Abstract
Aims:
To determine the effects of empagliflozin on adiposity indices among patients with type 2 diabetes mellitus.
Methods:
Changes in weight, waist circumference, estimated total body fat, index of central obesity and visceral adiposity index were assessed using analysis of covariance and testing of treatment by strata for age, sex and baseline waist circumference in patients with type 2 diabetes mellitus randomized to blinded treatment with empagliflozin versus placebo in clinical trials of 12 weeks (cohort 1) or 24 weeks (cohort 2) duration.
Results:
This study comprised 3300 patients (cohort 1, N = 823; cohort 2, N = 2477). Empagliflozin reduced weight, waist circumference and adiposity indices versus placebo in both cohorts. Adjusted mean (95% confidence interval) change from baseline in empagliflozin versus placebo was −1.7 kg (−2.1 to −1.4 kg) and −1.9 kg (−2.1 to −1.7 kg) for body weight (p < 0.001); −1.3 cm (−1.8 to −0.7 cm) and −1.3 cm (−1.7 to −1.0 cm) for waist circumference (p < 0.001); −0.2% (−0.7% to 0.3%; p = 0.45) and −0.3% (−0.7% to 0.0%; p = 0.08) for estimated total body fat; −0.007 (−0.011 to −0.004) and −0.008 (−0.010 to −0.006) for index of central obesity (p < 0.001); and −0.3 (−0.5 to 0.0; p = 0.07) and −0.4 (−0.7 to −0.1; p = 0.003) for visceral adiposity index in cohorts 1 and 2, respectively. Adipose reductions were seen across most age, sex and waist circumference subgroups.
Conclusion:
Empagliflozin significantly reduced weight and adiposity indices with the potential to improve cardiometabolic risk among patients with type 2 diabetes mellitus.
Keywords: Obesity, body fat distribution, visceral adipose tissue, empagliflozin, sodium glucose co-transporter 2 inhibitor
Introduction
Although improvements have been observed in type 2 diabetes mellitus (T2DM) risk factors over the last decade, many risk factors remain sub-optimally controlled or poorly recognized.1 For example, data suggest that intra-abdominal (visceral) adipose tissue (VAT) may be a primary driver of the cardiometabolic complications of obesity, including T2DM.2 Multiple factors including very low calorie diets, exercise3 and bariatric surgery4 result in significant reductions in VAT, potentially explaining some of the improvements in glycaemic control and resolution of T2DM seen with lifestyle and surgical interventions.5 Unfortunately, some of the available therapies for T2DM, in particular sulphonylureas, thiazolidinediones and insulin therapy, result in weight gain and may exacerbate the adverse effects of VAT in patients with T2DM.6
Empagliflozin (EMPA) is a potent and selective sodium glucose co-transporter 2 inhibitor (SGLT-2i) used for the treatment of T2DM shown to improve glycaemic control and reduce blood pressure and body weight in clinical trials.7–11 In a dedicated body composition study, EMPA treatment led to significant reductions in abdominal fat compared with glimepiride over 104 weeks.12 Multiple surrogate indices of visceral adiposity have been developed that do not require advanced imaging techniques, are more readily applied in the clinical setting and have been validated with metabolic risk factors and imaging modalities;13 however, generalizability of many equations is limited due to a lesser degree of validation among various race or ethnic groups and at the extremes body composition. As data describing the effects of EMPA on these more clinically available and, therefore, more relevant indices of VAT are lacking, we aimed to determine its effects compared with placebo on body weight, waist circumference (WC) and indices of total body fat and visceral adiposity over a short and intermediate treatment term among patients with T2DM enrolled in five clinical trials.
Materials and methods
Study population
Data from two cohorts of patients participating in randomized trials, one treated with double-blind EMPA versus placebo for 12 weeks (cohort 1) and one treated with double-blind EMPA versus placebo for 24 weeks (cohort 2), were analysed. Cohort 1 comprised participants in the EMPA-REG BP™ trial; details of the trial population and design have been published previously.7 Briefly, patients with T2DM [glycosylated haemoglobin (HbA1c) ⩾7% and ⩽10%], hypertension (defined as mean seated office systolic blood pressure 130–159 mmHg and diastolic blood pressure 80–99 mmHg) and body mass index (BMI) ⩽45 kg/m2 were randomized to receive EMPA 10 mg, EMPA 25 mg or placebo once daily for 12 weeks. Patients underwent 24-h ambulatory blood pressure monitoring ⩽7 days prior to randomization and at week 12. Background antihypertensive therapy was continued throughout the study. Cohort 2 comprised participants in four trials: EMPA-REG PIO™,8 EMPA-REG MONO™,9 EMPA-REG METSU™10 and EMPA-REG MET™;11 details of the trial populations and designs are published elsewhere. Briefly, all participants in these trials generally had HbA1c ⩾ 7% and ⩽10% and BMI ⩽ 45 kg/m2 and were randomized to receive EMPA 10 mg, EMPA 25 mg or placebo as monotherapy (EMPA-REG MONO™) or add-on to background therapies metformin, metformin plus sulphonylurea or pioglitazone with or without metformin (EMPA-REG MET™, EMPA-REG METSU™ and EMPA-REG PIO™) for 24 weeks. All participants in these studies provided written informed consent, and all studies were approved by the Institutional Review Boards and Independent Ethics Committees and Competent Authorities of the participating centres in compliance with the Declaration of Helsinki and in accordance with the International Conference on Harmonization Harmonized Tripartite Guideline for Good Clinical Practice.
Variable definitions and biomarker measurements
Blood pressure was measured three times (approximately 2 min apart) after 5 min of rest in the seated position, using the same arm, method and device throughout the trials. HbA1c and fasting plasma glucose were assessed from blood samples taken during visits at the trial sites before breakfast and before daily dose of study medication and analysed at a central laboratory using validated assays (Variant™ II Turbo ion-exchange high-performance liquid chromatography, Bio-Rad Laboratories, Hercules, CA, for HbA1c measurements; Gluco-quant Roche/Hitachi Modular glucose analyser, Roche Diagnostics, Mannheim, Germany, for plasma glucose measurements).
Body composition endpoints
Apart from body weight (kg) and WC (cm) that were measured directly, the following previously published indices were selected based on the evidence available to reflect abdominal fat depots,13 ‘ease-of-use’ from a clinical practice point of view and availability of data comprising the formulae in the EMPA phase III clinical trial database and were used to estimate body fat content and distribution: estimated total body fat (eTBF, YMCA formula): 100 × [−98.42 + (4.15 × WC (in)) − (0.082 × weight (lbs))]/weight for men and 100 × [−76.76 + (4.15 × WC) − (0.082 × weight)]/weight for women;14,15 index of central obesity (ICO): WC/body height, constructed to account for race- and sex-specific cut-offs for WC reflecting variability in average heights in these populations;16 and visceral adiposity index (VAI): [WC (cm)/(39.68 + (1.88 × BMI (kg/m2)))] × [triglycerides (TGs, mmol/L)/1.03] × [1.31/high-density lipoprotein cholesterol (HDL-C, mmol/L)] for men; [WC/(36.58 + (1.89 × BMI))] × (TG/0.81) × (1.52/HDL-C) for women, derived to incorporate the atherogenic dyslipidemia seen with excess visceral adiposity in addition to anthropometric indices.17 These indices have been shown to closely correlate with cardiometabolic risk factors in multiple investigations18,19 and in some cases proved superior to existing indices of central obesity to predict metabolic syndrome.20 Furthermore, in a post hoc analysis of a subset of the 1549 patients with T2DM randomized to treatment with EMPA versus glimepiride in the EMPA-REG H2H SUTM trial, we found strong correlations between eTBF (YMCA formula) and total body fat by dual x-ray absorptiometry and between WC and ICO and abdominal VAT by magnetic resonance imaging (MRI), validating the use of these indices in relation to direct measures of VAT and total body fat in a similar population of middle-aged, overweight and obese patients with T2DM.13 Moreover, the individual parameters used to derive these markers are readily available in the clinical setting and can be applied across a broad spectrum of populations. All endpoints were assessed at baseline and at week 12 (cohort 1) or week 24 (cohort 2).
Statistical analyses
Baseline characteristics of each cohort and treatment assignment are presented as mean (standard deviation) or proportion where appropriate. Changes in weight, WC and adiposity indices were assessed between baseline and week 12 (cohort 1) or week 24 (cohort 2). For each cohort, data from patients in the EMPA 10 mg and EMPA 25 mg groups were pooled. These changes from baseline were analysed using an analysis of covariance (ANCOVA) with baseline HbA1c and the baseline value of the adiposity measure as linear covariables, and baseline estimated glomerular filtration rate [eGFR by modification of diet in renal disease (MDRD)], region and treatment as fixed effects. The number of antihypertensive medications at baseline was an additional fixed effect in the analysis of cohort 1, and the individual study was an additional fixed effect in the analysis of cohort 2. Analyses were conducted on the full-analysis set; for cohort 1, this was defined as randomized patients who received at least one dose of study drug and had baseline HbA1c and mean 24-h systolic blood pressure values. For cohort 2, this was defined as randomized patients who received at least one dose of study drug and had a baseline HbA1c value. Values observed after initiation of additional anti-hyperglycaemic rescue therapy were set to missing. A last observation carried forward (LOCF) approach was used to impute missing data. Since impact on treatment effects for body weight and visceral adiposity markers are generally perceived to be greater in certain subpopulations, we also stratified effects of EMPA by age (<50, 50–64, 65–74 and ⩾75 years), sex and degree of abdominal obesity at baseline (WC < 88, 88–102 and >102 cm) and compared changes with placebo using adjusted means by ANCOVA with interaction testing of treatment by strata. For all statistical testing, a two-sided p-value < 0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.2 software (SAS Corporation, Cary, NC).
Results
Baseline characteristics of the two study cohorts by treatment assignment are shown in Table 1. The adjusted mean [standard error (SE)] HbA1c change from baseline at week 12 in cohort 1 was 0.03% (0.04%) with placebo compared with −0.61% (0.02%) with EMPA [adjusted mean (95% confidence interval (CI) difference versus placebo: −0.64% (−0.72% to −0.55%), p < 0.001]. In cohort 2, the adjusted mean (SE) HbA1c change from baseline at week 24 was −0.08% (0.03%) with placebo compared with −0.73% (0.02%) with EMPA [adjusted mean (95% CI) difference versus placebo: −0.65% (−0.71% to −0.59%), p < 0.001].
Table 1.
Baseline characteristics of the study population by cohort and treatment assignment (N = 3300).
| Baseline characteristics | Cohort 1a |
Cohort 2b |
||
|---|---|---|---|---|
| Placebo (N = 271) | Empagliflozin 10 or 25 mg (N = 552) | Placebo (N = 825) | Empagliflozin 10 or 25 mg (N = 1652) | |
| Age (years) | 60.3 (8.8) | 60.2 (9.1) | 55.7 (10.1) | 55.6 (10.2) |
| Male (%) | 168 (62.0) | 327 (59.2) | 424 (51.4) | 927 (56.1) |
| Race (%) | ||||
| White | 256 (94.5) | 515 (93.3) | 337 (40.8) | 686 (41.5) |
| Asian | 1 (0.4) | 7 (1.3) | 468 (56.7) | 923 (55.9) |
| Other | 14 (5.2) | 30 (5.4) | 20 (2.4) | 43 (2.6) |
| Time since T2DM diagnosis (years) | ||||
| ⩽1 | 7 (2.6) | 21 (3.8) | 112 (13.6) | 273 (16.5) |
| >1–5 | 70 (25.8) | 135 (24.5) | 301 (36.5) | 560 (33.9) |
| >5–10 | 83 (30.6) | 182 (33.0) | 234 (28.4) | 454 (27.5) |
| >10 | 111 (41.0) | 214 (38.8) | 178 (21.6) | 365 (22.1) |
| HbA1c (%) | 7.90 (0.72) | 7.90 (0.74) | 8.02 (0.86) | 7.97 (0.85) |
| Fasting glucose (mg/dL) | 160.1 (35.3) | 159.9 (38.0) | 153.7 (35.9) | 152.6 (34.1) |
| Systolic BP (mmHg) | 142.0 (12.4) | 142.1 (12.3) | 128.6 (14.6) | 129.3 (15.1) |
| Diastolic BP (mmHg) | 83.7 (7.1) | 84.0 (7.0) | 78.0 (8.8) | 78.5 (8.8) |
| Triglycerides (mg/dL) | 170.3 (98.7) | 172.2 (113.4) | 164.5 (111.5) | 173.2 (154.2) |
| HDL cholesterol (mg/dL) | 47.8 (12.9) | 48.7 (12.6) | 48.7 (12.6) | 49.1 (12.6) |
| Weight (kg) | 95.2 (17.5) | 95.2 (18.6) | 78.0 (18.8) | 78.9 (18.8) |
| Body mass index (kg/m2) | 32.4 (4.9) | 32.7 (5.2) | 28.6 (5.5) | 28.7 (5.5) |
| Waist circumference (cm) | 110.1 (13.8) | 110.1 (13.9) | 97.7 (13.9) | 98.0 (13.6) |
| Index of central obesity | 0.65 (0.08) | 0.65 (0.08) | 0.59 (0.08) | 0.59 (0.08) |
| Visceral adiposity index | 2.85 (2.13) | 2.91 (2.79) | 2.73 (2.46) | 2.91 (4.19) |
| Estimated total body fat (%) | 34.8 (9.8) | 35.2 (9.7) | 33.8 (11.1) | 32.9 (11.0) |
T2DM: type 2 diabetes mellitus; HbA1c: glycosylated haemoglobin; BP: blood pressure; HDL: high-density lipoprotein; SD: standard deviation.
Data are mean (SD) or n (%).
All randomized and treated patients who had a baseline HbA1c value and a baseline mean 24-h systolic blood pressure value.
All randomized and treated patients who had a baseline HbA1c value.
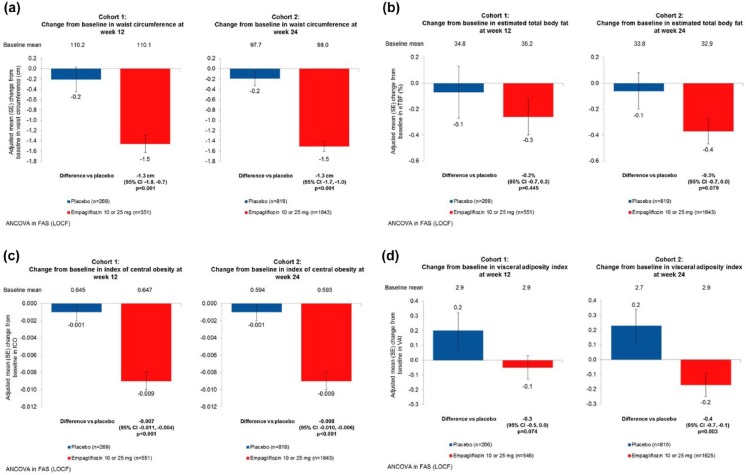
There were significantly greater reductions in body weight, WC and most but not all indices of adiposity in the EMPA-treated groups compared with placebo in both study cohorts (Figure 1(a) to (d)). The adjusted mean (95% CI) difference in body weight with EMPA versus placebo was −1.7 kg (−2.1 to −1.4 kg) in cohort 1 and −1.9 kg (−2.1 to −1.7 kg) in cohort 2 (p < 0.001 for both). The adjusted mean (95% CI) difference in WC with EMPA versus placebo was −1.3 cm (−1.8 to −0.7 cm) in cohort 1 and −1.3 cm (−1.7 to −1.0 cm) in cohort 2 (p < 0.001 for both). There was no difference in eTBF in cohort 1 for EMPA compared with placebo: adjusted mean difference −0.2% (−0.7% to 0.3%; p = 0.45), but in cohort 2, there was a trend towards greater reduction in eTBF for EMPA versus placebo: −0.3% (−0.7% to 0.0%; p = 0.08). The adjusted mean (95% CI) difference in ICO with EMPA versus placebo was −0.007 (−0.011 to −0.004) in cohort 1 and −0.008 (−0.010 to −0.006) in cohort 2 (p < 0.001 for both). For VAI, there was a trend for greater adjusted mean (95% CI) difference with EMPA versus placebo was −0.3 (−0.5 to 0.0; p = 0.07) in cohort 1, and a statistically greater reduction of −0.4 (−0.7 to −0.1; p = 0.003) in cohort 2.
Figure 1.
Effect of empagliflozin treatment on markers of visceral adiposity and estimated total body fat compared with placebo. Changes from baseline to week 12 (cohort 1) and week 24 (cohort 2) are shown for (a) waist circumference, (b) estimated total body fat, (c) index of central obesity and (d) visceral adiposity index. Greater reductions were seen in adiposity measures and indices in the empagliflozin treated groups compared with placebo in both study cohorts. The p-values for adjusted means based on ANCOVA with LOCF imputation.
ANCOVA: analysis of covariance; FAS: full-analysis set; LOCF: last observation carried forward.
EMPA reduced weight, WC and indices of adiposity when stratified by age (<50, 50–64, 65–74 and ⩾75 years; Table 2), sex (Table 3) and degree of abdominal obesity at baseline (WC < 88, 88–102, >102 cm; Table 4), albeit of greater magnitude with advanced age and with more severe abdominal obesity. Statistically significant interactions were seen with the effect of EMPA by age on weight (p-interaction = 0.028), WC (p-interaction = 0.010) and ICO (p-interaction = 0.010) and by degree of abdominal obesity on weight (p-interaction = 0.002) in cohort 2. Results stratified by age, sex and degree of abdominal obesity in cohort 1 were directionally consistent with those seen in cohort 2 (Supplemental Tables S1 to S3).
Table 2.
Impact of empagliflozin versus placebo on weight, waist circumference and indices of adiposity stratified by age at baseline in cohort 2.
| <50 years |
50–64 years |
65–74 years |
⩾75 years |
Treatment by age p-interaction | |||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo | Empagliflozin 10 or 25 mg | Placebo | Empagliflozin 10 or 25 mg | Placebo | Empagliflozin 10 or 25 mg | Placebo | Empagliflozin 10 or 25 mg | ||
| Body weight (kg), n | 222 | 464 | 459 | 871 | 119 | 276 | 25 | 41 | 0.03 |
| Adjusted mean change from baselinea | 0.0 | −2.0 | −0.3 | −2.1 | −0.6 | −2.3 | 0.9 | −2.8 | |
| Difference versus placebo (95% CI) | −2.0 (−2.4 to −1.6) | −1.8 (−2.1 to −1.5) | −1.7 (−2.2 to −1.1) | −3.7 (−4.9 to −2.4) | |||||
| Waist circumference (cm), n | 221 | 461 | 456 | 866 | 118 | 275 | 24 | 41 | 0.01 |
| Adjusted mean change from baselinea | 0.4 | −1.5 | −0.5 | −1.5 | −0.7 | −1.5 | 2.1 | −1.6 | |
| Difference versus placebo (95% CI) | −1.9 (−2.6 to −1.3) | −1.1 (−1.5 to −0.6) | −0.8 (−1.6 to 0.1) | −3.6 (−5.7 to −1.6) | |||||
| Index of central obesity, n | 221 | 461 | 456 | 866 | 118 | 275 | 24 | 41 | 0.01 |
| Adjusted mean change from baselinea | 0.0 | −0.01 | 0.0 | −0.01 | 0.0 | −0.01 | 0.01 | −0.01 | |
| Difference versus placebo (95% CI) | −0.01 (−0.02 to −0.01) | −0.01 (−0.01 to 0.0) | 0.00 (−0.01 to 0.00) | −0.02 (−0.03 to −0.01) | |||||
| Visceral adiposity index, n | 220 | 455 | 449 | 856 | 117 | 273 | 24 | 41 | 0.12 |
| Adjusted mean change from baselinea | 0.76 | −0.17 | 0.14 | −0.03 | −0.25 | −0.52 | −0.52 | −0.89 | |
| Difference versus placebo (95% CI) | −0.93 (−1.43 to −0.42) | −0.17 (−0.53 to 0.19) | −0.27 (−0.95 to 0.41) | −0.37 (−1.95 to 1.21) | |||||
CI: confidence interval.
Adjusted mean from ANCOVA with last observation carried forward imputation in patients randomized, received ⩾1 dose of study medication and with a baseline HbA1c value.
Table 3.
Impact of empagliflozin versus placebo on weight, waist circumference and indices of adiposity stratified by sex in cohort 2.
| Male |
Female |
Treatment by sex p-interaction | |||
|---|---|---|---|---|---|
| Placebo | Empagliflozin 10 or 25 mg | Placebo | Empagliflozin 10 or 25 mg | ||
| Body weight (kg), n | 424 | 927 | 401 | 725 | 0.14 |
| Adjusted mean change from baselinea | −0.3 | −2.1 | −0.2 | −2.3 | |
| Difference versus placebo (95% CI) | −1.8 (−2.1 to −1.5) | −2.1 (−2.4 to −1.8) | |||
| Waist circumference (cm), n | 421 | 921 | 398 | 722 | 0.53 |
| Adjusted mean change from baselinea | −0.2 | −1.5 | −0.2 | −1.6 | |
| Difference versus placebo (95% CI) | −1.2 (−1.7 to −0.8) | −1.4 (−1.9 to −1.0) | |||
| Index of central obesity, n | 421 | 921 | 398 | 722 | 0.38 |
| Adjusted mean change from baselinea | 0.00 | −0.01 | 0.00 | −0.01 | |
| Difference versus placebo (95% CI) | −0.01 (−0.01 to 0.00) | −0.01 (−0.01 to −0.01) | |||
| Visceral adiposity index, n | 419 | 911 | 391 | 714 | 0.43 |
| Adjusted mean change from baselinea | 0.02 | −0.27 | 0.46 | −0.05 | |
| Difference versus placebo (95% CI) | −0.29 (−0.65 to 0.07) | −0.50 (−0.89 to −0.12) | |||
CI: confidence interval.
Adjusted mean from ANCOVA with last observation carried forward imputation in patients randomized, received ⩾1 dose of study medication and with a baseline HbA1c value.
Table 4.
Impact of empagliflozin versus placebo on weight, waist circumference and indices of adiposity stratified by degree of abdominal obesity (WC) at baseline in cohort 2.
| <88 cm |
88–102 cm |
>102 cm |
Treatment by baseline WC p-interaction | ||||
|---|---|---|---|---|---|---|---|
| Placebo | Empagliflozin 10 or 25 mg | Placebo | Empagliflozin 10 or 25 mg | Placebo | Empagliflozin 10 or 25 mg | ||
| Body weight (kg), n | 207 | 357 | 346 | 762 | 266 | 524 | 0.002 |
| Adjusted mean change from baselinea | −0.6 | −2.3 | −0.4 | −2.0 | 0.2 | −2.2 | |
| Difference versus placebo (95% CI) | −1.7 (−2.2 to −1.3) | −1.6 (−1.9 to −1.3) | −2.4 (−2.8 to −2.1) | ||||
| Waist circumference (cm), n | 207 | 357 | 346 | 762 | 266 | 524 | 0.54 |
| Adjusted mean change from baselinea | 1.2 | −0.2 | −0.4 | −1.4 | −1.0 | −2.6 | |
| Difference versus placebo (95% CI) | −1.4 (−2.1 to −0.7) | −1.1 (−1.6 to −0.6) | −1.5 (−2.1 to −0.9) | ||||
| Index of central obesity, n | 207 | 357 | 346 | 762 | 266 | 524 | 0.54 |
| Adjusted mean change from baselinea | 0.0 | −0.01 | 0.0 | −0.01 | 0.0 | −0.01 | |
| Difference versus placebo (95% CI) | −0.01 (−0.01 to 0.0) | −0.01 (−0.01 to 0.0) | −0.01 (−0.01 to −0.01) | ||||
| Visceral adiposity index, n | 205 | 354 | 344 | 755 | 261 | 516 | 0.33 |
| Adjusted mean change from baselinea | 0.09 | −0.55 | 0.06 | −0.12 | 0.56 | 0.01 | |
| Difference versus placebo (95% CI) | −0.63 (−1.17 to −0.09) | −0.18 (−0.59 to 0.22) | −0.55 (−1.02 to −0.08) | ||||
CI: confidence interval.
Adjusted mean from ANCOVA with last observation carried forward imputation in patients randomized, received ⩾1 dose of study medication and with a baseline HbA1c value.
Discussion
In this study, we found that among 3300 patients with T2DM enrolled in five clinical trials, EMPA compared with placebo significantly reduced body weight, WC and multiple indices of overall and of visceral adiposity in patients with T2DM. Reductions in adiposity markers with EMPA were generally seen across all subgroups of age, sex and degree of abdominal obesity, with statistically significant heterogeneity of effects observed such that the effects of EMPA on reductions in body weight, WC and ICO were greater with increasing age; and reductions in body weight were greater with more severe abdominal obesity in those patients treated for 24 weeks. There was no heterogeneity of the effects of EMPA on body weight, WC or indices of visceral adiposity by sex. These results suggest that treatment with EMPA may reduce VAT and lead to changes in body composition associated with improved cardiometabolic risk profiles. Given that VAT is strongly associated with increased risk of T2DM, atherosclerotic cardiovascular disease (ASCVD) and cardiac function, if any degree of that association is causal, our findings could have potentially important clinical implications for the prevention and treatment of visceral adiposity-related cardiometabolic complications and warrant further investigation. Furthermore, in the context of previous studies directly measuring changes in VAT, these results suggest that WC and the indices of ICO and VAI might be useful for research and for clinical purposes as a surrogate for VAT. Further studies directly comparing these indices of adiposity with gold standard imaging assessments of VAT and other adipose depots are currently underway.13
Dedicated imaging sub-studies in clinical trials of SGLT-2 have demonstrated improvements in total fat mass and size of abdominal fat depots. In a 2-year study of EMPA versus glimepiride, total fat mass (by dual x-ray absorptiometry) and visceral and abdominal subcutaneous adipose tissue (SAT) volumes (by MRI) were significantly decreased among those treated with EMPA versus glimepiride.12 Similar findings were seen in trials of canagliflozin versus glimepiride21 and dapagliflozin versus placebo.22 The findings of these three dedicated imaging sub-studies align well with other results of weight reducing interventions3 and have been recently summarized.23 Our results here extend these observations to more clinically applicable indices of visceral adiposity that can be simply applied in the clinical setting and followed through treatment.
In contrast to prior dedicated body composition studies that measured total body fat directly with imaging, we did not observe a statistically significant reduction in eTBF with EMPA treatment using the referenced formula. This discrepancy could be due to the fact that the eTBF formula used in this study may be too imprecise to accurately quantify smaller relative changes in total body fat or may not be accurately calibrated for the trial population. However, in light of the presence of a trend towards a reduction in total body fat using the estimating equation in addition to prior evidence of a positive effect of EMPA and other drugs in this class on reducing body fat, the reduction of visceral and abdominal subcutaneous fat could have potential important clinical implications for cardiovascular and metabolic risk reduction.
Strengths of our study include the pooling of several clinical trials together with a large sample size and dedicated follow-up with precise measures of multiple markers used in the estimation of visceral adiposity by multiple published, validated indices. Several limitations also merit comment. First, although VAT is strongly associated with cardiometabolic disease and adverse outcomes, this study cannot demonstrate whether reduction in VAT with EMPA contributes to altered glycometabolic, ASCVD or heart failure risk. Second, with the large majority of study participants being Caucasian, our findings may not be generalizable to African-American patients who are more likely to have a lower visceral fat burden compared with other races even at higher BMIs or other racial minorities not represented or underrepresented in the present series of trials. Third, other anthropomorphic and imaging markers of VAT were not assessed in this study, so we are unable to comment on their relation with EMPA treatment. Fourth, although the use of an LOCF approach to impute missing data may lead to bias in the estimation of treatment effects and greater type 1 error, when we examined the similarity between LOCF and a mixed-effect model repeated measure approach in the individual trials, results were generally consistent.
In conclusion, our findings demonstrate that EMPA compared with placebo significantly reduced body weight, WC and indices of total and visceral adiposity in 3300 patients with T2DM. Whether changes in body composition induced by EMPA will be associated with reduced ASCVD and heart failure risk remains to be determined. The recently reported EMPA-REG OUTCOME™ trial (NCT01131676)24 assessing cardiovascular safety of EMPA versus placebo in a high-cardiovascular risk patient population provides further insight into this important clinical question and will help define the role of EMPA in the prevention and treatment of obesity and T2DM.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and study sites that participated in the five trials that form the basis for this analysis. Research materials may be accessed by contacting the corresponding author.
Footnotes
Declaration of conflicting interests: I.J.N. has nothing to disclose; D.K.M. reports research support and consultancy honoraria from Boehringer Ingelheim; Lilly USA; Janssen Research and Development LLC; Sanofi Aventis Groupe; Genentech, Inc.; Merck Sharp and Dohme Corp.; Daiichi Sankyo, Inc.; Novo Nordisk; GlaxoSmithKline; Takeda Pharmaceuticals North America; Bristol-Myers Squibb; AstraZeneca; Orexigen; Lexicon; Eisai; Regeneron; Pfizer; and Genfit; R.C. reports consulting fees from Pfizer, Bristol-Myers Squibb, Merck Sharp and Dohme, Takeda, Boston Scientific and Boehringer Ingelheim; and S.C., S.S.L., H.J.W., U.C.B. and O.E.J. are employees of Boehringer Ingelheim. S.S.L. owns shares in Novo Nordisk A/S and shares in dynamically traded investment funds which may own stocks from pharmaceutical companies.
Funding: Dr Neeland is supported by a Dedman Family Scholarship in Clinical Care from UT Southwestern and by grant 1K23DK106520-01 from the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health.
References
- 1. Ford ES. Trends in the control of risk factors for cardiovascular disease among adults with diagnosed diabetes: findings from the National Health and Nutrition Examination Survey 1999–2008*. J Diabetes 2011; 3: 337–347. [DOI] [PubMed] [Google Scholar]
- 2. Despres JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008; 28: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 3. Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes 2008; 32: 619–628. [DOI] [PubMed] [Google Scholar]
- 4. Gaborit B, Jacquier A, Kober F, et al. Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol 2012; 60: 1381–1389. [DOI] [PubMed] [Google Scholar]
- 5. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012; 366: 1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bays HE. Adiposopathy, diabetes mellitus, and primary prevention of atherosclerotic coronary artery disease: treating ‘sick fat’ through improving fat function with antidiabetes therapies. Am J Cardiol 2012; 110: 4B–12B. [DOI] [PubMed] [Google Scholar]
- 7. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015; 38: 420–428. [DOI] [PubMed] [Google Scholar]
- 8. Kovacs CS, Seshiah V, Swallow R, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab 2014; 16: 147–158. [DOI] [PubMed] [Google Scholar]
- 9. Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2013; 1: 208–219. [DOI] [PubMed] [Google Scholar]
- 10. Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2013; 36: 3396–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2014; 37: 1650–1659. [DOI] [PubMed] [Google Scholar]
- 12. Ridderstrale M, Andersen KR, Zeller C, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol 2014; 2: 691–700. [DOI] [PubMed] [Google Scholar]
- 13. Neeland IJ, Eliasson B, Ridderstrale M, et al. Comparison of adipose distribution indices with gold standard body composition assessments in the EMPA-REG H2H SU Trial. Diabetes Ther 2015, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilmore JH, Behnke AR. An anthropometric estimation of body density and lean body weight in young women. Am J Clin Nutr 1970; 23: 267–274. [DOI] [PubMed] [Google Scholar]
- 15. Wilmore JH, Behnke AR. An anthropometric estimation of body density and lean body weight in young men. J Appl Physiol 1969; 27: 25–31. [DOI] [PubMed] [Google Scholar]
- 16. Parikh RM, Joshi SR, Menon PS, et al. Index of central obesity – a novel parameter. Med Hypotheses 2007; 68: 1272–1275. [DOI] [PubMed] [Google Scholar]
- 17. Amato MC, Giordano C, Galia M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010; 33: 920–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luo W, Guo Z, Wu M, et al. Index of central obesity as a parameter to replace waist circumference for the definition of metabolic syndrome in predicting cardiovascular disease. J Cardiovasc Med 2014; 15: 738–744. [DOI] [PubMed] [Google Scholar]
- 19. Parikh RM, Mohan V. Changing definitions of metabolic syndrome. Indian J Endocrinol Metab 2012; 16: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parikh RM, Joshi SR, Pandia K. Index of central obesity is better than waist circumference in defining metabolic syndrome. Metab Syndr Relat Disord 2009; 7: 525–527. [DOI] [PubMed] [Google Scholar]
- 21. Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013; 382: 941–950. [DOI] [PubMed] [Google Scholar]
- 22. Bolinder J, Ljunggren O, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 2014; 16: 159–169. [DOI] [PubMed] [Google Scholar]
- 23. Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res 2015; 12: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. Epub ahead of print 17 September 2015. DOI: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.