Abstract
In gastric cancer, the non‐canonical Wnt signaling pathway is activated by Wnt5a, which has a critical role in disease outcome. Previous studies have shown that Wnt5a mediates the expression of the extracellular matrix protein laminin γ2 through Rac and JNK activation to promote gastric cancer progression. However, the mechanism of this regulatory pathway has not been completely addressed. The scaffold protein Dvl is a major component of the Wnt signaling pathway. Here, we show that Dvl‐associating protein with a high frequency of leucine residues (Daple) mediates Wnt5a‐induced laminin γ2 expression. Immunohistochemical analysis showed marked expression of Daple in advanced clinical stages of gastric cancer, where it highly correlated with Wnt5a/b and laminin γ2 expression, the depth of wall invasion, and the frequency of lymph node metastasis. In cultured cancer cells, Daple depletion led to the suppression of Wnt5a‐induced Rac and JNK activation, laminin γ2 expression, and cell migration and invasion. Accordingly, Daple depletion also suppressed liver metastasis in a mouse xenograft model of gastric cancer. These results suggest that the non‐canonical Wnt signaling pathway contributes to gastric cancer progression at least in part via Daple, which provides a new therapeutic opportunity for the treatment of the disease.
Keywords: Daple, gastric cancer, invasion, metastasis, Wnt signaling
Significant progress has been made in the diagnosis and treatment of gastric cancer, leading to a decrease in the mortality rate of patients with the disease. Nonetheless, many cases with delayed diagnosis and metastasis are intractable, making gastric cancer the third leading cause of cancer deaths worldwide.1 To date, multiple genes, proteins and signaling pathways have been found to be deregulated in gastric cancer.2, 3, 4, 5 However, the mechanisms underlying the tumorigenesis, heterogeneity and metastasis of gastric cancer are less well understood.
Previous studies have demonstrated that Wnt signaling represents one of the deregulated pathways in gastric cancer.6 Wnt signaling is essential for embryonic development and adult tissue homeostasis and is involved in cancer initiation and progression. It consists of two distinct branches that signal intracellularly: canonical and non‐canonical pathways.7, 8, 9 Activation of the canonical pathway induces β‐catenin nuclear accumulation and Wnt target gene transcription.10 Mutations in components of the canonical pathway, such as β‐catenin, adenomatous polyposis coli and Axin genes, are involved in human cancer initiation.11, 12 The non‐canonical pathway is independent of β‐catenin; instead, members of the Rho family of small GTPases, including Rac and Rho, and JNK transmit the signals to promote cell motility.13, 14 Accordingly, aberrant activation of non‐canonical pathway components has been implicated as well in invasion and metastasis in human malignancies.15
High levels of Wnt5a, a ligand that utilizes the non‐canonical pathway, have been reported to promote invasion in advanced gastric cancer.16, 17, 18 Wnt5a has been shown to induce the expression of laminin γ2, a subunit of the extracellular matrix laminin 5 protein that constitutes the epithelial basement membrane, through the activation of Rac, JNK and the transcription factor jun D.17 In turn, laminin γ2 expression promotes cancer cell adhesion and invasion.19 Importantly, cytoplasmic staining for laminin γ2 has been observed at the invasive front of gastric cancer and correlated with Wnt5a expression, indicating the relevance of the Wnt5a/laminin γ2 pathway in gastric cancer progression.17, 20 However, the mechanisms by which Wnt5a induces this process remain unclear.
In the present study, we investigated the involvement of Dvl‐associating protein with a high frequency of leucine residues (Daple) in the Wnt5a/laminin γ2 pathway in gastric cancer. Daple was originally identified as a binding protein for Dvl, which is a scaffold protein essential for transducing both Wnt signalling pathways.21 Daple is a large 226 kDa protein that has unique end‐terminal domains that flank a central long coiled‐coil domain. We previously reported that Daple controls the non‐canonical Wnt signaling pathway to regulate cell motility.22 Daple mediates the Wnt5a‐induced interaction of Dvl with atypical protein kinase C (aPKC), which promotes Rac activation and lamellipodia formation in migrating fibroblasts. Consistent with this was the finding that a Xenopus paralogue of Daple (xDal) is pivotal for the movements of convergent extension during gastrulation.23 To date, the reported involvement of Daple in the development and progression of human diseases constitutes a missense mutation in the human Daple gene (CCDC88C) that activates JNK and causes spinocerebellar ataxia.24
Here, using tissue sections from patients with gastric cancer, we demonstrated the relevance of Daple expression to gastric cancer progression. We also clarified, using cultured cancer cells and a xenograft mouse tumor model, that Daple mediates Wnt5a‐induced laminin γ2 expression and regulates gastric cancer invasion and metastasis.
Materials and Methods
Tissue samples and histological analysis
We obtained 130 tissue samples from patients with gastric cancer who underwent surgical treatment at Nagoya University Hospital between 2001 and 2006. Pathological diagnosis was made following classification of each case by the World Health Organization (WHO) and Lauren systems.25 Diffuse‐type cases, which correspond to poorly differentiated adenocarcinomas, were further divided into diffuse‐scattered and adherent types.16, 26 The Mucin phenotype was estimated by immunostaining with CD10, MUC2, MUC6 and MUC5AC antibodies.27, 28 Tumor staging was performed based on the TNM classification system. The study was approved by the Ethics Committee of Nagoya University.
Immunohistochemistry
Formalin‐fixed and paraffin‐embedded tissue sections were stained with anti‐Daple (1:100; IBL, Gumma, Japan), anti‐Wnt5a/b (1:50; Cell Signaling Technology, Danvers, MA, USA), anti‐laminin γ2 (1:200; Millipore, Bedford, MA, USA) and anti‐β‐catenin (1:1000; BD Transduction Laboratories, San Jose, CA, USA) antibodies. The sections were pretreated by boiling in citrate buffer (pH 7.0) for Daple, Wnt5a/b and β‐catenin staining or by incubation with proteinase K for laminin γ2 staining. After blocking with Protein Block Serum Free (Dako, Glosturp, Denmark), the sections were incubated with primary antibodies overnight at 4°C, then with secondary antibodies (Envision+, Dako). Reaction products were visualized using diaminobenzidine (Dako).
Cell culture, proliferation assay and RNA interference
Gastric cancer cell lines MKN45 and KKLS were purchased from the ATCC (Rockville, MD, USA) and provided by the Human Cancer Cell Line Bank (Cancer Research Institute of Kanazawa University, Japan), respectively. Cells were grown in RPMI 1640/10% FBS. For cell proliferation assays, KKLS cells were seeded at 5 × 104 cells per 35‐mm dish; after 12 h, the medium was replaced with RPMI 1640/1% FBS. The cells were counted every 24 h for 3 days. For RNA interference‐mediated depletion (knockdown) of Daple, human Daple‐specific (target sequence, 5′‐TCCAGCTGCGCGTTGTTCAGTGAGG‐3′) and control siRNAs were synthesized by Qiagen (Hilden, Germany). The siRNAs were transfected into MKN45 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to manufacturer instruction. The target sequences for shRNA mediated Daple knockdown were as follows (only the sense sequence is shown): Daple #1, 5′‐GGTGCAAGCTCGATGTGTA‐3′; Daple #2, 5′‐GCACCAAAGGCTATAACTC‐3′ and Daple #3, 5′‐GCCTGGAGCGTGACAACAA‐3′. The oligonucleotide pairs were inserted into the pSIREN‐RetroQ retroviral shRNA expression vector (Clontech, Palo Alto, CA, USA) to generate recombinant retroviruses as previously described,29 followed by infection of KKLS cells and puromycin selection.
Western blot analysis
Cells were lysed in SDS sample buffer. GTP loading of Rac was determined by pull‐down assay using GST‐PAK‐PBD (Cytoskeleton, Denver, CO, USA). Samples were separated by SDS‐PAGE, and proteins were transferred to PVDF membranes (Millipore). The membranes were blocked in 4% skimmed milk, and probed with anti‐Daple (1:500), anti‐laminin γ2 (1:1000), anti‐Wnt5a/b (1:1000), anti‐Rac1 (1:1000; Millipore) and anti‐phospho JNK and anti‐JNK (1:100; Cell Signaling Technology) antibodies. After incubation with HRP‐conjugated secondary antibodies (Dako), immunoreactivity was detected with an enhanced chemiluminescence system (Amersham Biosciences, Piscataway, NJ, USA).
Quantitative RT‐PCR
Total RNA was extracted from KKLS cells with TRIzol reagent (Invitrogen) according to manufacturer protocol. The RNA was then reverse transcribed into cDNA using the Rever Tra Ace qPCR RT kit (Toyobo, Osaka, Japan), following manufacturer protocol. Gene expression levels were quantitatively measured using the Thunderbird SYBR qPCR mix (Toyobo) and analyzed with a MX Pro 3000P Quantitative PCR System and MX Pro software (Stratagene, La Jolla, CA, USA). The primer sequences were as follows: human LAMC2 (laminin γ2 gene), forward, 5′‐ACCGTGTGGACAGAGGAGGC‐3′, reverse, 5′‐GGATGCGGAGGGCTGTGAGA‐3′; and human 18S, forward, 5′‐AGTCCCTGCCCTTTGTACACA‐3′, reverse, 5′‐CGATCCGAGGGCCTC‐3′.
Cell migration and invasion assays
Cell migration was examined with transwell assays using 8‐μm pore polyethylene terephthalate membranes (BD Bioscience). Chambers were coated with 10 μg/mL fibronectin or 10 μg/mL type 1 collagen for KKLS or MKN45 cells, respectively. The cells (2.5 × 104) were suspended in 100 μL RPMI/0.1% BSA and seeded in the upper chamber. In the lower chamber, RPMI/10% FBS was added. After 6 h, migrated cells were fixed and counted. KKLS cell invasion was assayed using Biocoat Matrigel invasion chambers (8‐μm; Corning, Corning, NY, USA). KKLS cells (2.5 × 104) were added to the upper chambers and allowed to invade for 48 h.
Xenograft mouse tumor model and metastasis assay
All animal studies were approved by the Animal Care and Use Committee of Nagoya University Graduate School of Medicine, and all the experiments were performed in accordance with institutional guidelines and regulations. In metastasis assays to investigate spontaneous metastasis of gastric cancer cells to the liver from the spleen, we injected 5 × 105 KKLS cells into the spleen of 6‐week old nude mice (BALB/cSls‐nu/nu) through a 29‐gauge needle. After 5 weeks of injection, the liver was enucleated, and the numbers of metastatic nodules with diameter >2 mm were counted. The size of metastatic nodules with diameter >1 mm was measured from tissue sections.
Statistical analysis
All statistical analysis was performed using GraphPad Prism 6 software (GraphPad, San Diego, CA, USA). The χ2‐test was used to analyze correlations between Daple expression and clinicopathological parameters. The overall survival was defined as the time between the date of surgery and the last date of follow up. Kaplan–Meier survival curves were created, wherein the differences between groups were evaluated by a log‐rank test. For in vitro experiments on cultured cells, statistical analyses were carried out using Student's t‐test. Mann–Whitney's U‐test was used for the analysis of metastasis assays. P‐values < 0.05 were considered statistically significant.
Results
Daple is highly expressed in advanced stages of gastric cancer
To evaluate the relevance of Daple expression in the progression of gastric cancer, we performed immunohistochemical analysis on tissue sections from 130 patients with gastric cancer and tissue array slides of human normal stomach (SuperBiochips Laboratories, Seoul, Korea). Preliminary experiments showed no or weak staining for Daple in the epithelia of normal stomach or in cancer cells from the early stage of gastric cancer, whereas Daple expression was clearly observed in cancer cells at more advanced stages (Fig. 1a). To statistically evaluate Daple expression, we constructed a scoring system in analogy with the Allred scoring system for estrogen and progesterone receptor expression in breast cancer,30 where the intensity and frequency (proportion) of cytoplasmic Daple expression was graded by intensity score (IS) (0–3) (Fig. 1b) and proportion score (PS) (0–3) (Fig. 1c). A total score (TS) (representing the sum of IS and PS) >3 was defined as Daple positive (Fig. 1c); these constituted 85/130 (65.38%) cases.
Figure 1.
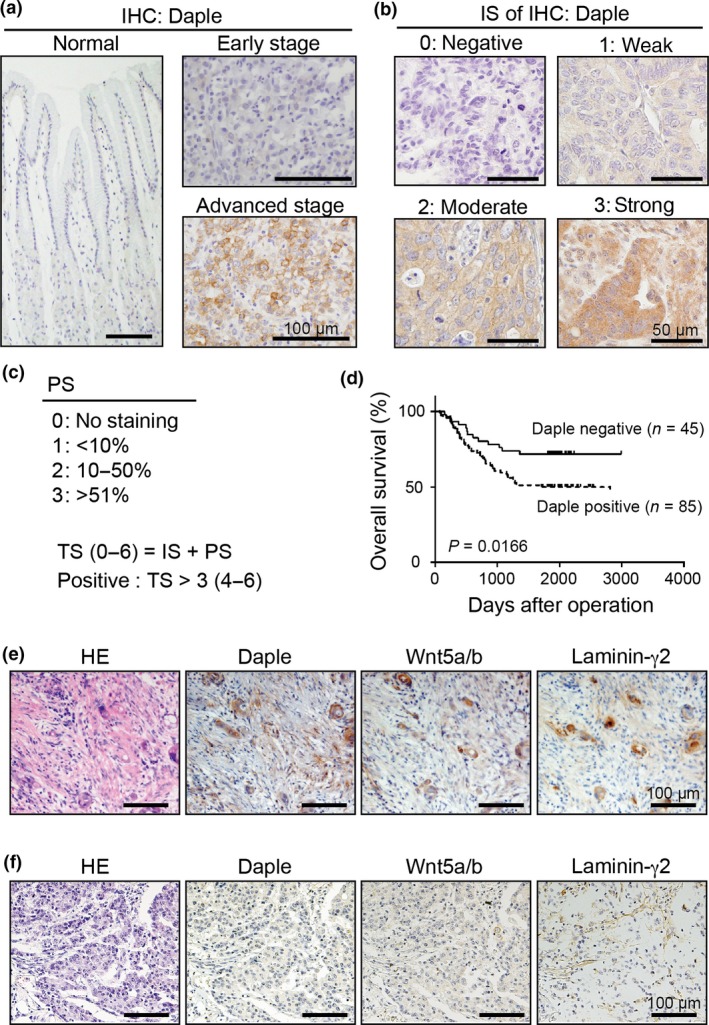
Expression of Daple in gastric cancer. (a) Representative images of immunohistochemical (IHC) staining for Daple. Sections as indicated including an invasive region of the advanced stage of gastric cancer (bottom right) were stained with anti‐Daple antibody. Scale bars, 100 μm. (b) Representative images for representative Daple staining intensity for each intensity score (IS) (0–3). Scale bars, 50 μm. (c) Frequency and distribution of Daple expression was judged with the proportion score (PS) as indicated in the panel. The sum of IS and PS was used as a total score (TS) for the determination of Daple positivity (box). TS > 3 was judged as positive. (d) Kaplan–Meier survival curves of patients with gastric cancer segregated by Daple expression status. (e,f) Representative images for Daple, Wnt5a/b and laminin γ2 expression in diffuse‐scattered (e) or diffuse‐adherent (f) types of gastric cancer. Scale bars, 100 μm.
We next analyzed the correlation between Daple positivity and clinicopathological parameters in the current cohort (Table 1). No significant association was found with patient age, gender, tumor size, tumor location, WHO classification or Mucin type. In contrast, Daple expression was statistically correlated with the depth of gastric wall invasion (the T component of the TNM classification) (P = 0.001), the frequency of lymph node metastasis (the N component) (P = 0.0162) and clinical stage (P = 0.0037). Specifically, Daple positivity rate was significantly high in patients at T2–T4 (76.1%), with lymph node metastasis‐positive (74.3%) and at clinical stage II–IV (76.5%). Furthermore, the Kaplan–Meier survival curve showed that the postoperative survival rate was significantly lower for patients who were Daple‐positive rather than Daple‐negative (P = 0.0166 by log‐rank test) (Fig. 1d). It should be noted that the overall survival was significantly influenced by other factors, including the depth of wall invasion, positive rate for lymph node metastasis and TNM stage (P < 0.0001) (Table S1), suggesting that Daple positivity does not independently regulate prognosis for patients with gastric cancer but has a synergistic interaction with other factors. Nonetheless, the results support the possible involvement of Daple in gastric cancer progression.
Table 1.
Correlation of Daple expression with clinicopathological characteristics in patients with gastric cancer
| Total | Daple positive | P‐value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Age | |||
| ≤60 | 62 (48.1) | 36 (58.1) | 0.1392 |
| >60 | 67 (51.9) | 48 (71.6) | |
| Sex | |||
| Male | 99 (76.2) | 68 (68.7) | 0.1952 |
| Female | 31 (23.8) | 17 (54.8) | |
| Size | |||
| ≤6 cm | 74 (67.3) | 51 (68.9) | 0.5193 |
| >6 cm | 36 (32.7) | 22 (61.1) | |
| Location | |||
| Cardia | 20 (15.6) | 15 (75.0) | 0.337 |
| Corpus | 33 (25.8) | 23 (69.7) | |
| Antrum | 34 (26.6) | 18 (52.9) | |
| Whole | 41 (32.0) | 27 (65.9) | |
| WHO classification | |||
| Well differentiated type | 11 (9.3) | 7 (63.6) | 0.0508 |
| Moderately differentiated type | 41 (34.7) | 33 (80.5) | |
| Poorly diffrentiated type | 66 (55.9) | 38 (57.6) | |
| Mucin type | |||
| Gastric type | 44 (33.8) | 28 (63.6) | 0.7508 |
| Gastrointestinal type | 8 (6.2) | 5 (62.5) | |
| Intestinal type | 54 (41.5) | 38 (70.4) | |
| Null type | 24 (18.5) | 14 (58.3) | |
| Gastric wall invasion | |||
| T1 | 38 (29.2) | 15 (39.5) | 0.001 |
| T2 | 14 (10.8) | 11 (78.6) | |
| T3 | 25 (19.2) | 20 (80.0) | |
| T4 | 53 (40.8) | 39 (73.6) | |
| Lymph node metastasis | |||
| Negative | 56 (43.1) | 30 (53.6) | 0.0162 |
| Positive | 74 (56.9) | 55 (74.3) | |
| TNM stage | |||
| Stage I | 45 (34.6) | 20 (44.4) | 0.0037 |
| StageII | 23 (17.7) | 18 (78.3) | |
| StageIII | 27 (20.8) | 21 (77.8) | |
| Stage IV | 35 (26.9) | 26 (74.3) | |
Coexpression of Daple with Wnt5a/b and laminin γ2 in gastric cancer
We previously showed that Daple mediates Wnt5a‐induced Rac activation through the non‐canonical Wnt pathway.22 As Wnt5a expression was also shown to correlate with laminin γ2 expression and gastric cancer aggression,17 we investigated whether Daple expression is also correlated with Wnt5a and laminin γ2 in our patient cohort. We evaluated Wnt5a/b expression by the same scoring system as used for Daple, and laminin γ2 expression was assessed as positive when signal was apparent in the cytoplasmic region of cancer cells. In addition, considering previous findings that altered expression and mutational activation of β‐catenin were found in gastric cancer,31 we monitored nuclear staining for β‐catenin, which is indicative of canonical Wnt signaling pathway activity. We found that Daple expression was significantly correlated with Wnt5a/b positivity (P < 0.001) but not with β‐catenin nuclear staining in our cohort (P = 0.3194) (Table 2), suggesting a role for Daple in the non‐canonical Wnt signaling pathway.
Table 2.
Correlation of Daple expression with Wnt5a/b, laminin‐γ2, and β‐catenin expression in patients with gastric cancer
| Total | Daple positive | P‐value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Wnt5a/b positivity | |||
| All cases | |||
| Wnt5a/b positive | 75 (57.7) | 66 (88.0) | <0.001 |
| Wnt5a/b negative | 55 (42.3) | 19 (34.5) | |
| Diffuse‐scattered type | |||
| Wnt5a/b positive | 20 (71.4) | 19 (95.0) | <0.001 |
| Wnt5a/b negative | 8 (28.6) | 2 (25.0) | |
| Other type | |||
| Wnt5a/b positive | 55 (53.9) | 47 (85.5) | <0.001 |
| Wnt5a/b negative | 47 (46.1) | 17 (36.2) | |
| Laminin‐γ2 cytoplasmic positivity | |||
| All cases | |||
| Cytoplasmic positive | 56 (43.1) | 42 (75.0) | 0.06 |
| Others | 74 (56.9) | 43 (58.1) | |
| Diffuse‐scattered type | |||
| Cytoplasmic positive | 17 (60.7) | 16 (94.1) | <0.01 |
| Others | 11 (39.3) | 5 (45.5) | |
| Other type | |||
| Cytoplasmic positive | 39 (38.2) | 26 (66.7) | 0.54 |
| Others | 63 (61.8) | 38 (60.3) | |
| β‐catenin activity | |||
| Nuclear/cytoplasm | 65 (50.0) | 29 (44.6) | 0.32 |
| Others | 65 (50.0) | 11 (16.9) | |
Previous studies classified poorly differentiated gastric cancer into the diffuse‐scattered type, where cancer cells exhibit weak intercellular adhesion, and diffuse‐adherent type, where cancer cells form connected group; therein, Wnt5a and laminin γ2 coexpression was apparent in diffuse‐scattered types but not in other types of gastric cancer.17 Given this finding, we differentially examined Daple expression in diffuse‐scattered type versus other types in our cohort. The results showed that both Wnt5a/b and cytoplasmic laminin γ2 expression significantly correlated with Daple positivity when limited to the diffuse‐scattered type (P < 0.001 and P < 0.01, respectively) (Table 2, Fig. 1e). In other types, although Daple and Wnt5a/b expression were significantly correlated (P < 0.001), significant correlation was not observed between Daple and cytoplasmic laminin γ2 expression (P = 0.54) (Table 2, Fig. 1f). These data, together with the association of Daple expression with clinicopathological features, suggest that Daple preferentially coexpresses with Wnt5a/b and laminin γ2 to regulate the non‐canonical Wnt signaling pathway in invasive gastric cancer.
Daple mediates Wnt5a‐induced laminin γ2 expression and invasion of gastric cancer cells
In gastric cancer cells, laminin γ2 expression is regulated by Wnt5a through Rac activation and JNK phosphorylation.17, 18 Therefore, we examined the effect of Daple knockdown on these effects in the MKN45 gastric cancer cell line. In addition, MKN45 cells exhibit weak endogenous Wnt5a expression.16 Thus, we can study the effect of exogenous Wnt5a; this increased laminin γ2 expression, Rac activation and JNK phosphorylation in control cells, all of which were abrogated by Daple knockdown (Fig. 2a). We previously reported that Daple knockdown attenuated Wnt5a‐induced migration in fibroblasts.22 Here, we showed that exogenous Wnt5a expression increased MKN45 migration and invasion through the Matrigel (Fig. 2b,c), which were significantly attenuated by Daple knockdown.
Figure 2.
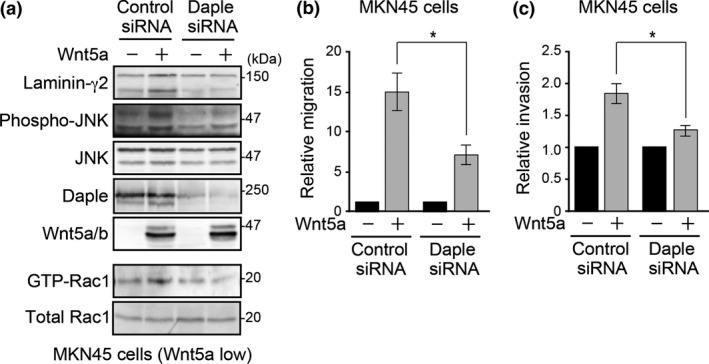
Daple regulates Wnt5a‐induced Rac/JNK activation and laminin γ2 expression in gastric cancer cells. (a) Daple knockdown inhibited Wnt5a‐induced laminin γ2 expression, Rac activation and JNK phosphorylation. Western blot analysis of total cell lysates from MKN45 cells transfected with the indicated combinations of plasmids (control or Wnt5a) and siRNA (control or Daple siRNA). For Rac activation analysis (lower two panels), GTP‐bound Rac1 was pulled down with GST‐PBD and precipitated samples were probed with Rac1 antibody. (b,c) Wnt5a‐induced migration or invasion was attenuated by Daple knockdown. Transwell migration (b) or invasion (c) assays of MKN45 cells transfected with the indicated combinations of plasmids. Migrated cell numbers were expressed as the relative migration divided by that of Wnt5a (−) cells. The results represent the means ± SE. *P < 0.05.
We further examined Daple function in the KKLS gastric cancer cell line, which expresses high levels of Wnt5a.17 We generated control and Daple‐depleted KKLS cells by retrovirus‐mediated transduction of control and three different Daple‐specific shRNAs (#1–3). Variable Daple knockdown efficiency was observed in stably transduced cells without affecting Wnt5a expression (Fig. 3a). Quantitative RT‐PCR analysis showed that Daple knockdown was accompanied by a decrease in LAMC2 expression (Fig. 3b). Of note, KKLS cells transduced with Daple shRNA#3, in which mild knockdown of endogenous Daple was observed, did not exhibit significant changes in laminin γ2 expression, showing the specificity of the experiment.
Figure 3.
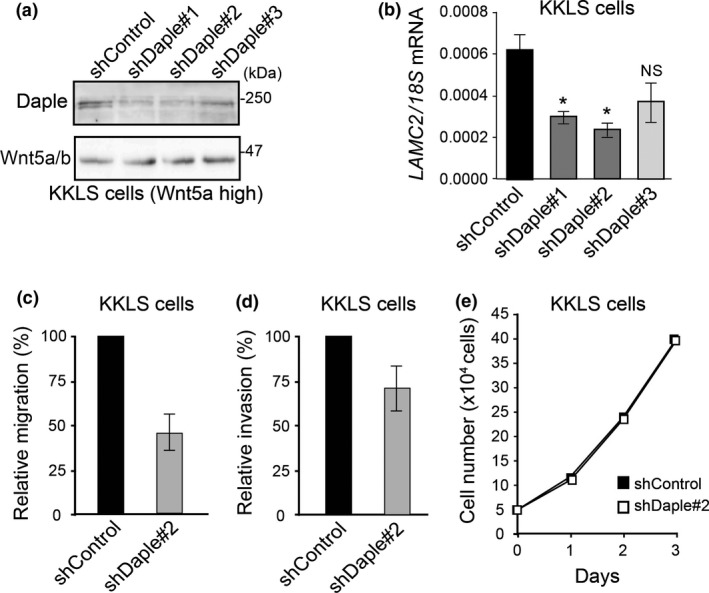
Daple knockdown attenuates migration and invasion of high‐Wnt5a‐expressing KKLS cells. (a) Western blot of total cell lysates of control and Daple knockdown KKLS cells using three different shRNA (shDaple#1‐3). (b) LAMC2 expression in control and Daple knockdown KKLS cells quantified by real‐time RT‐PCR, normalized to 18S ribosomal RNA (18S) expression. The results represent the means ± SE. *P < 0.05 compared with control shRNA. NS, not significant. (c,d) Migration (c) and invasion (d) assays of control shRNA or Daple shRNA#2‐expressing KKLS cells. The number of migrated cells was expressed as relative migration (%) divided by that of control cells. The results represent the means ± SE. (e) Daple knockdown had no effect on KKLS cell proliferation. 1 × 105 KKLS cells stably expressing control (closed square) or Daple (open square) shRNA were cultured for 3 days; cell numbers were counted each day.
We next examined the effect of Daple knockdown on KKLS migration and invasion, using Daple shRNA#2 (Figs 3c,d and 4). Daple depletion significantly decreased migration and invasion compared with control cells. However, cell proliferation was not affected, showing the specific role of Daple for cell motility in gastric cancer cells (Fig. 3e).
Figure 4.
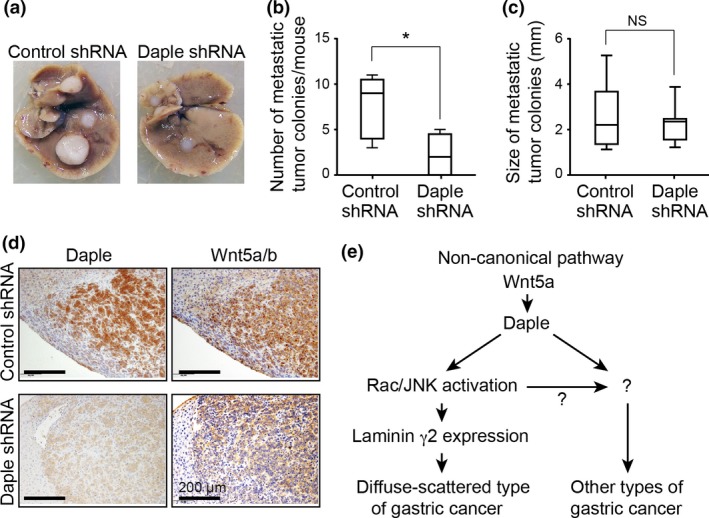
Daple knockdown attenuates gastric cancer cell metastasis. (a) Representative images of metastatic tumors that developed in the liver of nude mice at 5 weeks after intrasplenic injection of control (left) or Daple knockdown (right) KKLS cells. (b) Total numbers of metastatic hepatic nodules per mouse (n = 6) were counted and quantified. The box plot shows median (horizontal line), 25th to 75th percentile (box), and total range (bars). *P < 0.05 (Mann–Whitney test). (c) Metastatic nodule size (n = 12) was measured in each group. NS, not significant (Mann–Whitney test). (d) Representative images of anti‐Daple and anti‐Wnt5a/b antibody‐stained metastatic tumor tissues. Scale bars, 100 μm. (e) Proposed model for Daple function in the non‐canonical Wnt signaling pathway. In particular gastric cancers including the diffuse‐scattered type, Daple induces laminin γ2 expression through Rac and JNK activation downstream of Wnt5a stimulation (left). The current results also suggest the existence of laminin γ2‐independent roles in other types of gastric cancer.
Daple is involved in gastric cancer cell metastasis
Given that Daple expression was correlated with lymph node metastasis (Table 1), we examined the effect of Daple knockdown on metastasis in a xenograft tumor mouse model (Fig. 4). We utilized the KKLS cell line because it was established from a human primary gastric cancer with liver metastasis.32 Accordingly, control KKLS cells transplanted intrasplenically exhibited the propensity for liver metastasis in immunocompromised mice (Fig. 4a). In contrast, Daple knockdown KKLS cells rarely metastasized to the liver, and the number of metastatic nodules was significantly decreased compared with control cells (P = 0.0397) (Fig. 4a,b). However, metastatic nodule size was not significantly affected by Daple knockdown (Fig. 4c). Immunohistochemical analysis of metastatic tissues showed coexpression of Daple and Wnt5a/b in control but not Daple‐depleted tumors (Fig. 4d). Wnt5a/b expression was comparable between groups, suggesting that Daple functions downstream of Wnt5a/b, consistent with our biochemical data on cultured cancer cells (Fig. 2a). Taken together, these findings suggest that Daple mediates Wnt5a‐expressing gastric cancer cell metastasis.
Discussion
Despite recent advances in gastric cancer treatments, the prognosis of advanced disease remains poor because of the high frequency of metastasis to distant organs and dissemination,33 highlighting the importance of understanding the underlying mechanisms for cell motility in this disease. Here, we showed that Daple is highly expressed in advanced gastric cancer, where its expression significantly correlated with the depth of gastric wall invasion, frequency of lymph node metastasis and poor prognosis. We also demonstrated that Daple mediates the non‐canonical Wnt signaling pathway to regulate laminin γ2 expression, previously shown to be critical for gastric cancer progression.17, 19 These findings offer an opportunity for the development of new therapeutics for advanced gastric cancer.
Although Daple and Wnt5a/b expression were correlated in our immunohistochemical study (Table 2), MKN45 cells showed high Daple but low Wnt5a/b expression (Fig. 2a). In addition, stimulation with exogenous Wnt5a did not affect Daple expression in MKN45 cells, suggesting that Wnt5a does not directly regulate Daple expression in gastric cancer cells. Daple has been listed among Wnt target genes, the transcription of which is regulated by β‐catenin downstream of the canonical Wnt signaling pathway.34 However, this was not supported by our present study, wherein Daple expression was not correlated with β‐catenin nuclear localization (Table 2); in MKN45 cells, β‐catenin was localized at the cell membrane (data not shown). Alternatively, the estrogen receptor α (ERα) has been shown to interact with the human Daple gene to regulate its transcriptional activity in human breast cancer cells.35 Given that altered ERα expression is involved in the increased metastatic potential of gastric cancer,36 the role of ERα‐mediated signaling in Daple expression induction in gastric cancer as well as the crosstalk between the non‐canonical Wnt signaling pathway and ERα‐mediated signaling should be investigated.
Our data showed that Daple expression significantly correlates with laminin γ2 only in diffuse‐scattered type gastric cancer (Table 2), which notably was also shown to exclusively exhibit correlation of Wnt5a with laminin γ2.16 One plausible hypothesis to explain such histologically‐specific functioning is that, because laminin γ2 is a major component of laminin 5, which constitutes the cancer stroma that supports cancer cell invasion,19, 37 laminin γ2 expression might be specifically important for the invasion of small cancer cell nests that accompany the desmoplastic reaction and fibrosis of cancer stroma as occurs in the diffuse‐scattered type of gastric cancer. Furthermore, Daple expression correlated with Wnt5a/b expression independently of histological type (Table 2), suggesting that the Wnt5a/Daple pathway has multifaceted, laminin γ2‐independent roles in cancer progression (Fig. 4e).
In addition, in the non‐canonical Wnt signaling pathway, the mechanisms of laminin γ2 induction by Rac activation and subsequent JNK phosphorylation have been established.17, 18 However, the mechanism of Rac activation by Daple is complex and not completely understood. We previously showed that Daple regulates the subcellular localization of Dvl/aPKC complex to regulate Rac activity.22 However, another recent hypothesis is that Daple links non‐canonical Wnt stimulation to tripartite G‐protein activation, which enhances Rac activation and contributes to colorectal cancer invasiveness.38 Overall, these disparate possibilities indicate that further studies are required to reveal the biochemical mode of Daple function downstream of non‐canonical Wnt stimulation in various types of gastric cancer.
Disclosure statement
The authors have no conflict of interest to declare.
Supporting information
Table S1. Correlation of clinicopathological characteristics with overall survival in the cohort study.
Acknowledgments
We gratefully acknowledge A. Kikuchi and H. Yamamoto (Osaka University) for providing the Wnt5a expression plasmid. This work was supported by a Grant‐in‐Aid for Young Scientists (B) (to M. Takagishi) and Grants‐in‐Aid for Scientific Research (S) and (A) (to M. Takahashi) commissioned by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. M. Takagishi is a fellow of the Japan Society for the Promotion of Science.
Cancer Sci 107 (2016) 133–139
Funding Information Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 2. Deng N, Goh LK, Wang H et al A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co‐occurrence among distinct therapeutic targets. Gut 2012; 61: 673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang W, Raufi A, Klempner SJ. Targeted therapy for gastric cancer: molecular pathways and ongoing investigations. Biochim Biophys Acta 2014; 1846: 232–7. [DOI] [PubMed] [Google Scholar]
- 4. El‐Rifai W, Powell SM. Molecular biology of gastric cancer. Semin Radiat Oncol 2002; 12: 128–40. [DOI] [PubMed] [Google Scholar]
- 5. Gotoda T, Yanagisawa A, Sasako M et al Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3: 219–25. [DOI] [PubMed] [Google Scholar]
- 6. Katoh M. Dysregulation of stem cell signaling network due to germline mutation, SNP, Helicobacter pylori infection, epigenetic change and genetic alteration in gastric cancer. Cancer Biol Ther 2007; 6: 832–9. [DOI] [PubMed] [Google Scholar]
- 7. Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 2003; 1653: 1–24. [DOI] [PubMed] [Google Scholar]
- 8. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20: 781–810. [DOI] [PubMed] [Google Scholar]
- 9. Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013; 13: 11–26. [DOI] [PubMed] [Google Scholar]
- 10. Clevers H. Wnt/β‐catenin signaling in development and disease. Cell 2006; 127: 469–80. [DOI] [PubMed] [Google Scholar]
- 11. Kikuchi A. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci 2003; 94: 225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev 2007; 17: 45–51. [DOI] [PubMed] [Google Scholar]
- 13. Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev 2003; 17: 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Veeman MT, Axelrod JD, Moon RT. A second canon: functions and mechanisms of β‐catenin‐independent Wnt signalling. Dev Cell 2003; 5: 367–77. [DOI] [PubMed] [Google Scholar]
- 15. Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol (Oxf) 2012; 204: 17–33. [DOI] [PubMed] [Google Scholar]
- 16. Kurayoshi M, Oue N, Yamamoto H et al Expression of Wnt‐5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 2006; 66: 10439–48. [DOI] [PubMed] [Google Scholar]
- 17. Yamamoto H, Kitadai Y, Yamamoto H et al Laminin γ2 mediates Wnt5a‐induced invasion of gastric cancer cells. Gastroenterology 2009; 137: 242–52. [DOI] [PubMed] [Google Scholar]
- 18. Hanaki H, Yamamoto H, Sakane H et al An anti‐Wnt5a antibody suppresses metastasis of gastric cancer cells in vivo by inhibiting receptor‐mediated endocytosis. Mol Cancer Ther 2012; 11: 298–307. [DOI] [PubMed] [Google Scholar]
- 19. Miyazaki K. Laminin‐5 (laminin‐332): unique biological activity and role in tumor growth and invasion. Cancer Sci 2006; 97: 91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koshikawa N, Moriyama K, Takamura H et al Overexpression of laminin γ2 chain monomer in invading gastric carcinoma cells. Cancer Res 1999; 59: 5596–601. [PubMed] [Google Scholar]
- 21. Oshita A, Kishida S, Kobayashi H et al Identification and characterization of a novel Dvl‐binding protein that suppresses Wnt signalling pathway. Genes Cells 2003; 8: 1005–17. [DOI] [PubMed] [Google Scholar]
- 22. Ishida‐Takagishi M, Enomoto A, Asai N et al The Disheveled‐associating protein Daple controls the non‐canonical Wnt/Rac pathway and cell motility. Nat Commun 2012; 3: 859. [DOI] [PubMed] [Google Scholar]
- 23. Kobayashi H, Michiue T, Yukita A et al Novel Daple‐like protein positively regulates both the Wnt/β‐catenin pathway and the Wnt/JNK pathway in Xenopus. Mech Dev 2005; 122: 1138–53. [DOI] [PubMed] [Google Scholar]
- 24. Tsoi H, Yu AC, Chen ZS et al A novel missense mutation in CCDC88C activates the JNK pathway and causes a dominant form of spinocerebellar ataxia. J Med Genet 2014; 51: 590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lauren P. The two histological main types of gastric carcinoma, an attempt at a histoclinical classification. Acta Pathol Microbiol Scand. 1965; 64: 31–49. [DOI] [PubMed] [Google Scholar]
- 26. Shiroshita H, Watanabe H, Ajioka Y, Watanabe G, Nishikura K, Kitano S. Re‐evaluation of mucin phenotypes of gastric minute well‐differentiated‐type adenocarcinomas using a series of HGM, MUC5AC, MUC6, M‐GGMC, MUC2 and CD10 stains. Pathol Int 2004; 54: 311–21. [DOI] [PubMed] [Google Scholar]
- 27. Mizoshita T, Tsukamoto T, Nakanishi H et al Expression of Cdx2 and the phenotype of advanced gastric cancers: relationship with prognosis. J Cancer Res Clin Oncol 2003; 129: 727–34. [DOI] [PubMed] [Google Scholar]
- 28. Shimoyama Y, Hirohashi S. Expression of E‐and P‐cadherin in gastric carcinomas. Cancer Res 1991; 51: 2185–92. [PubMed] [Google Scholar]
- 29. Enomoto A, Asai N, Namba T et al Roles of disrupted‐in‐schizophrenia 1‐interacting protein girdin in postnatal development of the dentate gyrus. Neuron 2009; 63: 774–87. [DOI] [PubMed] [Google Scholar]
- 30. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998; 11: 155–68. [PubMed] [Google Scholar]
- 31. Woo DK, Kim HS, Lee HS, Kang YH, Yang HK, Kim WH. Altered expression and mutation of β‐catenin gene in gastric carcinomas and cell lines. Int J Cancer 2001; 95: 108–13. [DOI] [PubMed] [Google Scholar]
- 32. Tsuchiya Y, Sato H, Endo Y et al Tissue inhibitor of metalloproteinase 1 is a negative regulator of the metastatic ability of a human gastric cancer cell line, KKLS, in the chick embryo. Cancer Res 1993; 53: 1397–402. [PubMed] [Google Scholar]
- 33. Shah MA, Ajani JA. Gastric cancer–An enigmatic and heterogeneous disease. JAMA 2010; 303: 1753–4. [DOI] [PubMed] [Google Scholar]
- 34. Grote D, Boualia SK, Souabni A et al Gata3 acts downstream of β‐catenin signaling to prevent ectopic metanephric kidney induction. PLoS Genet 2008; 4: e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li G, Ruan X, Auerbach RK et al Extensive promoter‐centered chromatin interactions provide a topological basis for transcription regulation. Cell 2012; 148: 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takano N, Iizuka N, Hazama S, Yoshino S, Tangoku A, Oka M. Expression of estrogen receptor‐α and‐β mRNAs in human gastric cancer. Cancer Lett 2002; 176: 129–35. [DOI] [PubMed] [Google Scholar]
- 37. Pyke C, Salo S, Ralfkiaer E, Rømer J, Danø K, Tryggvason K. Laminin‐5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res 1995; 55: 4132–9. [PubMed] [Google Scholar]
- 38. Aznar N, Midde KK, Dunkel Y et al Daple is a novel non‐receptor GEF required for trimeric G protein activation in Wnt signaling. Elife 2015; 4: e07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Correlation of clinicopathological characteristics with overall survival in the cohort study.


