Abstract
In the last 5 years, immune checkpoint antibodies have become established as anticancer agents for various types of cancer. These antibody drugs, namely cytotoxic T‐lymphocyte‐associated antigen, programmed death‐1, and programmed death ligand‐1 antibodies, have revealed relatively high response rates, the ability to induce durable responses, and clinical efficacy in malignancies not previously thought to be susceptible to immune‐based strategies. However, because of its unique mechanisms of activating the host immune system against cancer as well as expensive cost, immune checkpoint blockade faces novel challenges in selecting appropriate patient populations, monitoring clinical responses, and predicting immune adverse events. The development of objective criteria for selecting patient populations that are likely to have benefit from these therapies has been vigorously investigated but still remains unclear. In this review, we describe immune checkpoint inhibition‐specific challenges with patient selection and monitoring, and focus on approaches to remedy these challenges. We also discuss applications of the emerging field of immunopharmacogenomics for guiding selection and monitoring for anti‐immune checkpoint treatment.
Keywords: Biomarkers, checkpoint inhibitors, immunopharmacogenomics, immunotherapy, T‐cell receptor
Introduction and principles of immune checkpoint blockade
Immunotherapies such as immune checkpoint antibodies have revolutionized cancer treatment. Rather than directly targeting cancer cells, immune checkpoint antibodies target proteins that inhibit the host's natural immune response towards cancer cells and then strongly activate the host immune response to eliminate cancer cells. At present, the three major target molecules for such blockade are cytotoxic T‐lymphocyte‐associated antigen‐4 (CTLA‐4), programmed death‐1 (PD‐1), and programmed death ligand‐1 (PD‐L1). Both CTLA‐4 and PD‐1/PD‐L1, which act through distinct mechanisms, are key regulators responsible for maintaining homeostasis during the T cell‐mediated immune response. To date, ipilimumab (anti‐CTLA‐4 antibody), nivolumab and pembrolizumab (anti‐PD‐1 antibodies) are approved for advanced melanoma, and nivolumab and pembrolizumab are also approved for advanced non‐small‐cell lung cancer (NSCLC).1, 2, 3
Application of antibodies against these immune checkpoint molecules in cancer therapy was first proposed in the 1990s.4, 5 A type I transmembrane protein, CTLA‐4 (also called CD152) is expressed on the surface of regulatory T cells and regulates the amplitude of the T cell response. Antigen‐presenting cells (APCs) display antigens on MHC to the T cell receptor (TCR) of T lymphocytes. Effective T cell responses require costimulatory signals transmitted through the engagement of CD28 on the surface of T cells with CD80 (also known as B7.1) and CD86 (B7.2) on APCs (Fig. 1).5 CTLA‐4 competes with CD28 for binding with CD80 and CD86, resulting in the inhibition of TCR signaling, suppression of effector T cell activation, and attenuation of the T cell‐mediated immune response.6 Because of these functions, CTLA‐4 plays indispensable roles in the prevention of autoimmunity and the perpetuation of self‐tolerance.4 Anti‐CTLA‐4 antibody was the first immune checkpoint antibody shown to have antitumor potential, as shown in a landmark study in mice in 1996.4, 5, 6 Clinical trials later proved that CTLA‐4 blockade could also be applied to have potent antitumor abilities in humans.7, 8
Figure 1.
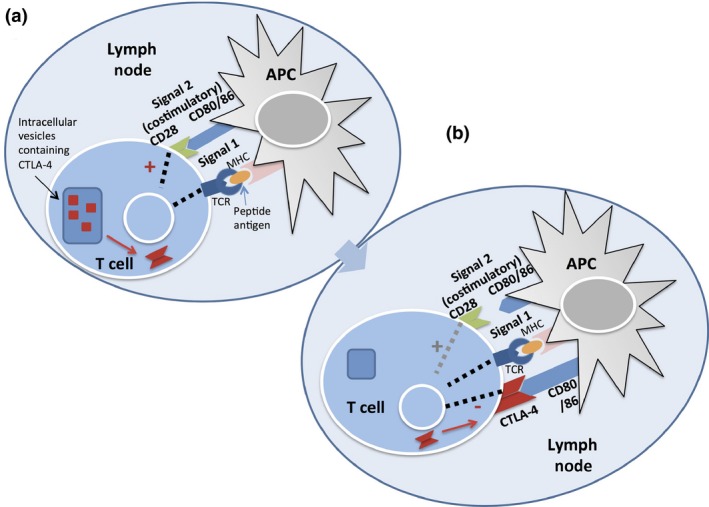
(a) T cell activation requires both signal 1, mediated by antigen presentation on MHC by dendritic cells to the T‐cell receptor (TCR), and costimulatory signals from CD80/86 engagement with CD28 on the surface of T cells. This initial activation sends signals to release cytotoxic T‐lymphocyte‐associated antigen‐4 (CTLA‐4) from intracellular vesicles. (b) Downregulation of T cell activity can occur distinctly through two mechanisms. CTLA‐4 is upregulated on the surface of T cells in response to initial activation, and outcompetes binding to CD80/86. In the periphery, tumor cells can present programmed death ligand‐1 to programmed death‐1 on the surface of T cells and also induce downregulation of T cell activity. APC, antigen‐presenting cell; MHC, major histocompatibility complex.
Programmed death‐1 is also a negative regulator of the T cell immune response that physiologically functions to avoid collateral damage caused by an overactive T cell response in peripheral tissues (Fig. 2). PD‐1 is expressed on activated T cells as well as B cells and natural killer cells, and engages with B7 family ligand partners, either PD‐L1 (also known as B7‐H1 and CD274) or PD‐L2 (B7‐DC and CD273), found in peripheral tissue cells, APCs, and tumor cells.9 Binding of PD‐L1/2 to PD‐1 inhibits kinase signal pathways involved in T cell activation. Chronic antigen exposure, caused by chronic viral infection or cancer, was first shown to induce high expression of PD‐1 (considered to represent a state of T cell exhaustion or anergy) which can be reversed upon the blockade of the PD‐1/PD‐L1 interaction.10
Figure 2.
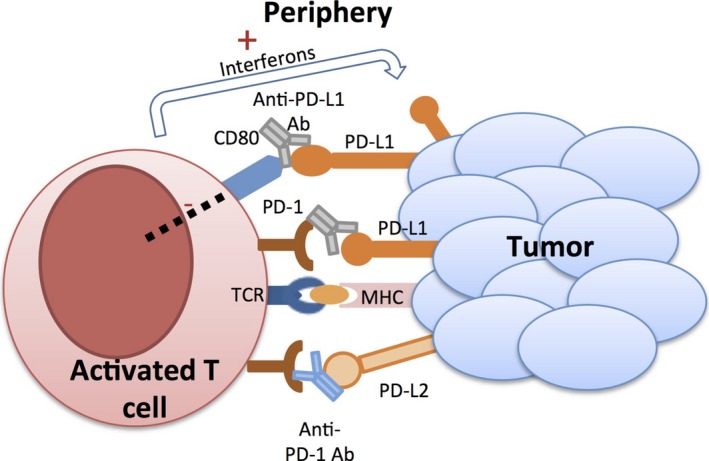
Schematic of the actions of anti‐programmed death‐1/programmed death ligand‐1 (PD‐1/PD‐L1) antibodies. In the periphery, PD‐1 can be inducibly expressed on the surface of T cells, as well as B cells and monocytes. T cell activation releases interferons that cause the upregulation of PD‐L1 and PD‐L2 on the surface of tumors (as well as on T cells, B cells, and antigen‐presenting cells, not shown here). Binding between PD‐1 and PD‐L1 causes downregulation of T cell activity and is intended to limit overly aggressive immune response in the periphery, but is capitalized by tumor cells to limit the antitumor response of the adaptive immune system. PD‐L1 on tumor cells also binds to CD80 on T cells, further initiating downregulation of activated T cells. Anti‐PD‐1 antibodies disrupt the PD‐1/PD‐L1 interaction, as well as PD‐1/PD‐L2 and PD‐L1/CD80 interaction. TCR, T‐cell receptor; ab, antibody; MHC, major histocompatibility complex.
Similar to CTLA‐4, cancer cells capitalize on the inhibitory role of the PD‐1/PD‐L1 interaction to evade host T cell immune attack on cancer cells.11 Quantitative analysis of 150 melanoma specimens revealed that PD‐L1 expression in tumor cells is tightly colocalized with tumor‐infiltrating lymphocytes in cancer tissues and is also upregulated geographically in areas of high γ‐interferon production.12 Therefore, cancer cells protect themselves from cytotoxic T cell attack by increasing expression of PD‐L1 on their surface, which anergizes activated T cells. Studies in mice showed that blockade of the PD‐1/PD‐L1 interaction could be used as a promising anticancer strategy.13 Of note, the mechanisms to block PD‐1 or PD‐L1 are not equivalent because blockade of PD‐L1 leaves the PD‐1/PD‐L2 interaction intact, which may maintain T cell anergy.
The U.S. FDA approved ipilimumab, a fully humanized monoclonal IgG1 antibody against CTLA‐4, in 2011 for treatment of advanced melanoma. At the time, ipilimumab was the first anti‐immune checkpoint agent to show survival benefit in patients with metastatic melanoma.14 Pembrolizumab, a PD‐1 antibody, was granted accelerated approval in 2014 by the FDA in advanced melanoma for patients previously treated with ipilimumab or BRAF inhibitors based on two studies.15, 16 Two anti‐PD‐1 antibodies, pembrolizumab and nivolumab, showed superior overall survival in ipilimumab‐refractory melanoma compared to chemotherapy, conclusively establishing these antibodies as standard of care after ipilimumab in advanced melanoma.17, 18
Progress in clinical trials: optimizing the regimen
After the approval of these three agents in advanced melanoma and NSCLC, these drugs have been tested in various cancer types. In some trials, the immune checkpoint blockade antibodies are combined with each other or other systemic therapies. For example, in both advanced and untreated melanoma, compelling evidence is emerging that PD‐1 blockade may be more efficacious than CTLA‐4 blockade. The first high‐profile phase III trial to compare pembrolizumab with ipilimumab showed improved progression‐free and overall survival rates in a pembrolizumab‐treated group, compared with ipilimumab alone, with lower incidence of drug‐related grade 3–5 adverse events.19 Combination trials published in 2015 have indicated that the combination of nivolumab and ipilimumab also has superior progression‐free survival over ipilimumab monotherapy,20, 21, 22 strongly suggesting PD‐1 blockade may be superior to CTLA‐4 blockade in melanoma.
Combining the immune checkpoint antibodies with other therapies, including systemic chemotherapies and molecular targeted therapies, has also been explored, although such combinations are often affected by higher incidences of adverse events.23, 24 For example, the combination of ipilimumab and dacarbazine has better survival than dacarbazine alone for untreated melanoma patients, but 56% of patients treated with the combination treatment experienced grade 3–4 treatment‐related adverse events.25
In addition to melanoma and NSCLC, immune checkpoint blockade is gaining traction in other cancer types, including refractory non‐Hodgkin's lymphoma, metastatic bladder cancer, intensively treated renal cell carcinoma, and colorectal cancers with mismatch repair deficiencies (Table 1).26, 27, 28, 29 With over 130 active clinical trials registered in the USA, it is beyond the scope of this review to highlight all the cancer types and combinations of immune checkpoint blockade therapies that are underway. Of note, while the immune checkpoint antibodies have shown positive results in a large number of clinical trials, at least one phase III trial using ipilimumab in metastatic castrate‐resistant prostate cancer failed to demonstrate a positive result.30
Table 1.
Major clinical trials with immune checkpoint blockade
| Therapy | Author, year, journal | Cancer type | Phase (no. of patients) | Findings, median PFS in months unless otherwise stated |
|---|---|---|---|---|
| Pembrolizumab versus ipilimumab | Robert, 2015, NEJM19 (KEYNOTE‐006) | Advanced melanoma | Phase 3 (834) | 5.5 (pembrolizumab every 2 weeks) versus 4.1 (pembrolizumab every 3 weeks) versus 2.8 (ipilimumab) |
| Nivolumab with ipilimumab versus nivolumab or ipilimumab monotherapy | Larkin, 2015, NEJM20 | Untreated melanoma | Phase 3 (945) | 2.9 (ipilimumab) versus 6.9 (nivolumab) versus 11.5 (combination) |
| Nivolumab plus ipilimumab versus ipilimumab monotherapy | Postow, 2015, NEJM21 | Untreated melanoma | Phase 2 (142) | Not reached (combination) versus 4.4 (ipilimumab) (BRAF‐WT tumors) |
| Nivolumab plus ipilimumab, concurrently and sequentially | Wolchok, 2013, NEJM22 | Advanced melanoma | Phase 1 (86) | ORR 40% (21/52) (concurrent) versus 20% (6/30) (sequential) |
| Pembrolizumab |
Garon, 2015, NEJM42
KEYNOTE‐001 |
NSCLC | Phase I (495) |
3.7 (all pts); 3.0 (previously untreated pts); 6.0 (previously untreated); PD‐L1 positive expression: PFS 6.3 |
| Pembrolizumab | Le, 2015, NEJM27 | Mismatch repair‐deficient cancers | Phase 2 (41) | Immune‐related PFS 78% (7/9) (mismatch repair deficient) versus 11% (2/18) (mismatch proficient) colorectal cancer |
| Pembrolizumab |
Ribas, 2015, Lancet Oncology
17
KEYNOTE‐002 |
Ipilimumab‐refractory melanoma | Phase 2 (540) | PFS at 6 months: 34% (2 mg/kg) versus 38% (10 mg/kg) versus 16% (ICC) |
| Lambrolizumab | Hamid, 2013, NEJM15 | Advanced melanoma | Phase 1 (135) | >7.0 (all patients) |
| Pembrolizumab | Robert, 2014, Lancet 16 | Ipilimumab‐refractory melanoma | Phase 1 (173) |
5.5 (pembrolizumab 2 mg/kg) versus 3.5 (10 mg/kg) Immune‐related response criteria: PFS 7.8 (2 mg/kg)versus 8.8 (10 mg/kg) |
| Ipilimumab | Hodi, 2010, NEJM14 | Advanced melaonma | Phase 3 (676) | Median OS 10.0 (ipilimumab + gp100) versus 6.4 (gp100 alone) |
| Ipilimumab | Robert, 2011, NEJM25 | Untreated melanoma | Phase 3 (502) | OS 11.2 (dacarbazine + ipilimumab) versus 9.1 (dacarbazine + placebo) |
| Nivolumab |
Weber, 2015, Lancet Oncology
18
Checkmate 037 |
Ipilimumab or BRAF inhibitor (BRAF mutated)‐refractory melanoma | Phase 3 (272) | 4.7 (nivolumab) versus 4.2 (ICC) |
| Nivolumab | Motzer, 2015, JCO28 | Clear‐cell, previously treated renal cell carcinoma | Phase 2 (168) | 2.7 (0.3 mg/kg) versus 4.0 (2 mg/kg) versus 4.2 (10 mg/kg) |
| Nivolumab | Robert, 2015, NEJM19 | Untreated melanoma without BRAF mutation | Phase 3 (418) | 5.1 (nivolumab) versus 2.2 (dacarbazine) |
| Nivolumab |
Rizvi, 2015, Lancet Oncology
57
Checkmate‐063 |
Advanced refractory NSCLC | Phase 2 (117) | PFS 1.9; OS 8.2 |
| Nivolumab | Brahmer, 2015, NEJM58 | Advanced squamous cell NSCLC | Phase 3 (272) chemo |
3.5 (nivolumab) versus 2.8 (docetaxel) OS 9.2 (nivolumab) versus 6.0 (docetaxel) |
| Nivolumab | Topalian, 2012, NEJM40 | Multiple solid tumors | Phase 1 (296) | Objective responses noted across varying doses in NSCLC, melanoma, and renal cell cancer; none in colorectal or prostate cancer |
| Nivolumab | Ansell, 2015, NEJM26 | Relapsed or refractory Hodgkin's Lymphoma | Phase I (23) | PFS at 6 months, 86% |
| MPDL3280A (anti‐PDL1) | Powles, 2014, Nature 39 | Metastatic bladder cancer | Phase 1 (68) | ORR at 6 weeks, 43% among PD‐L1 positive tumors and 11% for negative tumors |
| MPDL3289A (anti‐PDL1) | Herbst, 2014, Nature 43 | Multiple advanced cancers | Phase 1 (277) | Objective responses (complete or partial) in all tumor types tested |
| BMS‐936559 (anti‐PDL1) | Brahmer, 2012, NEJM59 | Advanced cancers | Phase 1 (207) | Objective responses seen in melanoma, NSCLC, renal cell cancer, ovarian cancer (none in colorectal or pancreatic) |
A representative, though not comprehensive, list of high‐profile clinical trials with immune checkpoint blockade is detailed. The therapy, authors and journal information, phase, and major findings are provided. BRAF‐WT: B‐raf wild‐type; ICC: investigator's choice chemotherapy; JCO, Journal of Clinical Oncology; NEJM, New England Journal of Medicine; NSCLC, non‐small‐cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival.
Immunotherapy‐specific challenges in clinical evaluation
One of the most exciting characteristics of the checkpoint inhibitors is that the clinical responses observed have been remarkably durable even after cessation of treatment.31 In a pooled analysis of 1861 patients treated with ipilimumab, 22% survived for at least 3 years, with the Kaplan–Meier survival curve achieving a plateau that extended from 3 to 10 years after treatment.32 Similar durability has been seen with anti‐PD‐1 inhibitors. In two major NSCLC trials, 28% and 27% of patients survived at least 18 months.33 In melanoma, 43% of patients survived at least 2 years.34 Hence, patient selection and monitoring for immunotherapy, if established, has the potential to offer unprecedented durable responses in other refractory stages of disease.
The first immune checkpoint blockade‐specific challenge in patient selection is how to define clinical efficacy with immune checkpoint inhibitors. In general, tumor responses to immunotherapies have shown tendencies to deviate from the widely used Response Evaluation Criteria in Solid Tumors (RECIST), meaning that a subset of patients who ultimately survive on therapy do not meet criteria for objective response during the trial period. The primary biological response of immune checkpoint blockade is blockage of immune suppressive molecules, resulting in activation of intratumoral infiltrated T lymphocytes and increase in cytokine production. Due to the inflammation caused by these secreted cytokines, tumor sizes may initially enlarge (termed pseudo‐progression)31 on radiographic assessment early after the initiation of treatment, before causing tumor shrinkage.35, 36 Accordingly, a delayed separation of survival curves from control arms has been observed in multiple trials. For example, in follow‐up reports of the earliest CTLA‐4 blockade trials, the average time among all patients to achieve complete response was 30 months, a period before which clinical trials often conclude.37 Therefore, immune checkpoint blockade therapy requires innovative strategies for monitoring and evaluating patient response based on the mode of action of the drugs.
Patient selection: predictive biomarkers
In the era of precision medicine, it is critically important to address how to select the patients that are likely to derive benefit from immune checkpoint blockade therapy.38, 39 Investigation into identifying factors involved in the immune checkpoint pathways in the tumor immune microenvironment has been extensively carried out and several molecules have been proposed to predict clinical response.
Intratumoral PD‐L1 expression for patient selection
A number of studies investigating the safety and efficacy of PD‐1/PD‐L1 antibodies reasonably hypothesized that tumor cell expression levels of PD‐L1 would predict response to both therapies. An early phase I trial suggested that tumors with positive PD‐L1 staining by immunohistochemistry showed better response to anti‐PD‐1 agents than PD‐L1‐negative tumors, with an objective response rate of 36% versus 0%.40 A meta‐analysis of 1475 patients concluded that PD‐L1‐positive tumors also have an improved response rate compared to PD‐L1‐negative tumors (34% vs 19.9%).41 However, no clear threshold for positivity of PD‐L1 has been defined. Although many trials applied 5% staining in tumor cells as positive, a phase I study of patients with NSCLC treated with pembrolizumab applied a cut‐off of 50% positive staining in tumor cells and obtained an objective response rate of 45.2% with a median overall survival of 26 months in PD‐L1‐positive patients. Although the authors concluded that the PD‐L1 positivity in >50% of tumor cells is a promising biomarker, tumors with as few as 1% of tumors cells staining positive for PD‐L1 still showed a median overall survival of >8 months.42 In addition, multiple trials have shown no correlation or inconclusive correlation between the clinical response and PD‐L1 status in cancer tissues (Table 2). Therefore, the mechanism of how PD‐L1‐negative patients respond to anti‐PD‐1 treatment still needs to be clarified. Of interest, pembrolizumab was recently approved specifically for use in NSCLC for PD‐L1‐positive tumors as defined by a commercial immunohistochemical diagnostic assay.3
Table 2.
Programmed death ligand‐1 (PD‐L1) status as predictive biomarker
| Therapy | Cancer | Author, year, journal | Antibody and PD‐L1+ definition | % Tumors PD‐L1‐positive | PD‐L1‐positive | PD‐L1‐negative | Conclusion | |
|---|---|---|---|---|---|---|---|---|
| Pembro and ipi | Advanced Melanoma | Robert, 2015, NEJM |
Merck 223C 1% tumor cells |
>80 | PFS and overall response not stated between the two groups | Insufficient sample size (too few PD‐L1‐negative tumors) to draw conclusions | ||
| Nivo and ipi in combination versus monotherapy | Untreated melanoma | Larkin, 2015, NEJM |
Dako 28‐8 clone (BMS assay) >5% tumor cells positive |
23.6 | PFS, months | For PD‐L1‐ patients, combo treatment may be most beneficial | ||
|
Nivo Combo Ipi |
14 14 3.9 |
5.3 11.2 2.8 |
||||||
| Nivo and ipi in combo versus ipi monotherapy | Untreated melanoma | Postow, 2015, NEJM |
Dako 28‐8 (BMS) >5% tumor cells |
30 | ORR, % | In combo therapy, PD‐L1 status not prognostic, but may be beneficial in pts receiving ipi alone | ||
|
Combo Ipi |
58 18 |
55 4 |
||||||
| Concurrent versus sequential combo treatment with nivo and ipi | Advanced melanoma | Wolchok, 2013, NEJM |
Dako 28‐8 (BMS) >5% tumor cells |
38 | ORR, % | Objective responses seen regardless of PD‐L1 status | ||
|
Concurrent Seq |
46 50 |
41 7 |
||||||
| Pembro | NSCLC | Garon, 2015, NEJM |
Merck 22C3 >50% membrane staining |
>50%: 23.3 1–49%: 37.5 <1%: 39.2 |
PFS, months | PD‐L1 staining >50% may be a valuable biomarker, but PD‐L1 neg pts still derive benefit | ||
| 6.3 | 4.0 | |||||||
| Nivolumab | Advanced melanoma | Weber, 2015, Lancet Oncology | Dako 28‐8 (BMS)>5% tumor cells | ~50% | ORR, % | PD‐L1 appeared to be associated with response, but small sample sizes | ||
| Nivo‐treated | 43.6 | 20.3 | ||||||
| Nivolumab | Previously treated clear‐cell renal‐cell carcinoma | Motzer, 2015, JCO |
Dako 28‐8 (BMS) >5% tumor cells |
27 | PFS, months/ORR, % | PD‐L1 status was associated with response, but not conclusively since PD‐L1 negative patients also responded | ||
| Nivo‐treated | 4.9/31 | 2.0/28 | ||||||
| Nivolumab | Melanoma | Robert, 2015, NEJM | Dako 28‐8 (BMS)>5% tumor cells | 35.4 | ORR, % | PD‐L1 status was not as important as nivolumab's superiority over dacarbazine | ||
| Nivo‐treated | 52.7 | 33.1 | ||||||
| Nivolumab | NSCLC | Brahmer, 2015, NEJM | Dako 28‐8 (BMS)>1, 5, and 10% of tumor cells | ORR, % | PD‐L1 was neither predictive nor prognostic | |||
| Nivo‐treated | 17 | 17 | ||||||
| Nivolumab | Advanced cancers | Topalian, 2012, NEJM |
5H1 >5% tumor cells |
59% | ORR, %: | PD‐L1 may be a prognostic marker | ||
| Nivo‐treated | 36 | 0 | ||||||
| Nivolumab | Non‐Hodgkin's lymphoma | Ansell, 2015, NEJM | 10 patient samples underwent PDL1 and PDL2 copy number analysis | All had 3–15 copy number gains in PDL1 and PDL2 | Pathway activation of PDL1/2 copy number gain prognostic for response | |||
| MPDL3280A | Metastatic bladder cancer | Powles, 2014, Nature | >5% of tumor or tumor‐infiltrating immune cells |
27% on immune cells 4% on both immune and tumor cells |
ORR, % | Immune cell PD‐L1 staining may be prognostic for MPDL3280A response | ||
| Immune cell staining | 43 | 11 | ||||||
| MPDL3280A | Advanced cancers | Herbst, 2014, Nature | Ventana clone SP142)>5% of tumor or tumor‐infiltrating immune cells | 12–36% (immune cell expression) depending on tumor type; 1–24% (tumor cell) depending on tumor type |
Correlation between immune cell IHC staining (P = 0.007 all patients) No correlation found with tumor cell staining |
Immune cell PD‐L1 staining may be prognostic for MPDL3280A response | ||
Summary of a subset of clinical trials that have examined the correlation between PD‐L1 immunohistochemistry (IHC) on either tumor or immune cells with clinical response. BMS, Bristol‐Myers Squibb; combo, combination; ipi, ipilimumab; JCO, Journal of Clinical Oncology; NEJM, New England Journal of Medicine; nivo, nivolumab; NSCLC, non‐small cell lung cancer; ORR, objective response rate; pts, patients; pembro, pembrolizumab; PFS, progression‐free survival; Seq, sequential.
While many studies define PD‐L1 positivity according to tumor cell expression, PD‐L1 expression on immune cells, including macrophages, dendritic cells, and T lymphocytes, has also been investigated. One study of anti‐PD‐L1 antibody in multiple tumor types reported significant association between PD‐L1 expression in tumor‐infiltrating immune cells and clinical response (P < 0.007).43 However, a different study of 41 patients comprised of five solid‐tumor types treated with nivolumab showed no association between PD‐L1 expression in immune cells with objective response.12
The variability of the results listed above is generated by a number of factors, including the complexity and dynamic nature of the PD‐1/PD‐L1 interaction, subjectivity of PD‐L1 staining, variations in techniques and assays used, tumor heterogeneity, and use of archival tissues that may not reflect the status of PD‐1/PD‐L1 interaction at time of treatment.44 Because of the variability of results across trials and the inability to exclude patients with negative PD‐L1 expression as poor responders, there is still no solid evidence for applying PD‐L1 expression level on tumor or immune cells as a sole criterion for patient selection.45
Intratumoral and tumor microenvironment correlate as predictors
Intensive efforts have also highlighted specific factors in the tumor microenvironment as potential predictive biomarkers because of their influence on drug action. Herbst et al.43 analyzed pretreatment and on‐treatment tumor biopsies from patients treated with MPDL3280A across seven solid malignancies and found an association between high CTLA‐4 expression in pretreatment tissue samples and good therapeutic response. Other investigators have similarly shown that high intratumoral baseline expression of FoxP3 and indoleamine 2,3‐dioxygenase, a metabolic enzyme that inhibits the immune responses through depletion of amino acids, is associated with clinical activity of ipilimumab.46
Anti‐PD‐1/PD‐L1 therapies may have improved prognosis in patients whose tumors have pre‐existing coalitions of cytotoxic T lymphocytes that are in an anergic state.31 Serial on‐treatment biopsies from 46 patients with melanoma treated with pembrolizumab revealed that patients who responded well to pembrolizumab had higher densities of CD8+ T cells at the invasive tumor margin and center and that the densities of CD8+ T cells in close proximity to PD‐1+/PD‐L1+ tumor cells increased after treatment.47
However, while it is not clinically practical to obtain serial biopsy samples to monitor patient immune response, pretreatment biopsy samples may be acceptable surrogates for capturing a signature of the balance between active and suppressive immune elements. Quantitative and standardized means of assessing the balance between active and suppressive immune factors are critically important for the development of validated and robust criteria for selecting and monitoring patients for immune checkpoint inhibitors.
Mutational landscape of tumors as predictors of neoantigens
Advances in sequencing technology in the clinical setting have also ushered in new strategies for identifying biomarkers to immune checkpoint blockade treatment.48 Non‐synonymous somatic mutations are considered to be the basis for generation of cancer‐specific neoantigens, which are likely to be recognized by and induce clonal expansion of certain T cells. This hypothesis was indirectly supported by findings that mutations in DNA‐damage repair genes increases somatic mutation burden, and are associated with longer recurrence‐free survival in surgically resected muscle‐invasive bladder cancer patients.49 A high somatic mutation burden should theoretically increase the probability of generating neoantigens that can be presented with HLA molecules on the surface of cancer cells, recognized by CD8+ T cells, and can induce clonal expansion of cytotoxic T cells. Indeed, in tumors treated with pembrolizumab, the overall mutational burden correlated with response to therapy; interestingly, the absolute burden of predicted neoantigens seemed to be a better predictor.50
Bioinformatics approaches provide useful tools for predicting neoantigens from whole exome and transcriptome sequencing data. For example, in a study of mice injected with the d42m1‐T3 sarcoma cell line, mutations occurring in the Lama4 and Alg8 genes were successfully identified as d42m1‐T3‐specific neoepitopes that stimulated a CD8+ T cell response.51 In these methods, prediction of binding to individual HLA molecules is essential for identifying possible neoantigens. Although the total number of somatic missense mutations correlated with long‐term response to ipilimumab, a signature of preserved tetrapeptides in neoepitope polymers was a more accurate predictor of clinical response in melanoma.52
Avenues for future direction: immunopharmacogenomics
The work carried out thus far in patient selection and monitoring in immune checkpoint therapy has underlined the importance of deeply understanding both the immune and genetic landscape of tumors in order to predict clinical response. The next step will be integrating the knowledge gained from these studies and applying it to modulating and improving clinical response. We have proposed a new study field, termed immunopharmacogenomics, which links the pharmacological response to cancer genomics with immunogenomics using massively parallel next‐generation sequencing of the TCR repertoire. Immunopharmacogenomics has shown promise in both serving as a pharmacodynamics marker of immunotherapeutic activity and potentially modulating the clinical response. The TCR sequencing of tumor‐infiltrating lymphocytes (TILs) from pretreatment biopsy samples, with comparison of on‐treatment or post‐treatment biopsy samples, can provide critical information about the changes in TIL repertoire during immune checkpoint inhibitor therapy. For example, deep sequencing of TCR repertoires from serial tumor tissue biopsies on treatment showed a 10‐fold clonal expansion in cancer tissues in responders, but less or no expansion of clonal T cells in non‐responsive patients.47 While serial tissue biopsies are difficult to obtain, peripheral blood samples collected from patients on anti‐CTLA antibody therapy showed an increase in TCR diversity for most patients on therapy, suggesting that TCR sequencing can be a tool for pharmacodynamics monitoring.53 Deep sequencing of the TCR, both within the tumor and in the peripheral blood, can therefore provide direct quantification of the clonality and specificity of T cells.38
In addition, identifying TCR sequences that are expanded in tumors of patients treated with immune checkpoint blockade has the potential for new therapeutic interventions such as production of genetically engineered T cells targeting cancer cells. Particularly, there is significant interest and progress in identifying T cell clones that recognize neoantigens generated by somatic missense mutations in cancer cells.48 The oligoclonal expansion of these T cells, which recognize neoantigens, may be potential immune responses against cancer. T‐cell receptor deep sequencing has already been used to identify oligoclonal expansion of CD8+‐PD‐1+ TILs in melanoma tumors that are specific for mutated antigens.54 Therefore, immunopharmacogenomics may both offer insight into patient selection and monitoring on immune checkpoint blockade as well as offer avenues to enhance the clinical response.55, 56 Tissue and blood samples, collected from patients on immune checkpoint antibody therapy, are needed to further validate this work.
Conclusions
Although the immune checkpoint inhibitors are already successes as anticancer agents, we are still far from knowing which patients may benefit from the use of immune checkpoint monotherapies or from knowing at what point to alter the direction of treatment. Immunopharmacogenomics may have a strong foothold in addressing lingering questions about predictive biomarkers for immunotherapy.
In summary, the class of immune checkpoint inhibitors has already changed how we think of anticancer strategies. In chess, the point of victory is called checkmate, stemming originally from the Russian phrase, “shakh mat” or “death to the king.” In the balance between natural immunity and cancer tissues, immune checkpoint inhibitors, by unleashing the body's armament of self‐defense already poised for action, may have the potential to, at last, bring death to cancer. There remains much work to do, however, to bring that potential to its full realization.
Disclosure Statement
The authors have no conflict of interest.
Cancer Sci 107 (2016) 107–115
Funding Information
No sources of funding were declared for this study.
References
- 1. FDA expands approved use of Opdivo to treat lung cancer. FDA Press Release (Serial online) 2015 March 1(1): [Cited September 10, 2015.] Available from URL: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm436534.htm
- 2. Webster RM. The immune checkpoint inhibitors: where are we now? Nat Rev Drug Discovery 2014; 13: 883–4. [DOI] [PubMed] [Google Scholar]
- 3. Peddicord S. FDA approves Keytruda for advanced non‐small cell lung cancer. FDA Press Release (Serial online) 2015 October 1(1): [Cited October 9, 2015]. Available from URL: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm465444.htm
- 4. Waterhouse P, Penninger JM, Timms E et al Lymphoproliferative disorders with early lethality in mice deficient in Ctla‐4. Science 1995; 270: 985–8. [DOI] [PubMed] [Google Scholar]
- 5. Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol 1996; 14: 233–58. [DOI] [PubMed] [Google Scholar]
- 6. Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA‐4 is a second receptor for the B cell activation antigen B7. J Exp Med 1991; 174: 561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hodi FS, Mihm MC, Soiffer RJ et al Biologic activity of cytotoxic T lymphocyte‐associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci 2003; 100: 4712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phan GQ, Yang JC, Sherry RM et al Cancer regression and autoimmunity induced by cytotoxic T lymphocyte‐associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci 2003; 100: 8372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Freeman GJ, Long AJ, Iwai Y et al Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192: 1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barber DL, Wherry EJ, Masopust D et al Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439: 682–7. [DOI] [PubMed] [Google Scholar]
- 11. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD‐L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD‐L1 blockade. Proc Natl Acad Sci 2002; 99: 12293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taube JM, Klein A, Brahmer JR et al Association of PD‐1, PD‐1 ligands, and other features of the tumor immune microenvironment with response to anti‐PD‐1 therapy. Clin Cancer Res 2014; 19: 5064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strome SE, Dong H, Tamura H et al B7‐H1 blockade augments adoptive T‐cell immunotherapy for squamous cell carcinoma. Cancer Res 2003; 63: 6501–5. [PubMed] [Google Scholar]
- 14. Hodi FS, O'Day SJ, McDermott DF et al Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamid O, Robert C, Daud A et al Safety and tumor responses with lambrolizumab (anti‐PD‐1) in melanoma. N Engl J Med 2013; 369: 134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Robert C, Ribas A, Wolchok JD et al Anti‐programmed‐death‐receptor‐1 treatment with pembrolizumab in ipilimumab‐refractory advanced melanoma: a randomised dose‐comparison cohort of a phase 1 trial. Lancet 2014; 384: 1109–17. [DOI] [PubMed] [Google Scholar]
- 17. Ribas A, Puzanov I, Dummer R et al Pembrolizumab versus investigator‐choice chemotherapy for ipilimumab‐refractory melanoma (KEYNOTE‐002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015; 16: 908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weber JS, D'Angelo SP, Minor D et al Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti‐CTLA‐4 treatment (CheckMate 037): a randomised, controlled, open‐label, phase 3 trial. Lancet Oncol 2015; 16: 375–84. [DOI] [PubMed] [Google Scholar]
- 19. Robert C, Schachter J, Long GV et al Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372: 2521–32. [DOI] [PubMed] [Google Scholar]
- 20. Larkin J, Chiarion‐Sileni V, Gonzalez R et al Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Postow MA, Chesney J, Pavlick AC et al Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015; 372: 2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolchok JD, Kluger H, Callahan MK et al Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369: 122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med 2013; 368: 1365–6. [DOI] [PubMed] [Google Scholar]
- 24. Twyman‐Saint Victor C, Rech AJ, Maity A et al Radiation and dual checkpoint blockade activate non‐redundant immune mechanisms in cancer. Nature 2015; 520: 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robert C, Thomas L, Bondarenko I et al Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364: 2517–26. [DOI] [PubMed] [Google Scholar]
- 26. Ansell SM, Lesokhin AM, Borrello I et al PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015; 372: 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le DT, Uram JN, Wang H et al PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015; 372: 2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Motzer RJ, Rini BI, McDermott DF et al Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 2015; 33: 1430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Powles T, Eder JP, Fine GD et al MPDL3280A (anti‐PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515: 558–62. [DOI] [PubMed] [Google Scholar]
- 30. Kwon ED, Drake CG, Scher HI et al Ipilimumab versus placebo after radiotherapy in patients with metastatic castration‐resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184‐043): a multicentre, randomised, double‐blind, phase 3 trial. Lancet Oncol 2014; 15: 700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schadendorf D, Hodi FS, Robert C et al Pooled analysis of long‐term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015; 33: 1889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Follow‐up Data from Two Pivotal Opdivo (nivolumab) Trials Demonstrates Sustained Survival Results in Patients with Previously Treated Squamous Non‐Small Cell Lung Cancer. Business Wire (Serial online). 2015 September 1(1); [Cited 9/12/15 2015.] Available from URL: http://news.bms.com/press-release/follow-data-two-pivotal-opdivo-nivolumab-trials-demonstrates-sustained-survival-result
- 34. Topalian SL, Sznol M, McDermott DF et al Survival, durable tumor remission, and long‐term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32: 1020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoos A, Eggermont AM, Janetzki S et al Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 2010; 102: 1388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolchok JD, Hoos A, O'Day S et al Guidelines for the evaluation of immune therapy activity in solid tumors: immune‐related response criteria. Clin Cancer Res 2009; 15: 7412–20. [DOI] [PubMed] [Google Scholar]
- 37. Prieto PA, Yang JC, Sherry RM et al CTLA‐4 blockade with ipilimumab: long‐term follow‐up of 177 patients with metastatic melanoma. Clin Cancer Res 2012; 18: 2039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ribas A, Tumeh PC. The future of cancer therapy: selecting patients likely to respond to PD1/L1 blockade. Clin Cancer Res 2014; 20: 4982–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Powles T. Immunotherapy: the development of immunotherapy in urothelial bladder cancer. Nat Rev Clin Oncol 2015; 12: 193–4. [DOI] [PubMed] [Google Scholar]
- 40. Topalian SL, Hodi FS, Brahmer JR et al Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carbognin L, Pilotto S, Milella M et al Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death‐ligand‐1 (PD‐L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS ONE 2015; 10: e0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garon EB, Rizvi NA, Hui R et al Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015; 372: 2018–28. [DOI] [PubMed] [Google Scholar]
- 43. Herbst RS, Soria JC, Kowanetz M et al Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mahoney KM, Freeman GJ, McDermott DF. The next immune‐checkpoint inhibitors: PD‐1/PD‐L1 blockade in melanoma. Clin Ther 2015; 37: 764–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 33: 1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hamid O, Schmidt H, Nissan A et al A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Tranl Med 2011; 9: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tumeh PC, Harview CL, Yearley JH et al PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348: 69–74. [DOI] [PubMed] [Google Scholar]
- 49. Yap KL, Kiyotani K, Tamura K et al Whole‐exome sequencing of muscle‐invasive bladder cancer identifies recurrent mutations of UNC5C and prognostic importance of DNA repair gene mutations on survival. Clin Cancer Res 2014; 20: 6605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rizvi NA, Hellmann MD, Snyder A et al Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015; 348: 124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gubin NM, Zhang X, Schuster H et al Checkpoint blockade cancer immunotherpay targets tumor‐specific mutant antigens. Nature 2014; 528: 577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Snyder A, Makarov V, Merghoub T et al Genetic basis for clinical response to CTLA‐4 blockade in melanoma. N Engl J Med 2014; 371: 2189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Robert L, Tsoi J, Wang X et al CTLA4 blockade broadens the peripheral T‐cell receptor repertoire. Clin Cancer Res 2014; 20: 2424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gros A, Robbins PF, Yao X et al PD‐1 identifies the patient‐specific CD8(+) tumor‐reactive repertoire infiltrating human tumors. J Clin Invest 2014; 124: 2246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rapoport AP, Stadtmauer EA, Binder‐Scholl GK et al NY‐ESO‐1‐specific TCR‐engineered T cells mediate sustained antigen‐specific antitumor effects in myeloma. Nat Med 2015; 21: 914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kirsch I, Vignali M, Robins H. T‐cell receptor profiling in cancer. Mol Oncol 2015; 9: 2063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rizvi NA, Mazieres J, Planchard D et al Activity and safety of nivolumab, an anti‐PD‐1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non‐small‐cell lung cancer (CheckMate 063): a phase 2, single‐arm trial. Lancet Oncol 2015; 16: 257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brahmer JR, Tykodi SS, Chow LQ et al Safety and activity of anti‐PD‐L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]


