting. Kitteringham24 observed that SLR angle improvements were not significantly different at 6 weeks after lumbar surgical decompression between two groups of patients treated with different frequencies of NDM exercises performed in the SLR direction. Scrimshaw and Maher25 noted a lack of additional benefit from NDM (SLR) on global perceived effect, pain and disability in a 12-month follow-up trial when compared to standard physiotherapy treatment after spinal surgery (laminectomy, discectomy and fusion). Conversely, Bertolini et al.26 observed a greater decrease of pain in rats with experimentally induced sciatica following NDM (SLR + ankle alternately moved in passive plantarflexion/dorsiflexion) as compared to hindlimb static stretching (SLR with 70° of hip flexion, maximum knee extension and ankle dorsiflexion). Nagrale et al.27 examined the effects of NDM in patients with non-radicular LBP who received 3 weeks of physiotherapy. They found that patients receiving slump stretching in addition to lumbar spinal mobilisation with exercise experienced greater improvements in Oswestry disability index, pain and fear-avoidance belief questionnaires at a 6-week follow-up versus patients who received spinal mobilisation and stabilisation exercises without slump stretching.27
In spite of the clinical influence of NDM on patients' symptoms, the underlying mechanisms for this response are unclear. Initial considerations lead clinicians to suspect a mechanical influence. The perspective of movement and gliding may apply in the context of peripheral nerves within the limbs23,28; however, Ridehalgh et al.29 indicated that the mobility of the lumbar roots were not available using their measurement techniques, and findings by Smith et al.30 were obtained incorporating significant dissection, specifically, laminectomy and facetectomy. Gilbert et al.31,32 found that an in situ SLR produced minimal displacement ( < 1.0 mm) of the L4, L5 and S1 nerve roots, with 60° of hip flexion needed to induce significant movement of the roots. It is doubtful that such small movements during NDM would free up scar tissue/fibrotic adhesions within the intervertebral foramen or lateral recess and increase lumbar nerve root mobility, and while selected patients continue to experience clinical benefits in response to NDM, the mechanisms behind the symptom improvement remain unclear. A recent in situ cadaveric investigation showed that repetitive ankle plantar and dorsiflexion NDM-induced intraneural fluid dispersion within the tibial nerve, suggesting a passive mechanical effect of NDM on peripheral intraneural fluid behaviour.6 A similar finding was noted with in vitro peripheral nerve tissue using a standardised mechanically delivered simulated NDM strategy.33 However, the effect of NDM on intraneural fluid dispersion within lumbar nerve roots has not been investigated.
The purpose of this study was to assess the effects of repetitive SLR neural mobilisation on the fluid dynamics within the fourth lumbar nerve root in unembalmed cadavers. We hypothesised that NDMs performed through passive SLR would induce fluid dispersion in the lateral recess/foraminal region of the fourth lumbar root.
Methods
Experimental design
A pretest–posttest repeated measures design.
Dissection and specimens
This study included seven unembalmed cadavers (four females; three males) with a mean age of 74.0 ± 10.3 years, height of 173.8 ± 8.9 cm and weight of 73.1 ± 12.0 kg (Table 1). Cadaveric specimens were handled in accordance with university policy and State of Texas regulations as determined by the Texas Anatomical Board.
Table 1.
Cadaver demographics
| Cadaver number | Sex | Age (years) | Height (cm) | Weight (kg) |
|---|---|---|---|---|
| 1 | Female | 65 | 167.6 | 59.1 |
| 2 | Male | 70 | 182.9 | 90.9 |
| 3 | Female | 66 | 167.7 | 68.2 |
| 4 | Male | 86 | 182.9 | 75.1 |
| 5 | Male | 86 | 180.3 | 72.2 |
| 56 | Female | 82 | 175.3 | 85.7 |
| 7 | Female | 63 | 160.1 | 60.5 |
| Mean | 74.0 | 173.8 | 73.1 | |
| SD | 10.3 | 8.9 | 12.0 |
SD: standard deviation.
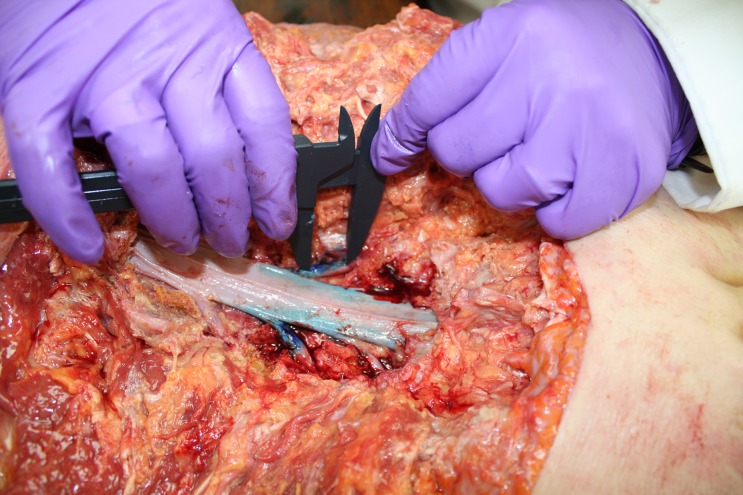
Cadavers were positioned prone and the skin, subcutaneous tissue, and fascia were reflected from the lumbar region from the first lumbar (L1) to first sacral (S1) levels. The fourth lumbar (L4) nerve roots were exposed bilaterally via posterior dissection (laminectomy and facetectomy) from L3 to L5. After dissection and clear visualisation of the L4 root, 0.2 cc of dye solution (2:1 human plasma: 1% Toluidine Blue Stock Solution)34 was injected into the L4 nerve root just proximal to the dorsal root ganglion expansion. This injection was administered via a syringe (1 cc) and a 0.45 × 23 mm needle at a location just deep to the epineurium. The initial injection longitudinal dye spread was measured using a digital calliper (Digimax 6″ precision digital calliper, Wiha Quality Tools, LLC, Monticello, MN, 55362 USA). Measurements were taken as described previously,6,33 including the most distinct proximal and distal borders of the dye spread (Fig. 1). One investigator was responsible for dye spread measurements and was blinded to the measurement results. The investigators previously established the high reliability of this dye spread measurement technique (r = 0.987).6,33
Figure 1.
Data collection: dye spread measurement at the right L4 nerve root using digital calliper – notice the dye spread in the intradural space (darker colour)
Pretest measurements
The initial longitudinal dye spread was noted, and the injected fluid spread was allowed to stabilise for 5 to 15 minutes. In order to maximise measurement reliability and decrease error, three separate dye spread measurements were taken at each interval (5 minutes) and values were averaged. As previously described,6,33 the dye spread was considered stabilised when the average of three measurements at each time frame (5-minute intervals) was within 0.5 mm of the previous measurement.
Experimental nerve segments
Once the dye spread was stabilised, the NDMs (stretch/relax cycles) were applied. One researcher performed 5 minutes of NDM (intervention consisting of prone SLR with the experimental lower limb off the side of the table) on the experimental limb at a rate of one stretch/relaxation cycle every 2 seconds (e.g., 150 stretch/relax cycles across 5 minutes). Mobilisation was induced from resting position to 90° hip flexion with the knee and ankle/foot in maximal extension and dorsiflexion, respectively. After the mobilisation cycles, three longitudinal dye spread measurements were taken and averaged. This ‘posttest’ measurement was compared to the pretest measurement to determine if a change in longitudinal dye spread occurred from the initially stabilised condition within the experimental nerve segment. While the prone position for SLR NDM differs from the typical clinical application, a supine position would have disallowed the calliper measurements. Care was taken to allow pelvic movement during SLR NDM as would have occurred in a supine position.
Control nerve segments
Previous studies indicated no significant longitudinal dye spread movement within an immobilised control limb or nerve segment.6,33 Therefore, the current study focussed on the comparison between the pre-mobilisation and post-mobilisation measurement on the experimental limb. In addition, a preliminary observation of the control behaviour in the lumbar roots was conducted on four cadavers. In this case, the control limb underwent the same dye stabilisation procedures described above followed by a 5-minute ‘non-mobilisation’ period, where no NDM (stretch/relaxation) was performed. The ‘posttest’ measurements were taken at the end of the 5-minute ‘non-mobilisation/waiting’ period in the fashion previously described.
Statistical analysis
The mean, standard deviation, ranges and confidence intervals were calculated for the length of the longitudinal dye spread within the experimental nerve segments during pretest and posttest conditions.
Inferential analysis (α = 0.05) was conducted using SPSS (v17.0). The independent variable was time with two levels: pretest versus posttest. The dependent variable was the length of longitudinal dye spread. Although the sample size was small, the data were parametric in nature and met assumptions for normality, based on Shapiro–Wilk test outcomes of W = 0.827, P = 0.159 and W = 0.89, P = 0.384 (pre- and post-mobilisation spread measurements, respectively). In response, a paired samples t-test was used to compare pre-mobilisation measurements to post-mobilisation measurements.
Results
Quantitative findings
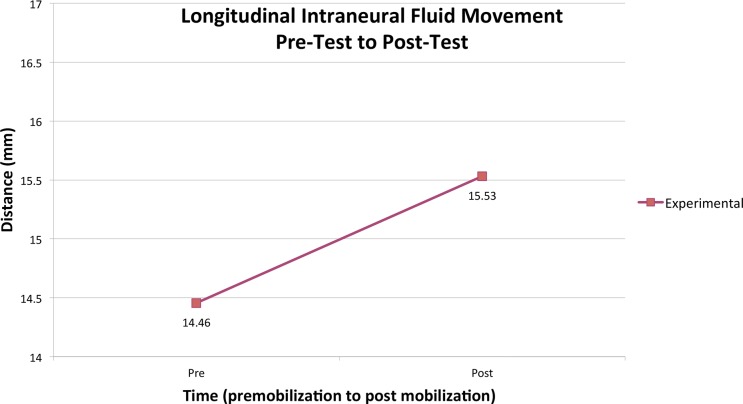
The intraneural fluid longitudinal dye spread increased as a result of NDM. The mean experimental posttest measurement was 1.1 ± 0.9 mm greater than the initial stabilised pretest measurements (Fig. 2). These increases ranged from 0.0 to 2.6 mm and represented an average of 7.9% and up to an 18.1% increase in longitudinal dye spread (Table 2). The results of the paired samples t-test demonstrated that these differences were statistically significant (t = − 3.157; P = 0.02). Concerning the preliminary control nerve segments, the mean change of the dye spread length was 0.2 ± 0.6 mm, which was not statistically significant (t = − 0765; P = 0.500) nor was the average spread greater than the criterion used for a ‘stabilised’ dye spread (i.e. less than 0.5 mm on an average of three measures at 5-minute increments as described in the Methods section of this manuscript as well as those of Brown et al.6 and Gilbert et al.6,33
Figure 2.
Longitudinal intraneural fluid dispersion (pretest to posttest).
Table 2.
Descriptive statistics – experimental slide
| Range | Mean | SD | 95% CI | |
|---|---|---|---|---|
| Initial dye spread (mm) | 9.9–16.7 | 14.4 | 2.2 | 1.8 |
| Stabilized dye spread (mm) | 9.3–17.0 | 14.5 | 2.5 | 2.0 |
| Time to stabilize (minutes) | 10.0–15.0 | 11.4 | 2.4 | 2.0 |
| Post-mobilization dye spread (mm) | 10.8–18.0 | 15.5 | 2.5 | 2.0 |
| Total dye spread length change post-mob (mm) | 0.0–2.6 | 1.1 | 0.9 | 0.7 |
| Percent change post-mob dye spread (%) | − 0.2–18.1 | 7.9 | 6.9 | 5.6 |
SD: standard deviation; CI: confidence interval.
Qualitative observations
At rest, the dura appeared relatively slack, likely due to the reduced cerebrospinal fluid within the dural sheath. During the mobilisation cycles, the dura filled with air/fluid upon return (to hip extension) from each lower extremity neural mobilisation (to hip flexion). In addition, the mobilisation cycles appeared to spread a portion of the injected dye proximally into the dural sac. However, we were unable to quantify the degree and extent of this fluid dispersion with the calliper. Additionally, on several cadavers, we noted increased blood leakage from surrounding tissues and vessels with the mobilisation, possibly from the lumbar venous plexus or surrounding vessels damaged by dissection. This ‘bleeding’ appeared to occur only during mobilisation cycles and was not present during the resting state once dissection was completed.
Discussion
This is the first study, to our knowledge, to investigate the effect of neural mobilisation using the SLR on fluid dispersion in the lateral recess/foraminal area of a lumbar nerve root. Results indicate that intraneural fluid within the neural tissue of L4 nerve root dispersed following repetitive SLR in the cadaveric specimens.
While previous studies of NDM investigated quantitative findings related to the lumbar nerve root mechanics of displacement and strain during lower limb movement,30–32 they provided no perspective regarding the mechanisms responsible for the perceived benefits of incorporating NDM. Intraneural oedema is a common response to nerve damage,2,4,35–37 regardless of the aetiology. Previous studies reported the common presence of neural oedema/inflammation surrounding compromised nerve roots both in animals38 and human beings.39,40 In human beings, the location of the swollen lumbar nerve root corresponded with the pain locations indicated in pain drawings from patients with sciatica.39 Therefore, researchers and clinicians have geared their management strategies towards therapeutic injections,41,42 low velocity mobilisations, and high velocity thrust manipulative therapies,43,44 all aimed at reducing LBP and nerve root irritation. Our hypothesis that NDM would lead to increased fluid dispersion in the L4 nerve root was supported. Longitudinal dye dispersion increases were statistically significant post-mobilisation compared to the pre-mobilisation stabilised longitudinal dye spread. These results are consistent with similar studies that examined a comparison between these pretest/posttest conditions, with matched controls (contralateral side).6,33 In these previous studies, the matched controls demonstrated no significant longitudinal dye spread during the ‘no-mobilisation’ period. The preliminary control segments tested in this study indicated the same general behaviour of no significant change in longitudinal dye spread movement during the ‘no-mobilisation’ period.
The qualitative observations expand our understanding of neural tissue response to NDM. We observed a proximal/cranial migration of the fluid into the dura in all cadaveric specimens following the SLR neural mobilisations. While the fluid dispersion was significant, the distal dispersion appeared to be limited to the level of the dorsal root ganglia. While our study did not objectify the reason for this behaviour, perhaps the dorsal root ganglion served as a mechanical barrier to further distal intraneural passive fluid movement. Future research should examine the specific mechanical influence of the dorsal root ganglion on fluid dispersion throughout the intraneural space.
The mechanisms responsible for the longitudinal dye spread during NDM may include fluid movement resulting from transverse narrowing of the nerve and/or intrafascicular gliding as it lengthens.45 During the NDM technique, the L4 nerve root underwent elongation and shortening cycles which may have produced a temporary rise and subsequent fall in intraneural pressure (i.e. an intraneural tissue pump as described by Gilbert et al.).33 It is possible that this repetitive stretch/relax cycle, or ‘pumping’ action, may have induced a flushing effect of the dye and possibly an intraneural pressure modification with intraneural fluid dispersion.
Changes in intraneural pressure appear to be clinically important as evidenced by studies demonstrating the adverse effects of increased intraneural pressure levels on neural tissue health and function.3,35 Neurodynamic techniques may promote healthy nerve function by promoting reduced oedema and changing intraneural pressure, which lead to improvement in axonal transport and prevention of the deposit of mechanosensitivity factors that result in pain and limitations in neural movement.46,47 During the initial stages of neural tension or compression injury, the prevention or reduction of oedema may limit compromise of blood flow.3,35 Improved blood flow may limit impaired axonal transport,36,48 demyelination,49,50 loss of elasticity due to fibrosis or adhesions,45,51 and ultimately, decrease nerve structural and functional compromise.1,49,52,53 The promotion of intraneural fluid dispersion through the incorporation of lower limb movement may provide a mechanism to disrupt the cycle of oedema formation and the subsequent consequences that contribute to fibrosis.54
In the current study, the cadavers were positioned prone with one limb maintained on the table. The treatment limb was positioned with the hip flexed to 90°, knee extended, and the ankle in 0° dorsiflexion. In this position, the SLR movement was performed at a rate of one mobilisation repetition every 2 seconds or 30 repetitions per minute for 5 minutes. Rhythmic mobilisation (stretch/relax) cycles were chosen as the mode of intervention since previous experiments in animals indicate a cessation of blood flow (arteriole and venous) and alterations in nerve conduction velocity during prolonged static stretching,55,56 as well as limitations in axonal transport during graded compression and sustained vibration.57,58 Since symptomatic patients are often unable to reach 90° of hip flexion during the SLR, future research should determine the minimum and optimal degree of hip flexion during SLR NDM that is necessary to obtain such fluid dispersion. Various degrees of ankle dorsiflexion during repeated SLR could be evaluated as well, as previous research indicated greater nerve root strain during the SLR with the ankle dorsiflexed,32 which may lead to greater fluid dispersion than with the ankle plantar flexed.
Outcomes-based clinical trial studies would be beneficial in assessing the outcomes of NDM in normal patients as well as those presenting with discogenic LBP and/or signs of nerve root compression and irritation. Additionally, studies examining various limb and spine positions on intraneural fluid dispersion would be beneficial, especially if these studies quantify intraneural pressure alterations.
Limitations of this study include the inability to generalise the findings to live patients based on three facts. First, because our study utilised cadaveric tissue, we could not account for the influence of active physiological processes within the nerve on fluid spread. However, by eliminating these processes, we were able to infer that the study's effects were consequent to a mechanical influence. Second, the posterior approach for dissection, including laminectomy/foraminectomy, was necessary to examine the fluid dynamics of the root level. We recognise that this level of dissection is substantial, and creates limitations to generalisability. Finally, we cannot generalise the findings to pathologically involved nerves, such as those with signs of decreases in elasticity due to fibrosis or adhesions,45,51 as these conditions may alter the manner in which fluid is dispersed. Future studies should examine these queries, as many patients with root-related pain exhibit such alterations.
In conclusion, this study demonstrated that passive NDM, in the form of repetitive SLR, led to a significant increase in longitudinal fluid dispersion in the L4 nerve root of human cadaveric specimens. Lower limb NDM may aid in preserving nerve function through an alteration of intraneural fluid accumulation within a nerve root after injury or irritation, preventing the negative effects of intraneural oedema and resultant pressure.
Acknowledgements
The authors respectfully honour the unselfish men and women who donate their bodies to science. The authors thank Claude Lobstein, Director of the Willed Body Program as well as the Texas Tech University Health Sciences Center and the School of Allied Health Sciences for the use of Gross Anatomy and the Clinical Anatomy Research Labs, respectively. The authors thank Epimed International for financial support of this project.
Disclaimer Statements
ContributorsAll authors contributed to this work in substantial ways. Gilbert, Smith, Brismée and Sobzcak – design and data collection; Gilbert, James, Sizer and Brismée – analysis; Gilbert, Smith, James, Brismée, Sobzcak and Sizer – manuscript preparation.
FundingThis study was supported by a grant from Epimed International, Farmers Branch, Texas 75234.
Conflicts of interestThe authors declare that they have no conflict of interest.
Ethics approvalWe followed our university guidelines related to cadaveric research (which does not require IRB approval). Our lab is certified by the Institutional Biohazard Committee. We followed the rules and regulations regarding use of cadaver material as set forth by the Anatomical Board for the State of Texas.
References
- 1.Dahlin LB, Archer DR, McLean WG. Axonal transport and morphological changes following nerve compression. An experimental study in the rabbit vagus nerve. J Hand Surg Br. 1993;18(1):106–10. [DOI] [PubMed] [Google Scholar]
- 2.Lundborg G, Dahlin LB, Danielsen N, Hansson HA, Necking LE, Pyykko I. Intraneural edema following exposure to vibration. Scand J Work Environ Health. 1987;13(4):326–9. [DOI] [PubMed] [Google Scholar]
- 3.Lundborg G. Structure and function of the intraneural microvessels as related to trauma, edema formation, and nerve function. J Bone Joint Surg Am. 1975;57(7):938–48. [PubMed] [Google Scholar]
- 4.Lundborg G, Dahlin LB. Anatomy, function, and pathophysiology of peripheral nerves and nerve compression. Hand Clin. 1996;12(2):185–93. [PubMed] [Google Scholar]
- 5.Lundborg G, Myers R, Powell H. Nerve compression injury and increased endoneurial fluid pressure: a “miniature compartment syndrome.”. J Neurol Neurosurg Psychiatry. 1983;46(12):1119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown CL, Gilbert KK, Brismee J-M, Sizer PS, Roger James C, Smith MP. The effects of neurodynamic mobilization on fluid dispersion within the tibial nerve at the ankle: an unembalmed cadaveric study. J Man Manip Ther. 2011;19(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleland JA, Hunt GC, Palmer JA. Effectiveness of neural mobilization in the treatment of a patient with lower extremity neurogenic pain: a single-case design. J Man Manip Ther. 2004;12:143–52. [Google Scholar]
- 8.Cleland JA, Childs JD, Palmer JA, Eberhart S. Slump stretching in the management of non-radicular low back pain: a pilot clinical trial. Man Ther. 2006;11(4):279–86. [DOI] [PubMed] [Google Scholar]
- 9.Murphy DR, Hurwitz EL, Gregory AA, Clary R. A non-surgical approach to the management of lumbar spinal stenosis: a prospective observational cohort study. BMC Musculoskelet Disord. 2006;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis RF, Hing WA. Neural mobilization: a systematic review of randomized controlled trials with an analysis of therapeutic efficacy. J Man Manip Ther. 2008;16(1):8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sizer PS Jr, Phelps V, Dedrick G, Matthijs O. Differential diagnosis and management of spinal nerve root-related pain. Pain Pract. 2002;2(2):98–121. [DOI] [PubMed] [Google Scholar]
- 12.Nee R, Butler D. Management of peripheral neuropathic pain: integrating neurobiology, neurodynamics, and clinical evidence [corrected] [published erratum appears in PHYS THER SPORT May;7(2):110-1]. Phys Ther Sport. 2006;7(1):36–49. [Google Scholar]
- 13.Coppieters MW, Kurz K, Mortensen TE, Richards NL, Skaret IA, McLaughlin LM et al. , The impact of neurodynamic testing on the perception of experimentally induced muscle pain. Man Ther. 2005;10(1):52–60. [DOI] [PubMed] [Google Scholar]
- 14.Herrington L. Effect of different neurodynamic mobilizations techniques on knee extension range of motion in the slump position. J Man Manip Ther. 2006;14:101–7. [Google Scholar]
- 15.Butler DL. The sensitive nervous system. Adelaide, Australia: Noigroup; 2000. [Google Scholar]
- 16.Shacklock M. Clinical neurodynamics: a new system of musculoskeletal treatment. New York: Elsevier Butterworth-Heinemann; 2005. [Google Scholar]
- 17.Akalin E, El O, Peker O, Senocak O, Tamci S, Gülbahar S et al. , Treatment of carpal tunnel syndrome with nerve and tendon gliding exercises. Am J Phys Med Rehabil. 2002;81(2):108–13. [DOI] [PubMed] [Google Scholar]
- 18.Allison GT, Nagy BM, Hall T. A randomized clinical trial of manual therapy for cervico-brachial pain syndrome – a pilot study. Man Ther. 2002;7(2):95–102. [DOI] [PubMed] [Google Scholar]
- 19.Coppieters MW, Stappaerts KH, Wouters LL, Janssens K. Aberrant protective force generation during neural provocation testing and the effect of treatment in patients with neurogenic cervicobrachial pain. J Manipulative Physiol Ther. 2003;26(2):99–106. [DOI] [PubMed] [Google Scholar]
- 20.Coppieters MW, Stappaerts KH, Wouters LL, Janssens K. The immediate effects of a cervical lateral glide treatment technique in patients with neurogenic cervicobrachial pain. J Orthop Sports Phys Ther. 2003;33(7):369–78. [DOI] [PubMed] [Google Scholar]
- 21.Pinar L, Enhos A, Ada S, Güngör N. Can we use nerve gliding exercises in women with carpal tunnel syndrome? Adv Ther. 2005;22(5):467–75. [DOI] [PubMed] [Google Scholar]
- 22.Baysal O, Altay Z, Ozcan C, Ertem K, Yologlu S, Kayhan A. Comparison of three conservative treatment protocols in carpal tunnel syndrome. Int J Clin Pract. 2006;60(7):820–8. [DOI] [PubMed] [Google Scholar]
- 23.Coppieters MW, Crooke JL, Lawrenson PR, Khoo SJ, Skulstad T, Bet-Or Y. A modified straight leg raise test to differentiate between sural nerve pathology and Achilles tendinopathy. Man Ther. 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Kitteringham C. The effect of straight leg raise exercises after lumbar decompression surgery – a pilot study. Physiotherapy. 1996;82(2):115–23. [Google Scholar]
- 25.Scrimshaw SV, Maher CG. Randomized controlled trial of neural mobilization after spinal surgery. Spine. 2001;26(24):2647–52. [DOI] [PubMed] [Google Scholar]
- 26.Bertolini G, Silva T, Trindade D, Ciena A, Carvalho A. Neural mobilization and static stretching in an experimental sciatica model - an experimental study. Rev Bras Fisioter. 2009;13(6):493–8. [Google Scholar]
- 27.Nagrale AV, Patil SP, Gandhi RA, Learman K. Effect of slump stretching versus lumbar mobilization with exercise in subjects with non-radicular low back pain: a randomized clinical trial. J Man Manip Ther. 2012;20(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coppieters MW, Alshami AM, Babri AS, Souvlis T, Kippers V, Hodges PW. Strain and excursion of the sciatic, tibial, and plantar nerves during a modified straight leg raising test. J Orthop Res. 2006;24(9):1883–9. [DOI] [PubMed] [Google Scholar]
- 29.Ridehalgh C, Moore A, Hough A. Sciatic nerve excursion during a modified passive straight leg raise test in asymptomatic participants and participants with spinally referred leg pain. Man Ther. 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30.Smith SA, Massie JB, Chesnut R, Garfin SR. Straight leg raising. Anatomical effects on the spinal nerve root without and with fusion. Spine. 1993;18(8):992–9. [PubMed] [Google Scholar]
- 31.Gilbert KK, Brismee JM, Collins DL, James CR, Shah RV, Sawyer SF et al. , 2006 Young Investigator Award Winner: lumbosacral nerve root displacement and strain: part 1. A novel measurement technique during straight leg raise in unembalmed cadavers. Spine. 2007;32(14):1513–20. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert KK, Brismee JM, Collins DL, James CR, Shah RV, Sawyer SF et al. , Young Investigator Award Winner: lumbosacral nerve root displacement and strain: part 2. A comparison of 2 straight leg raise conditions in unembalmed cadavers. Spine. 2007;32(14):1521–5. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert KK, James CR, Apte G, Brown CL, Sizer PS, Brismee JM et al. , Effects of simulated neural mobilization on fluid movement in cadaveric peripheral nerve sections: implications for the treatment of neuropathic pain and dysfunction. J Man Manip Ther. 2015;23(4):219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Hoof T, Gomes GT, Audenaert E, Verstraete K, Kerckaert I, D'Herde K. 3D computerized model for measuring strain and displacement of the brachial plexus following placement of reverse shoulder prosthesis. Anat Rec (Hoboken). 2008;291(9):1173–85. [DOI] [PubMed] [Google Scholar]
- 35.Rydevik B, Lundborg G. Permeability of intraneural microvessels and perineurium following acute, graded experimental nerve compression. Scand J Plast Reconstr Surg. 1977;11(3):179–87. [DOI] [PubMed] [Google Scholar]
- 36.Tanoue M, Yamaga M, Ide J, Takagi K. Acute stretching of peripheral nerves inhibits retrograde axonal transport. J Hand Surg Br. 1996;21(3):358–63. [DOI] [PubMed] [Google Scholar]
- 37.Peer S, Kovacs P, Harpf C, Bodner G. High-resolution sonography of lower extremity peripheral nerves: anatomic correlation and spectrum of disease. J Ultrasound Med. 2002;21(3):315–22. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi S, Yoshizawa H, Yamada S. Pathology of lumbar nerve root compression. Part 1: intraradicular inflammatory changes induced by mechanical compression. J Orthop Res. 2004;22(1):170–9. [DOI] [PubMed] [Google Scholar]
- 39.Sirvanci M, Kara B, Duran C, Ozturk E, Karatoprak O, Onat L et al. , Value of perineural edema/inflammation detected by fat saturation sequences in lumbar magnetic resonance imaging of patients with unilateral sciatica. Acta Radiol. 2009;50(2):205–11. [DOI] [PubMed] [Google Scholar]
- 40.Eguchi Y, Ohtori S, Yamashita M, Yamauchi K, Suzuki M, Orita S et al. , Diffusion-weighted magnetic resonance imaging of symptomatic nerve root of patients with lumbar disk herniation. Neuroradiology. 2011;53(9):633–41. [DOI] [PubMed] [Google Scholar]
- 41.Manchikanti L, Buenaventura RM, Manchikanti KN, Ruan X, Gupta S, Smith HS et al. , Effectiveness of therapeutic lumbar transforaminal epidural steroid injections in managing lumbar spinal pain. Pain Physician. 2012;15(3):E199–E245. [PubMed] [Google Scholar]
- 42.Kim NR, Lee JW, Jun SR, Lee IJ, Lim SD, Yeom JS et al. , Effects of epidural TNF-α inhibitor injection: analysis of the pathological changes in a rat model of chronic compression of the dorsal root ganglion. Skeletal Radiol. 2012;41(5):539–45. [DOI] [PubMed] [Google Scholar]
- 43.Jayson MI, Sims-Williams H, Young S, Baddeley H, Collins E. Mobilization and manipulation for low-back pain. Spine. 1981;6(4):409–16. [DOI] [PubMed] [Google Scholar]
- 44.Mior S. Manipulation and mobilization in the treatment of chronic pain. Clin J Pain. 2001;17(4 Suppl):S70–6. [DOI] [PubMed] [Google Scholar]
- 45.Millesi H, Zoch G, Reihsner R. Mechanical properties of peripheral nerves. Clin Orthop. 1995. May; (314):76–83. [PubMed] [Google Scholar]
- 46.Greening J, Dilley A, Lynn B. In vivo study of nerve movement and mechanosensitivity of the median nerve in whiplash and non-specific arm pain patients. Pain. 2005;115(3):248–53. [DOI] [PubMed] [Google Scholar]
- 47.Dilley A, Bove GM. Disruption of axoplasmic transport induces mechanical sensitivity in intact rat C-fibre nociceptor axons. J Physiol. 2008;586(2):593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dahlin LB, McLean WG. Effects of graded experimental compression on slow and fast axonal transport in rabbit vagus nerve. J Neurol Sci. 1986;72(1):19–30. [DOI] [PubMed] [Google Scholar]
- 49.Rydevik B, Nordborg C. Changes in nerve function and nerve fibre structure induced by acute, graded compression. J Neurol Neurosurg Psychiatry. 1980;43(12):1070–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dahlin LB, Shyu BC, Danielsen N, Andersson SA. Effects of nerve compression or ischaemia on conduction properties of myelinated and non-myelinated nerve fibres. An experimental study in the rabbit common peroneal nerve. Acta Physiol Scand. 1989;136(1):97–105. [DOI] [PubMed] [Google Scholar]
- 51.Abe Y, Doi K, Kawai S. An experimental model of peripheral nerve adhesion in rabbits. Br J Plast Surg. 2005;58(4):533–40. [DOI] [PubMed] [Google Scholar]
- 52.Brown R, Pedowitz R, Rydevik B, Woo S, Hargens A, Massie J et al., Effects of acute graded strain on efferent conduction properties in the rabbit tibial nerve. Clin Orthop Relat Res. 1993; (296):288–94. [PubMed] [Google Scholar]
- 53.Dahlin LB, Lundborg G. The neurone and its response to peripheral nerve compression. J Hand Surg Br. 1990;15(1):5–10. [DOI] [PubMed] [Google Scholar]
- 54.Sakurai M, Miyasaka Y. Neural fibrosis and the effect of neurolysis. J Bone Joint Surg Br. 1986;68(3):483–8. [DOI] [PubMed] [Google Scholar]
- 55.Lundborg G, Rydevik B. Effects of stretching the tibial nerve of the rabbit. A preliminary study of the intraneural circulation and the barrier function of the perineurium. J Bone Joint Surg Br. 1973;55(2):390–401. [PubMed] [Google Scholar]
- 56.Driscoll PJ, Glasby MA, Lawson GM. An in vivo study of peripheral nerves in continuity: biomechanical and physiological responses to elongation. J Orthop Res. 2002;20(2):370–5. [DOI] [PubMed] [Google Scholar]
- 57.Rydevik B, McLean WG, Sjostrand J, Lundborg G. Blockage of axonal transport induced by acute, graded compression of the rabbit vagus nerve. J Neurol Neurosurg Psychiatry. 1980;43(8):690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan JG, Matloub HS, Sanger JR, Zhang LL, Riley DA. Vibration-induced disruption of retrograde axoplasmic transport in peripheral nerve. Muscle Nerve. 2005;32(4):521–6. [DOI] [PubMed] [Google Scholar]