Abstract
Background
Selective inhibitors of Kv1.5 channels are being developed for the treatment of atrial fibrillation (AF).
Objectives
The purpose of this study was to investigate the effects of the highly selective Kv1.5 inhibitor XEN-D0103 on human atrial action potentials (APs) at high excitation rates and to assess safety.
Methods
Intracellular APs (stimulation rates 1–5 Hz) were measured in right atrial trabeculae from patients in sinus rhythm (SR), chronic AF (cAF; AF of >6 months duration), and paroxysmal AF (pAF). The safety and tolerability of XEN-D0103 were tested in a double-blind, randomized, placebo-controlled phase 1 study.
Results
Depending on its concentration, XEN-D0103 elevated the plateau potential. At 1 Hz, XEN-D0103 (3 µM) shortened action potential duration at 90% repolarization (APD90) and effective refractory period (ERP) in SR preparations, but prolonged these parameters in cAF preparations. In SR and pAF preparations, the shortening effects on APD90 and ERP turned into prolongation at high rates. In cAF trabeculae, XEN-D0103 prolonged APD90 and ERP at 2 and 3 Hz. At high rates, more SR and pAF preparations failed to capture excitation in the presence of the drug than in its absence. XEN-D0103 (10 µM) did not significantly affect human ventricular APs. Even with plasma concentrations reaching 7000 ng/mL, XEN-D0103 did not increase ∆∆QTcF (QT interval corrected by the Fridericia formula) in the analysis of electrocardiograms of healthy volunteers, and no subjects receiving an active treatment had a QT or QTcF interval >450 ms, or increase in QTcF from baseline >30 ms.
Conclusion
APD prolongation and suppression of APs by XEN-D0103 at high stimulation rates in SR and pAF tissue, but not cAF, could be of therapeutic benefit for reducing AF burden. This concept needs to be confirmed in clinical trials.
Abbreviations: AF, atrial fibrillation; ANOVA, analysis of variance; AP, action potential; APA, action potential amplitude; APD20, APD50, APD90, action potential duration at 20%, 50%, and 90% of repolarization; cAF, chronic atrial fibrillation; DMSO, dimethyl sulfoxide; dV/dtmax, maximum upstroke velocity; ECG, electrocardiogram/electrocardiographic; ERP, effective refractory period; HR, heart rate; IKur, ultra-rapidly activating delayed-rectifier potassium current; pAF, paroxysmal atrial fibrillation; PLT20, plateau potential, that is, mean absolute membrane potential (mV) in the time window between 20% and 30% of APD90; QT, corrected QT; QTcF, QT interval corrected by the Fridericia formula; RMP, resting membrane potential; SR, sinus rhythm; TMC, time-matched control
Keywords: IKur inhibitor XEN-D0103, Atrial fibrillation, Frequency dependence, Human atrial action potentials, QTc, QTcF
Introduction
Drug treatment of atrial fibrillation (AF) aims to prevent stroke and restore and maintain sinus rhythm (SR). Experimental and clinical evidence suggest that AF is due to microreentry triggered by ectopic foci located in the proximity of the pulmonary veins1; hence, Na+ channel blockers (class I) for reducing excitability and K+ channel blockers for prolongation of the action potential duration (APD) and effective refractory period (ERP) (class III)2 are accepted therapeutic options. The multi-ion channel blocker amiodarone is currently the most efficacious antiarrhythmic agent for the pharmacological treatment of AF; nevertheless, the drug is poorly tolerated. Current AF drugs lack atrial specificity, producing proarrhythmic liability in the ventricles that can lead to torsades de pointes and sudden cardiac death.3 Thus, there is a clear unmet medical need for new pharmacological AF therapies with improved efficacy and safety.4
The ultra-rapidly activating delayed-rectifier potassium current (IKur) conducted by the Kv1.5 protein is a novel drug target because this channel is hardly expressed in ventricles.5, 6, 7 Therefore, selective inhibitors of IKur are expected to prolong atrial but not ventricular ERP and should be devoid of risk for ventricular proarrhythmia.
We have previously investigated the electrophysiological effects of several modestly selective IKur inhibitors, that is, 4-aminopyridine (low concentration), AVE0118, XEN-D0101, and MK-0448, all of which elevated the plateau phase of human atrial action potentials (APs) recorded in trabeculae of right atrial appendages of patients in SR or AF.8, 9, 10, 11 Interestingly, the expected actions of a K+ channel blocker, that is, prolongation of APD/ERP, were observed only in AF preparations, whereas in SR preparations these parameters were shortened.8, 9, 10, 11 Those experiments were performed with a stimulation frequency of 1 Hz (60 beats/min); however, it is unknown how IKur inhibitors affect human atrial APs at higher excitation rates as encountered in AF (ie, 228–440 beta/min, 4–8 Hz12).
The Kv1.5 inhibitor MK-0448 failed to affect ERP in young healthy volunteers,13 and the authors concluded that IKur block cannot be expected to exert therapeutically useful antiarrhythmic effects in AF. However, only rather long cycle lengths could be tested (≥400 ms, ≤150 beats/min),13 but like many conventional antiarrhythmic drugs, IKur inhibitors, too, could exhibit frequency-dependent actions.
We have published a preliminary report that XEN-D0103 (Servier S66913) selectively blocks human IKur and recombinant hKv1.5 current in CHO cells stably expressing the hKCNA5 gene.14 Since the effects of IKur inhibitors on ex vivo human atrial tissue paced at pathophysiological frequencies are unknown, the purpose of this study was to evaluate the effects of XEN-D0103 on atrial APs and ERP at normal and AF relevant frequencies (1–5 Hz) with standard microelectrode techniques in isolated atrial trabeculae from patients in SR and chronic AF (cAF) or paroxysmal AF (pAF). The safety and tolerability of XEN-D0103 were determined in a phase 1 study performed in healthy volunteers.
Methods
Studies reported here conform to the principles outlined in the Declaration of Helsinki and were reviewed and approved by relevant ethics committees; all human subjects gave written informed consent. Atrial tissue was obtained from patients receiving cardiac surgery (ethic committee approval number of TU Dresden: EK790799). The characteristics of the patients are summarized in Online Supplemental Table S1. The AP study included 22 preparations from patients in SR (SR group), 19 preparations from 17 patients in permanent AF (defined as cAF for >6 month at the time of tissue collection), and 12 preparations from 11 patients in pAF (episodes of AF terminating spontaneously within 7 days).
In vitro AP and ERP measurements
APs were measured with a sharp microelectrode (tip resistances 20–80 MΩ) in small pieces of right atrial appendages at 36°C±1°C, as described previously.9, 11 The basal rate of stimulation was 1 Hz. Each experiment was preceded by a 60-minute equilibration period during which the preparations were allowed to stabilize. Small pieces of the ventricular septum were removed during the surgical correction of outflow track hypertrophy.
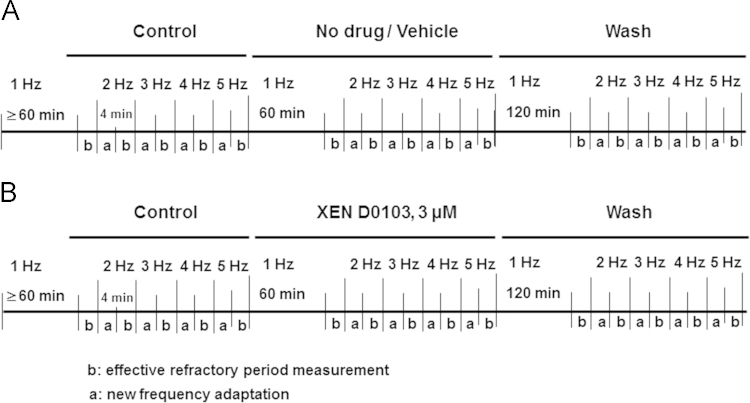
A schematic of experimental design for the study of frequency-dependent drug effects is given in Figure 1. Stimulation frequency was increased in 1-Hz steps up to 5 Hz for 2 minutes each. ERP was measured by an extrastimulus delivered after trains of 10 regular pulses with decreasing intervals in 5-ms steps until the extrastimulus failed to excite. Time-matched control (TMC) experiments in the absence of the drug or in the presence of vehicle (dimethyl sulfoxide [DMSO]) were conducted in a similar manner to assess time-dependent changes associated with the length of the experiment (“run-up/run-down”). Since no differences were detected between the 2 groups, the results were pooled.
Figure 1.
Schematic of the experimental procedure for action potential measurements in human atrial trabeculae. A: Time-matched control experiments: after an equilibration period of ≥60 minutes at a stimulation rate of 1 Hz, the effective refractory period was measured (b). Stimulation rate was then increased successively, with 2 minutes for stabilization (a) and 2 minutes for the measurement of effective refractory period (b). B: After returning to 1 Hz, preparations were exposed to XEN-D0103 for 60 minutes before going through another set of rate increases.
The following AP parameters were analyzed: resting membrane potential (RMP, in mV); action potential amplitude (APA, in mV); APD at 20%, 50%, and 90% of repolarization (APD20, APD50 and APD90, in ms); maximum rate of depolarization; maximum upstroke velocity (dV/dtmax, in V/s); and plateau potential (defined as the mean absolute membrane potential [in mV] in the time window between 20% and 30% of APD90 [PLT20]). Preparations with resting potentials less negative than −65 mV and amplitudes below 85 mV were discarded.
Stock solutions and drug application
Aliquots of 10 mM XEN-D0103 stock solution (in DMSO) were added directly to the reservoir of the circulating solution. The DMSO concentration never exceeded 0.2%, which did not induce changes when applied alone.
Corrected QT interval evaluation in healthy volunteers
A double-blind, randomized, placebo-controlled phase 1 single ascending dose study was performed in healthy male volunteers to determine the safety and tolerability of XEN-D0103 and to examine the relationship between the drug plasma concentration and the corrected QT (QTc) interval (for details, see Online Supplemental Methods). Doses investigated were 10, 30, 60, 120, and 200 mg of XEN-D0103. An assay of plasma XEN-D0103 was performed using a high-performance LC-MS/MS. The lower limit of quantification for the assay was 0.5 ng/mL. Safety electrocardiographic (ECG) telemetry (2-lead ECG) was monitored continuously from dosing until 24 hours after doing to monitor heart function and detect proarrhythmic events in real time.
Statistical analysis
For statistical analysis, drug effects on relative AP parameters (ΔERP, ΔAPD90, and ΔPLT) were compared with TMCs using an unpaired Student t test. Drug effects on absolute AP parameters were compared with predrug effects using a paired Student t test (Online Supplemental Tables S2–S7). Within 1 group, the frequency-dependent changes were analyzed using a repeated-measures analysis of variance (ANOVA) followed by Bonferroni multiple comparison test. Data are typically presented as mean ± standard error of the mean, and 95% confidence intervals are given, where appropriate.
Concentration-effect modeling was performed for QTcF (QT interval corrected by the Fridericia formula) evaluation in healthy volunteers, with ∆∆QTcF intervals being regressed onto the plasma concentrations of XEN-D0103 at the exact times as the continuous 12-lead ECGs. The effect of XEN-D0103 plasma concentration on ∆∆QTcF was investigated using a regression model and ANOVA. At each time of stable supine rest, XEN-D0103 plasma concentration was interpolated using nonparametric superposition. A mixed-effects model that included plasma concentration, treatment group, and an interaction between plasma concentration and treatment group as fixed factors and subject as a random factor was evaluated.
Results
Effects of XEN-D0103 on human cardiac APs and ERP
APs from patients in SR Figure 2 (upper panel) displayed the typical spike-and-dome shape.15 The concentration-dependent effects of XEN-D0103 (0.3–10 µM; Online Supplemental Figure S1) on SR APs at the basal stimulation frequency of 1 Hz showed that XEN-D0103 produced robust effects on electrophysiological parameters, consisting of a prominent elevation of the plateau accompanied by shortening of APD90. The maximum effects were reached within 40–50 minutes.
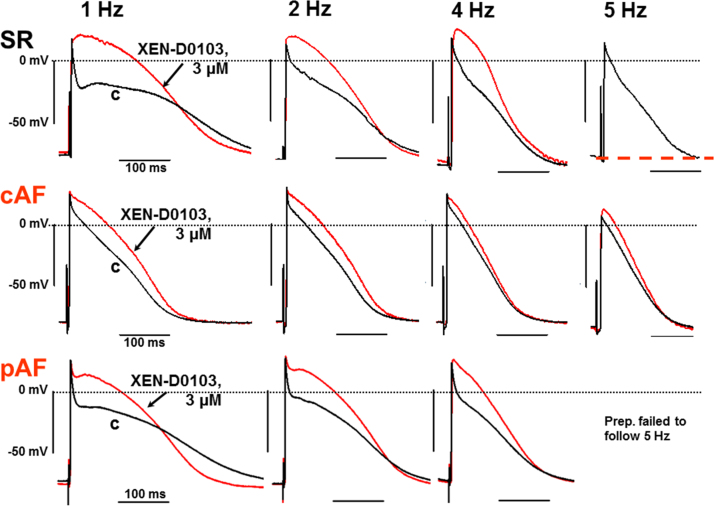
Figure 2.
Frequency dependence of XEN-D0103 effects (3 µM). Original traces of action potentials recorded in right atrial trabeculae from patients in sinus rhythm (SR), chronic atrial fibrillation (cAF), and paroxysmal AF (pAF). c, Predrug control; red line, in the presence of XEN-D0103. The red dashed line in the top-right panel indicates failure to follow stimulation at this rate in the presence of the drug. Calibration bars as indicated.
Effects of stimulation frequency on APs and ERP in SR and AF
In the absence of the drug, APs and ERP from patients in SR shortened with increasing stimulation rate, as reported by others.16 This adaptation response was attenuated in AF preparations (Online Supplemental Figure S2). ERP values and AP parameters (at 1–5 Hz) for SR, cAF, and pAF experiments and TMC experiments are summarized in Online Supplemental Tables S2–S7. A lager fraction of SR preparations than of AF preparations failed to follow 5 Hz, and since measurements of ERP were more prone to failures than were measurements of other AP parameters, numbers for ERP values are still lower at 4 and 5 Hz.
Effects of XEN-D0103 on APs and ERP in atrial tissue from patients in SR
In SR preparations, the ERP/APD90 shortening effect of XEN-D0103 (3 µM) at 1 and 2 Hz was converted into a significant prolonging effect at 3 and 4 Hz (Figure 2, upper panel, and Figure 3) and excitability was suppressed in the presence of the drug at 5 Hz.
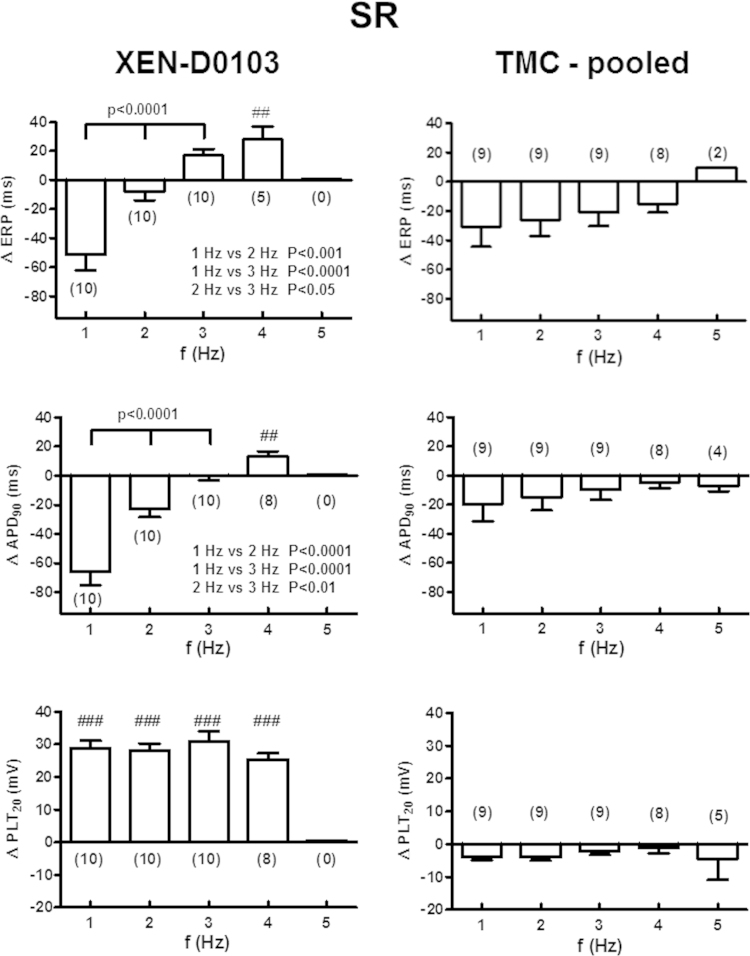
Figure 3.
Frequency-dependent effects of XEN-D0103 in right atrial trabeculae from patients in sinus rhythm (SR). Effects on effective refractory period (ERP), action potential duration at 90% repolarization (APD90), and plateau potential (defined as the mean absolute membrane potential [in mV] in the time window between 20% and 30% of APD90 [PLT20]) depicted as the difference between predrug control and in the presence of the drug (delta values). Note that in SR, none of the preparations followed stimulation at 5 Hz in the presence of XEN-D0103. Separate time-matched control (TMC) experiments without drug application are shown on the right side. Delta values in the presence of the drug were compared with the corresponding TMC data at each frequency using an unpaired Student t test. The results of this comparison are shown as ##P < .01 and ###P < .0001. Delta values at each frequency (1–3 Hz) in the presence of the drug were compared in a group to determine frequency-dependent drug effects using a repeated-measures analysis of variance (results shown as a line, comparing 1–3 Hz) with a Bonferroni posttest (results shown in the inset). Failure to capture action potentials in all preparations at 5 Hz precluded comparison of this group using a repeated-measures test.
Although the typical “spike-and-dome” shape of the SR AP converted to a triangular AF-like shape at 4 Hz, the maximum effect of XEN-D0103 on the plateau potential (ΔPLT20, Figure 3, lower left panel) was similar at all frequencies while APD20 and APD50 were both significantly prolonged at all frequencies tested. RMP was not altered by XEN-D0103 (see Online Supplemental Table S2); however, a reduction in dV/dtmax was observed.
The results of TMC experiments revealed a trend toward shortening of APD90 over the course of the experiment, as such the drug-induced prolongation of APD90 at 3 and 4 Hz is likely to be underestimated and the shortening of APD90 at lower pacing frequencies of 1 and 2 Hz may be overestimated. Data were not corrected for run-down because experiments were conducted in different tissue preparations.
Effects of XEN-D0103 on APs and ERP in atrial tissue from patients in cAF
In cAF trabeculae, XEN-D0103 also significantly elevated the PLT20 (Figure 2, middle panel). The increase in PLT20 was smaller compared to SR preparations and somewhat decreased with increasing stimulation frequencies (Figure 4), but in contrast to SR, XEN-D0103 prolonged APD at the 3 repolarization levels analyzed (prolongation statistically significant for APD90 at 2 Hz and for APD50 and APD20 at all frequencies tested) (Online Supplemental Table S4). APA was significantly increased at 1 Hz; RMP was significantly more negative at 1–4 Hz; and dV/dtmax was similar in the absence and presence of the drug at all frequencies (Online Supplemental Table S4).
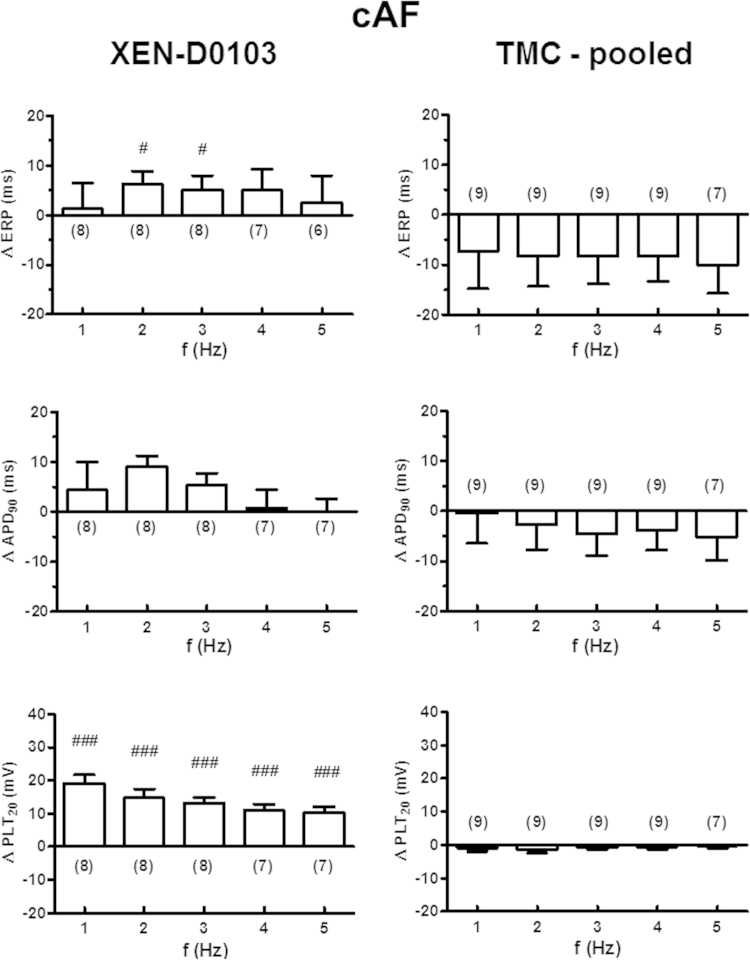
Figure 4.
Frequency-dependent effects of XEN-D0103 in right atrial trabeculae from patients in chronic atrial fibrillation (cAF). Layout as in Figure 3. Delta values in the presence of the drug were compared with the corresponding time-matched control data at each frequency using an unpaired Student t test. The results of this comparison are shown as #P < .05 and ###P < .0001. Note that repeated-measures analysis of variance with Bonferroni posttest did not result in any statistically significant differences between 1, 2, and 3 Hz. Abbreviations as in Figure 3.
With 3 µM XEN-D0103, ERP was significantly prolonged at all frequencies but only reached statistical significance at 2 Hz (Figure 4, left upper panel, and Online Supplemental Table S4). Similar to the results in SR tissue, ERP and APD90 shortened with time in TMC experiments (Online Supplemental Table S5), as such leading to the underestimation of drug effects on ERP and APD90.
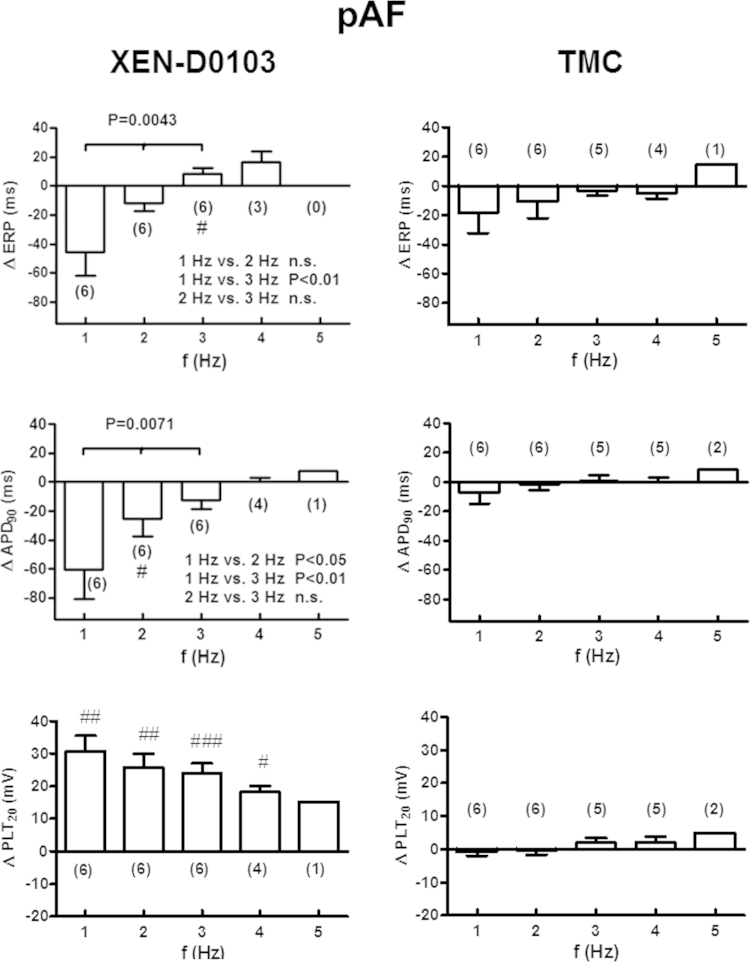
Effects of XEN-D0103 on APs and ERP in atrial tissue from patients in pAF
The APs recorded from pAF tissue displayed a spike-and-dome shape similar to that seen in SR tissue (Figure 2, lower panel). In pAF tissue, XEN-D0103 (3 µM) produced frequency-dependent effects similar to those in SR tissue (Figure 5). APA and RMP were not affected by XEN-D0103, but dV/dtmax was reduced in the presence of XEN-D0103 at 1–4 Hz (P < .05; Online Supplemental Table S6). PLT20 was elevated at all frequencies, and APD90 was shortened at 1 Hz and even slightly prolonged at 5 Hz (Figure 5). In the presence of the drug, only 4 and 1 of 6 preparations could be stimulated at 4 and 5 Hz, respectively (Online Supplemental Table S6), similar to SR tissue, whereas 5 and 3 of 6 trabeculae followed these rates in TMC (Online Supplemental Table S7).
Figure 5.
Frequency-dependent effects of XEN-D0103 in right atrial trabeculae from patients in paroxysmal atrial fibrillation (pAF). Layout as in Figure 3. Delta values in the presence of the drug were compared with the corresponding time-matched control data at each frequency using an unpaired Student t test. The results of this comparison are shown as #P < .05, ##P < .01, and ###P < .0001. Group comparison of delta values (1–3 Hz) in the presence of the drug as in Figure 3. Abbreviations as in Figure 3.
ERP significantly decreased in pAF tissue at the stimulation frequency of 1 Hz. This effect was diminished at 2 Hz and turned into a slight, though nonsignificant, prolongation at 3 and 4 Hz. In TMC pAF preparations, ERP tended to shorten from 1 to 4 (Figure 5 and Table S7).
Effects of XEN-D0103 on human ventricular AP parameters
For comparison purposes and for confirming the atrial selectivity of XEN-D0103, APs were recorded from 3 human ventricular septum preparations. XEN-D0103 (10 µM) had no significant effect on human ventricular AP parameters (Online Supplemental Figure S3).
Effects of XEN-D0103 on the QTc interval and drug-induced proarrhythmia in healthy human subjects
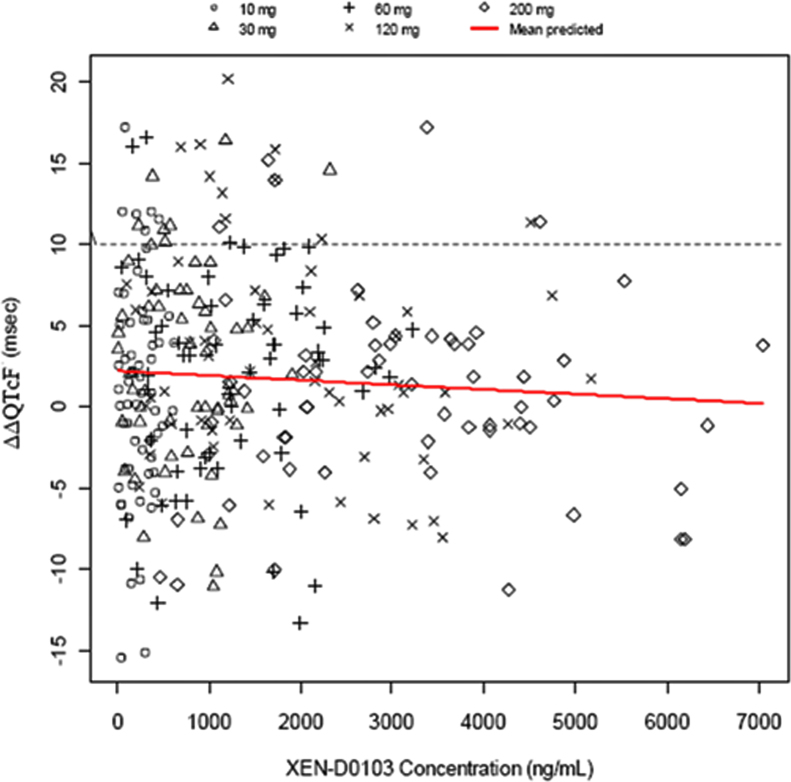
Comprehensive ECG recordings and ECG analysis were performed in a phase 1 clinical trial to determine whether XEN-D0103 prolongs the QTc interval in humans. As no meaningful change in heart rate (HR) was noted during the study, the Fridericia formula was used to correct the QT interval for the HR (QTcF). A plot of XEN-D0103 plasma concentration vs ∆∆QTcF is shown in Figure 6, and most notably, XEN-D0103 plasma concentrations reached 7000 ng/mL in some volunteers without increasing ∆∆QTc. A categorical analysis of QT interval and QTcF was performed, and no subjects receiving an active treatment had a QT interval or QTcF >450 ms, or increase in QTcF from baseline >30 ms. In addition to 12-lead Holter monitoring, 2-lead ECG telemetry monitoring was performed for 24 hours after dosing. All results of ECG telemetry were considered as normal by the investigator. There were no clinically significant changes, including induction of AF or ventricular proarrhythmia after XEN-D0103 dosing. No dose-related effects on PR interval, QRS complex, and HR as well as on T-wave and U-wave morphologies were noted.
Figure 6.
Baseline- and placebo-adjusted QTcF (∆∆QTcF) values plotted against XEN-D0103 plasma concentration derived from healthy volunteers (n = 63). Concentration effect modeling showed no evidence of a relationship between the plasma concentration of XEN-D0103 (in ng/mL) and ∆∆QTcF (in ms) in healthy volunteers orally dosed with XEN-D0103. QTcF = QT interval corrected by the Fridericia formula.
Discussion
We have investigated the effects of the novel highly selective IKur/Kv1.5 inhibitor XEN-D0103 (Servier S66913) on AP parameters and ERP over a pathophysiological relevant range of frequencies (1–5 Hz). Our major finding was that XEN-D0103 prolonged APD and ERP preferentially at high stimulation rates in SR and pAF preparations, providing novel mechanistic information on modulating IKur that is likely to be highly desirable for treating pAF.
In accordance with the literature,15, 17, 18 electrical remodeling during cAF caused triangulation of the AP shapes. Atrial tissue from patients in pAF was apparently not electrically remodeled since APs still exhibited the typical SR-like spike-and-dome configuration. We also found that atrial APD and ERP shortened with increasing frequency of stimulation, which has been related mainly to decrease in L-type Ca2+ current,19 and that adaptation to high rates was attenuated in preparations from patients in cAF.15, 16 The maximum rate of depolarization dV/dtmax declined significantly at high rates16 because of the incomplete restoration of RMP with an increasing number of preparations failing to capture stimulation, thereby limiting frequency testing to 5 Hz.
Channels conducting the human cardiac IKur are encoded by the Kv1.5 gene KCNA5, which is strongly expressed in atria, and although small amounts of messenger RNA have been reported in human ventricular tissue, no functional current has been reported.20 Consequently, IKur block exhibits an atrial-selective effect on cardiac repolarization and could be considered a drug intervention suited for the treatment of atrial rhythm disturbances without unwanted effects on ventricular function. This is suggested by the lack of impact of XEN-D0103 on ventricular AP. The absence of XEN-D0103–induced QTc prolongation as well as PR and QRS changes and the absence of arrhythmias, including AF, in healthy volunteers observed in the clinical study confirms the finding previously reported for XEN-D01019 and MK-044813 and also provides further support for IKur only being present and functional in the human atria and not the ventricle.
The characteristic changes in electrophysiological parameters observed with XEN-D0103 in human right atrial trabeculae (1 Hz) are consistent with block of Kv1.5 channels as previously reported for this14 and other less selective drugs (eg, 4-AP,7, 21, 22 DPO-1,23 AVE0118,8 XEN-D0101,9 and MK-044810). These drugs elevated the plateau potential in SR preparations and significantly shortened APD90 at 1 Hz but prolonged APD90 in cAF trabeculae. The shortening of APD90 and ERP by Kv1.5 inhibitors in SR is proposed to be caused by the marked elevation of the plateau potential into a voltage range where more hERG channels are activated, and although they inactivate rapidly, they can recover from inactivation at more negative potentials and thus hasten final repolarization.24 Since the most prominent effects of XEN-D0103 were elevation of PLT20, shortening of APD90 in SR at 1 Hz, and prolongation of APD20, APD50, APD 90 in cAF, we conclude that XEN-D0103 does block IKur in human atrial trabeculae. Furthermore, these characteristic electrophysiological changes appear to be a dominant class effect of IKur block, since they are observed with XEN-D0103 as well as with other less selective Kv1.5 inhibitors.
Interestingly, in SR preparations, the APD shortening effect of XEN-D0103 at the basal frequency of 1 Hz turns into an APD-prolonging effect accompanied by an increase in ERP at the higher stimulation rates of 3 and 4 Hz (Figure 3). In addition, fewer preparations could be stimulated at 4 Hz and excitation failed in all SR preparations at 5 Hz in the presence of XEN-D0103. In pAF, similar effects were observed, the PLT20 increased at all frequencies, and ERP was prolonged at 3–4 Hz (although this effect was significant only at 3 Hz) with all but one preparation failing to capture excitation at 5 Hz. These qualitatively similar responses suggest that tissue from patients in SR may be a useful surrogate for investigating the effects of drugs in pAF, especially given the scarcity of tissue from patients in pAF. The data in SR and pAF tissue provide novel evidence in support of IKur/Kv1.5 as a target for the management of pAF.
In cAF, qualitatively different effects were observed compared with those seen in SR and pAF. All but one preparation followed stimulation at 5 Hz both in the absence and in the presence of XEN-D0103. The ability to pace the cAF tissue at high frequencies suggests a remodeled phenotype that forms the substrate for the initiation and perpetuation of the arrhythmia in these patients. The shorter APD90 and ERP and the more negative RMP of cAF tissue have previously been reported by us and others9, 11, 16, 25, 26, 27, 30 at 1 Hz. This is likely to contribute to the ability of cAF tissue to capture higher stimulation frequencies, in the absence and presence of the drug, than to the ability of SR or pAF tissue because of the higher availability of Na+ channels at more negative membrane potential. In cAF tissue, XEN-D0103 prolonged the APD at all frequencies but effects on the ERP were significant only at 2 Hz. However, the TMC data suggest this was underestimated at all frequencies tested. Despite these effects on AP parameters in cAF preparations, we do not expect block of Kv1.5 channels by XEN-D0103 to convert cAF back to SR because of electrical and structural remodeling.6 Nevertheless, this effect may protect the atrial myocardium during the phase of reverse remodeling after cardioversion.
At present, it is not known whether and how block of IKur is related to ERP. We speculate that the primary reduction in IKur can cause a secondary effect on Na+ conductance, leading to increased postrepolarization refractoriness rather than having a direct effect on the cardiac Na+ channel. This is supported by the lack of effect on the recombinant peak Nav1.5 current at concentrations up to 10 μM.14 Given the lack of a direct effect on Na+ channels, the increase in postrepolarization refractoriness 28, 29 may be due to an indirect effect on Na+ channel availability owing to the marked change in the profile of the AP in the presence of the drug (ie, elevated plateau and triangulation of the AP shapes). However, this is speculative and further experiments are needed to clarify the mechanism of ERP modulation by XEN-D0103, but these are outside the scope of the present study. In any case, it is seems highly desirable to increase ERP in both SR and pAF to disrupt reentry and prevent AF recurrence. This, in turn, may be expected to facilitate the reverse remodeling process after cardioversion. Pharmacological interventions for interrupting microreentry have to prolong the ERP, while suppression of ectopic foci requires reduction of excitability. Given the pharmacological profile of XEN-D0103, it is more likely to increase atrial refractoriness than to reduce excitability.
It should be noted that although the reported effect of XEN-D0103 in cAF appears small, prolongation of ERP may be underestimated because of the concomitant reduction of ERP observed in TMC. A broader comparison to other AF antiarrhythmic drugs was not possible, as they would require evaluation using the methodology that evaluated APD parameters and ERP in SR and AF tissue at multiple pacing frequencies as well as assessment of the effect on ventricular tissue to confirm atrial selectivity. More importantly, the present data suggest some benefits of XEN-D0103 in pAF. A lack of effect of other IKur inhibitors in healthy volunteers13 could be due to the rather long cycle lengths (ie, >400 ms) used. According to our data, we would not have expected a prolonging effect of MK-0448 on atrial ERP at cycle lengths below 333 ms (3 Hz). Last but not least, although IKur had been originally discovered in dog atria, IKur could no longer be demonstrated during the past years31; hence, the absence of any effect of IKur inhibitors in dog models of AF is not surprising. In conclusion, we found the use of IKur inhibitors in treating AF that includes both pAF and recent-onset persistent AF, that is, AF of <6 months of duration.
Our data highlight the importance of pathophysiologically relevant pacing frequencies (SR and pAF) and an arrhythmogenic substrate (ionic and structural remodeling in cAF). Only at higher pacing frequencies in SR and pAF and at 2 and 3 Hz in cAF tissue did we observe significant increases in APD/ERP. On the basis of our results, we expect XEN-D0103 to be preferentially effective in non-remodeled atrial tissue (SR and pAF). The positive frequency dependence of ERP prolongation and loss of excitability at high frequencies (5 Hz) in SR preparations and an essentially similar pattern of effects in pAF suggest that IKur inhibitors could be effective for the termination of recent-onset AF (ie, suppression of high-frequency firing) and reduction of AF burden in pAF before electrical and structural remodeling has taken place.
To date, no clinical trials have been published on a selective human IKur inhibitor in a patient population with AF; however, based on these novel mechanistic findings for XEN-D0103, it is currently being evaluated in 2 phase 2 clinical studies to evaluate the therapeutic potential of a selective IKur inhibitor to prevent recurrences in patients in pAF. They both started in 2014.
The first trial (EudraCT no. 2013-004456-38) was a double-blind, randomized, placebo-controlled, crossover study assessing a single-dose level (150 mg twice daily) of XEN-D0103 in patients in pAF and patients with implanted pacemakers. The primary objective was to assess the reduction in AF burden over 28 days compared to placebo. The second phase 2 study in patients in pAF called DIAGRAF-IKUR (EudraCT no. 2013-004456-38) aimed to evaluate the efficacy of 3 doses of XEN-D0103 (S66913), vs placebo on AF and/or atrial tachycardia burden (AF/AT burden) in patients who have an indication for AF ablation and are implanted with insertable cardiac monitoring device.
Study limitations
In most of the preparations, basic parameters slightly declined with time. The classification of patients into SR and AF groups is not always reliable; that is, the SR group may include atrial tissue from patients that have had unrecognized episodes of pAF or recent-onset AF. Furthermore, cAF tissue will include patients in permanent AF who are known to be refractory to SR-maintenance therapy.
Conclusion
The selective IKur inhibitor XEN-D0103 consistently elevated the AP plateau in human atrial trabeculae irrespective of the initial shapes of the control APs and whether the preparations originated from patients in SR, pAF, or cAF. In SR and pAF preparations, the APD shortening effect observed at 1 Hz resulted in prolongation of APD90 and ERP at high stimulation rates with loss of excitability at 5 Hz, whereas in cAF preparations, ERP prolongation was modest. For the prevention of AF recurrence, frequency-dependent prolongation of ERP by XEN-D0103 in both SR and pAF preparations is a highly desirable mode of action that may protect the atrial myocardium during the phase of reverse remodeling after cardioversion. These data warrant the evaluation of XEN-D0103 in a clinical trial in patients in pAF.
Clinical Perspectives.
Pharmacological treatment of atrial fibrillation (AF) remains a clinical challenge owing to the lack of effective and safe drugs. The selective blockers of Kv1.5 channels were designed to fill this gap, yet their antiarrhythmic effects are yet to be reported in a clinical setting. To date, most Kv1.5 blockers have been shown to prolong only action potential duration (APD) and effective refractory period (ERP) at physiological beating rates in atrial tissue remodeled by persistent AF, whereas they actually shorten APD and ERP in sinus rhythm (SR) tissue. However, little is known about their efficacy at the high rates of excitation encountered during AF. Here we report that the highly selective Kv1.5 channel blocker XEN-D0103 prolongs APD and ERP measured with sharp microelectrodes in human right atrial trabeculae from patients in chronic AF in the frequency range 1–5 Hz. In trabeculae from patients in SR and paroxysmal AF, the ADP-shortening effect of XEN-D0103 at low stimulation rate (1 Hz) turns into APD prolongation at high rates, leading to failure of excitation response. We conclude that the XEN-D0103 exhibits positive frequency–dependent electrophysiological effects that could be clinically useful, because prolongation of APD and suppression of action potentials by XEN-D0103 at high stimulation rates in SR and paroxysmal AF tissue is expected to reduce AF burden. This concept needs to be confirmed in clinical trials. Our data cannot provide evidence for preventing recurrence or termination of permanent AF by XEN-D0103. This must be tested in appropriate models.
Acknowledgments
We gratefully acknowledge Konstanze Fischer, BSc, for technical assistance. We also acknowledge Julie Salzmann, PhD (Institut de Recherches Internationales Servier) and Catherine Thollon, PhD (Institut de Recherche Servier) for reviewing this manuscript.
Footnotes
This work was supported by Xention, the European Union (grant no. FP7 Health T2-2010 -261057 “EUTRAF” [European Network for Translational Research in Atrial Fibrillation]), and the Department of Pharmacology and Toxicology, Medical Faculty TU Dresden.
Dr Ford, Dr Milnes, and Dr El Haou are employees of Xention.
Dr Ravens and Dr Wettwer are consultants to Xention.
Dr Tyl is an employee of Institut de Recherches Internationales Servier.
Appendix
Supplementary materials
Supplementary Material
References
- 1.Haissaguerre M., Jais P., Shah D.C., Takahashi A., Hocini M., Quiniou G., Garrigue S., Le Mouroux A., Le Metayer P., Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1983;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Vaughan Williams E.M. A classification of antiarrhythmic actions reassessed after a decade of new drugs. J Clin Pharmacol. 1984;24:129–147. doi: 10.1002/j.1552-4604.1984.tb01822.x. [DOI] [PubMed] [Google Scholar]
- 3.Camm A.J., Savelieva I. Advances in antiarrhythmic drug treatment of atrial fibrillation: where do we stand now? Heart Rhythm. 2004;1:244–246. doi: 10.1016/j.hrthm.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Waldo A.L. A perspective on antiarrhythmic drug therapy to treat atrial fibrillation: there remains an unmet need. Am Heart J. 2006;151:771–778. doi: 10.1016/j.ahj.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Feng J., Wible B., Li G.R., Wang Z., Nattel S. Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes. Circ Res. 1997;80:572–579. doi: 10.1161/01.res.80.4.572. [DOI] [PubMed] [Google Scholar]
- 6.Ravens U., Wettwer E. Ultra-rapid delayed rectifier channels: molecular basis and therapeutic implications. Cardiovasc Res. 2011;89:776–785. doi: 10.1093/cvr/cvq398. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z., Fermini B., Nattel S. Sustained depolarization-induced outward current in human atrial myocytes: evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993;73:1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- 8.Christ T., Wettwer E., Voigt N., Hala O., Radicke S., Matschke K., Varro A., Dobrev D., Ravens U. Pathology-specific effects of the IKur/Ito/IK,ACh blocker AVE0118 on ion channels in human chronic atrial fibrillation. Br J Pharmacol. 2008;154:1619–1630. doi: 10.1038/bjp.2008.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford J., Milnes J., Wettwer E., et al. Human electrophysiological and pharmacological properties of XEN-D0101: a novel atrial-selective Kv1.5/IKur inhibitor. J Cardiovasc Pharmacol. 2013;61:408–415. doi: 10.1097/FJC.0b013e31828780eb. [DOI] [PubMed] [Google Scholar]
- 10.Loose S., Mueller J., Wettwer E., Knaut M., Ford J., Milnes J., Ravens U. Effects of IKur blocker MK-0448 on human right atrial action potentials from patients in sinus rhythm and in permanent atrial fibrillation. Front Pharmacol. 2014;5:26. doi: 10.3389/fphar.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wettwer E., Hala O., Christ T., Heubach J.F., Dobrev D., Knaut M., Varro A., Ravens U. Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation. 2004;110:2299–2306. doi: 10.1161/01.CIR.0000145155.60288.71. [DOI] [PubMed] [Google Scholar]
- 12.Bollmann A., Kanuru N.K., McTeague K.K., Walter P.F., DeLurgio D.B., Langberg J.J. Frequency analysis of human atrial fibrillation using the surface electrocardiogram and its response to ibutilide. Am J Cardiol. 1998;81:1439–1445. doi: 10.1016/s0002-9149(98)00210-0. [DOI] [PubMed] [Google Scholar]
- 13.Pavri B.B., Greenberg H.E., Kraft W.K., et al. MK-0448, a specific Kv1.5 inhibitor: safety, pharmacokinetics, and pharmacodynamic electrophysiology in experimental animal models and humans. Circ Arrhythm Electrophysiol. 2012;5:1193–1201. doi: 10.1161/CIRCEP.111.969782. [DOI] [PubMed] [Google Scholar]
- 14.Loose S., Muller J., Wettwer E., El-Haou S., Jackson C., Tang R., Madge D., Milnes J., Ravens U., Ford J. Positive frequency-dependent effects of highly selective Kv1.5 blockers (XEN-D0103 & MK-0448) in right atrial trabeculae from patients in sinus rhythm. Circulation. 2013;128:A18323. [Google Scholar]
- 15.Dobrev D., Ravens U. Remodeling of cardiomyocyte ion channels in human atrial fibrillation. Basic Res Cardiol. 2003;98:137–148. doi: 10.1007/s00395-003-0409-8. [DOI] [PubMed] [Google Scholar]
- 16.Workman A.J., Kane K.A., Rankin A.C. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res. 2001;52:226–235. doi: 10.1016/s0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- 17.Nattel S., Maguy A., Le Bouter S., Yeh Y.H. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 18.Van Wagoner D.R., Nerbonne J.M. Molecular basis of electrical remodeling in atrial fibrillation. J Mol Cell Cardiol. 2000;32:1101–1117. doi: 10.1006/jmcc.2000.1147. [DOI] [PubMed] [Google Scholar]
- 19.Li G.R., Nattel S. Properties of human atrial ICa at physiological temperatures and relevance to action potential. Am J Physiol. 1997;272:H227–H235. doi: 10.1152/ajpheart.1997.272.1.H227. [DOI] [PubMed] [Google Scholar]
- 20.Mays D.J., Foose J.M., Philipson L.H., Tamkun M.M. Localization of the Kv1.5 K+ channel protein in explanted cardiac tissue. J Clin Invest. 1995;96:282–292. doi: 10.1172/JCI118032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amos G.J., Wettwer E., Metzger F., Li Q., Himmel H.M., Ravens U. Differences between outward currents of human atrial and subepicardial ventricular myocytes. J Physiol. 1996;491:31–50. doi: 10.1113/jphysiol.1996.sp021194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escande D., Loisance D., Planche C., Coraboeuf E. Age-related changes of action potential plateau shape in isolated human atrial fibers. Am J Physiol. 1985;249:H843–H850. doi: 10.1152/ajpheart.1985.249.4.H843. [DOI] [PubMed] [Google Scholar]
- 23.Regan C.P., Wallace A.A., Cresswell H.K., Atkins C.L., Lynch J.J., Jr. In vivo cardiac electrophysiologic effects of a novel diphenylphosphine oxide IKur blocker, (2-isopropyl-5-methylcyclohexyl) diphenylphosphine oxide, in rat and nonhuman primate. J Pharmacol Exp Ther. 2006;316:727–732. doi: 10.1124/jpet.105.094839. [DOI] [PubMed] [Google Scholar]
- 24.Gintant G.A. Characterization and functional consequences of delayed rectifier current transient in ventricular repolarization. Am J Physiol Heart Circ Physiol. 2000;278:H806–H817. doi: 10.1152/ajpheart.2000.278.3.H806. [DOI] [PubMed] [Google Scholar]
- 25.Bosch R.F., Zeng X., Grammer J.B., Popovic K., Mewis C., Kuhlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–131. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 26.Dobrev D., Graf E., Wettwer E., Himmel H.M., Hala O., Doerfel C., Christ T., Schuler S., Ravens U. Molecular basis of downregulation of G-protein-coupled inward rectifying K(+) current (I(K,ACh) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced I(K,ACh) and muscarinic receptor-mediated shortening of action potentials. Circulation. 2001;104:2551–2557. doi: 10.1161/hc4601.099466. [DOI] [PubMed] [Google Scholar]
- 27.Van Wagoner D.R., Pond A.L., Lamorgese M., Rossie S.S., McCarthy P.M., Nerbonne J.M. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 28.Davidenko J.M., Antzelevitch C. Electrophysiological mechanisms underlying rate-dependent changes of refractoriness in normal and segmentally depressed canine Purkinje fibers: the characteristics of post-repolarization refractoriness. Circ Res. 1986;58:257–268. doi: 10.1161/01.res.58.2.257. [DOI] [PubMed] [Google Scholar]
- 29.Franz M.R., Costard A. Frequency-dependent effects of quinidine on the relationship between action potential duration and refractoriness in the canine heart in situ. Circulation. 1988;77:1177–1184. doi: 10.1161/01.cir.77.5.1177. [DOI] [PubMed] [Google Scholar]
- 30.Ford J.W., Milnes J.T. New drugs targeting the cardiac ultra-rapid delayed-rectifier current (I Kur): rationale, pharmacology and evidence for potential therapeutic value. J Cardiovasc Pharmacol. 2008;52:105–120. doi: 10.1097/FJC.0b013e3181719b0c. [DOI] [PubMed] [Google Scholar]
- 31.Qi X.Y., Diness J.G., Brundel B., Zhou X.B., Naud P., Wu C.T., Huang H., Harada M., Aflaki M., Dobrev D., Grunnet M., Nattel S. Role of small conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation. 2014;129:430–440. doi: 10.1161/CIRCULATIONAHA.113.003019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material