Abstract
The data contained within the electronic health record (EHR) is “big” from the standpoint of volume, velocity, and variety. These circumstances and the pervasive trend towards EHR adoption have sparked interest in applying big data predictive analytic techniques to EHR data. Acute kidney injury (AKI) is a condition well suited to prediction and risk forecasting; not only does the consensus definition for AKI allow temporal anchoring of events, but no treatments exist once AKI develops, underscoring the importance of early identification and prevention. The Acute Dialysis Quality Initiative (ADQI) convened a group of key opinion leaders and stakeholders to consider how best to approach AKI research and care in the “Big Data” era. This manuscript addresses the core elements of AKI risk prediction and outlines potential pathways and processes. We describe AKI prediction targets, feature selection, model development, and data display.
Keywords: Acute kidney injury, Electronic health record, Electronic medical record, Prediction, Big data, Predictive analytics
Abrégé
Les données figurant dans les dossiers médicaux électroniques (DMÉ) sont considérables, tant au point de vue du volume que du débit ou de la variété. Ces trois caractéristiques et la tendance générale à adopter les DMÉ ont soulevé un intérêt pour appliquer les techniques d’analyse prédictive des mégadonnées aux données contenues dans les dossiers médicaux électroniques. L’insuffisance rénale aiguë (IRA) est une maladie qui convient parfaitement à une méthode de prévision et de prévention des risques: non seulement la définition acceptée de cette affection permet-elle un ancrage temporel des événements ; mais il n’existe aucun traitement une fois que la maladie est déclarée, ce qui montre l’importance d’une détection précoce. L’Acute Dialysis Quality Initiative (ADQI) a convoqué un groupe de travail constitué de leaders d’opinion et autres intervenants du milieu pour se pencher sur la meilleure façon d’approcher la recherche et les soins offerts aux patients atteints d’IRA en cette ère de mégadonnées. Le présent article traite des éléments centraux de la prévention des risques et en expose les procédures potentielles. Nous y décrivons les cibles de prévention de l’IRA, la sélection des paramètres, l’élaboration des modèles et l’affichage des données.
Background
The term “big data” has traditionally been used to describe extraordinarily large and complex datasets. For many medical practitioners, this concept was initially epitomized by genomics – the colossal amount of discrete data generated by high throughput sequencing techniques required analytic methods ranging far beyond standard statistical approaches [1]. However, “omics” are now ubiquitous and “big data” has become vernacular in medicine [2, 3]. Clinical researchers are beginning to employ innovative, high-content analytic techniques capable of integrating and exploring the exceedingly large and diverse datasets contained within the electronic health record (EHR).
EHR data, which are generated through the routine provision of clinical care, are “big” from the standpoint of volume (number of discrete data points available), velocity (rate at which new data accumulates), and variety (myriad of data elements available for interrogation) [3, 4]. These aspects, along with its singular clinical relevance, make EHR data ideal for disease prediction and risk forecasting. In particular, acute kidney injury (AKI) is a syndrome which lends itself well to predictive modeling and early risk stratification (Fig. 1). The presence of a standard, consensus definition allows accurate and efficient AKI diagnosis [5]; temporal anchoring of the AKI event creates a distinct pre-disease dataset to which high-content, high-throughput predictive techniques can be applied (Fig. 1). Additionally, although AKI has been associated with poor short and long term outcomes in both adults and children, no treatments exist to mitigate or cure AKI once it has developed [6–13]. The ability to predict AKI in hospitalized patients would provide the opportunity to modify care pathways and implement interventions. This, in turn, could prevent AKI events, thereby reducing mortality, shortening length of stay, averting the development of chronic kidney disease, and potentially creating novel quality of care indicators [13, 14]. In this manuscript, we present evidence informed, consensus driven statements regarding the concepts of primary relevance when considering the capacity of EHR data to be used in AKI prediction applications.
Fig. 1.

Signal Identification for AKI Development and Progression. Current consensus AKI definitions allow AKI events to be precisely anchored from a temporal standpoint, clearly defining a pre-disease state. As the patient progresses from “No AKI” to “AKI,” the pattern of data generated within the EHR changes, creating an “AKI signal” which can be identified through advanced analytic techniques. This signal can be translated into a prediction model which is capable of identifying patients at high risk for AKI development. Reproduced with permission from ADQI
Methods
This consensus meeting following the established ADQI process, as previously described [15]. The broad objective of ADQI is to provide expert-based statements and interpretation of current knowledge for use by clinicians according to professional judgment and identify evidence care gaps to establish research priorities. The 15th ADQI Consensus Conference Chairs convened a diverse panel representing relevant disciplines from five countries from North America and Europe around the theme of “Acute Kidney Injury in the Era of Big Data” for a 2-day consensus conference in Banff, Canada on September 6–8, 2015. During the pre-conference phase of the meeting, each work group performed a comprehensive literature search to summarize areas where broad consensus exists, categorize knowledge gaps, and identify future priorities for research. Specifically for the AKI prediction workgroup, the literature search was conducted using the terms “acute kidney injury prediction”, “acute renal failure prediction”, and “AKI prediction” in MEDLINE using PUBMED as the search engine. This search yielded a total of 679 articles for review. Studies were limited to articles published in 2010–2015 to reflect more recent harmonized AKI definitions. Studies were included if they discussed a prediction model and did not isolate the analysis to identification of independent risk factors. Studies were excluded if the focus of the prediction model was novel biomarkers due to practical issues in using these markers in current clinical practice. Thirty-four articles were selected in the initial review. Upon reviewing the articles, there was a consensus amongst work group members to include seven additional articles published prior to 2010; these articles used earlier consensus definitions for AKI, laid the groundwork for the subsequently developed models, and were archetype models when published [16–22]. Four core questions/concepts were crafted for presentation to the entire ADQI consensus group during the conference (Table 1). During the conference our work group developed consensus positions, and plenary sessions involving all ADQI contributors were used to present, debate, and refine these positions. Following the conference this summary report was generated, revised, and approved by all members of the work group.
Table 1.
Core Questions for ADQI Consensus Group
| Question 1 | Across the spectrum of AKI, which event or events should be targeted for prediction? |
| Question 2 | For the purposes of predictive modelling, what paradigm should be used for variable identification and selection? |
| Question 3 | What is the optimal technical approach for model building and EHR integration? |
| Question 4 | What is the optimal output of an architype predictive model? |
Results
Question 1: Across the spectrum of AKI, which event or events should be targeted for prediction?
Prior to developing a model, it is important to carefully choose the target for prediction. From the outset, the consensus group believed it was imperative that, for the purposes of prediction, AKI be diagnosed and identified according to the generally accepted consensus definition and classification scheme, the KDIGO criteria [5]. This is the most current consensus definition, it harmonizes the previously proposed AKI criteria (RIFLE, pRIFLE, and AKIN), and is applicable to both adults and children [5, 23–25]. In order to build the strongest and most useful predictive model, we would recommend forecasting AKI events with a horizon of 48–72 h. While it would be advantageous to identify AKI events as early as possible, lengthening the event horizon reduces the accuracy of the model; we believe the suggested horizon gives practitioners adequate time to modify practice, optimize hemodynamics, and mitigate potential injury without sacrificing predictive power. The group additionally believed that rather than targeting all AKI, it would be initially advantageous to predict “moderate/severe” AKI as defined as KDIGO stage 2 or 3. While this recommendation is based on evidence-informed opinion, there are rational justifications for making it. First, this is consistent with the initial ADQI consensus statement which described the RIFLE criteria; operationally, Stage 1 KDIGO-defined AKI correlates with RIFLE stage “Risk” [24]. Treating KDIGO-defined Stage 1 AKI as “AKI risk,” allows it to become a subsequent predictor for moderate/severe AKI. Second, AKI predictors or risk factors have traditionally been more strongly associated with higher severity AKI [26, 27]. The greater strength of association will likely result in more powerful predictive modeling by reducing confounding; the development of robust models is of paramount importance for these initial big data attempts at predictive AKI analytics. Finally, while “mild” Stage 1 AKI has been associated with poorer outcomes, the association with these outcomes is significantly stronger for Stages 2/3 [6, 11, 27–31]. This ability to strongly link AKI with outcomes has an additional benefit as it will allow the models to predict not only AKI, but AKI-related outcomes as well. In one potential scenario proposed by the workgroup, a model would provide predictive AKI risk up until the occurrence of AKI then, at the inflection point of AKI development, it would provide a one-time predictive risk for patient-centered, clinically important outcomes. The workgroup acknowledges that if only Stage 2 and 3 AKI are targeted for prediction, early simulative subanalysis should be performed to evaluate the suitability of this approach.
Consensus Statement
For the purpose of developing AKI prediction models using the data contained within the EHR, the prototype should predict risk both for developing KDIGO-defined Stage 2/3 AKI as well as patient-centered and clinically important AKI-related outcomes.
Question 2: For the purposes of predictive modelling, what paradigm should be used for variable identification and selection?
Prior to applying “big data” analytics to AKI prediction, the consensus group believed it was important to appraise the AKI prediction models which had been developed to date. Based upon our predictive goals outlined in the prior section, model variables of particular interest would be causally and/or temporally associated both with the development of AKI and with AKI-related outcomes.
A number of investigators have approached AKI prediction using standard multivariable regression methodology [17–22, 32, 33]. Models have been developed for a variety of patient populations with a particular emphasis on cardiac surgery patients [34, 35]; notably, less work has been performed in general critical care populations despite the fact that they are also at high risk for AKI [36–38]. Even less established are prediction models in non-critically ill patients. However, given the ultimate goal of preventing AKI, we also need to consider predictive modeling in these populations in order to identify high-risk patients as early as possible [39, 40]. A fairly comprehensive list of studies and variables are shown in Table 2. Variables from patient-specific models are often constrained to the clinical care specific to that population; for example, models for cardiac surgery patients include cardiopulmonary bypass time and number of bypass grafts. However, a number of variables commonly appear across many of the existing models (i.e., age, baseline renal function, medications, diabetes, hypertension, etc.); these variables may be better suited for a generalized model. Most models had modest predictive success with area under the receiver operating curves (AUC) approximating 0.75; a few models reached AUCs as high as 0.9, although the sample sizes were smaller and there was a pre-selection of high-risk patients [41–44]. Regardless of their ultimate utility in defining predictive variables, these models give us a minimum AUC threshold to target for successful model development.
Table 2.
Selected list of Predictive Models Currently Available in the Literature
| Study | Population and sample Size | Variables in Model | Outcome |
|---|---|---|---|
| Aronson A et al, 2007 [91] | CABG patients n = 2381 |
Age, preoperative CHF, prior MI, preexisting renal disease, intraoperative inotropes, intraoperative intra-aortic balloon pump, bypass time, pre-operative pulse pressure. | postoperative creatinine ≥2 mg/dL w/ increase ≥0.7 mg/dL from baseline or dialysis |
| Basu RK et al. 2014 [29] | Pediatric critically ill patients n = 584 total in 4 cohorts |
Vasopressor/inotrope use, invasive mechanical ventilation, percent fluid overload, change in creatinine clearance | KDIGO Stage 2/3 AKI |
| Brown JR et al, 2007 [92] | CABG patients n = 8363 |
Age, female, diabetes, peripheral vascular disease, CHF, hypertension, prior CABG, preoperative IABP, elevated WBC count | eGFR <30 ml/min/1.73 m2 |
| Chawla LS et al, 2013 [37] Koyner JL et al, 2015 [93] |
Critically ill patients n = 77 |
Urine output measurement | Progression to AKIN stage III |
| Chong E et al, 2012 [94] | Patients w/ eGFR <60 ml/min/1.73 m2 undergoing percutaneous coronary intervention n = 770 | Age, baseline eGFR, post-percutaneous coronary intervention, creatinine kinase, contrast volume | Contrast-induced nephropathy defined as 25 % or 0.5 mg/dL increase from baseline creatinine within 48 h after PCI |
| Cruz DN et al. 2014 [95] | Critically ill patients n = 506 | Age, diabetes, cardiovascular disease, chronic kidney disease, hypertension, obesity, hyperbilirubinemia, cerebrovascular accident, AIDS, cancer, hypotension, high-risk surgery, nephrotoxin exposure, sepsis | AKI stage II and III defined by AKIN |
| Demirjian S et al, 2012 [44] | Cardiac surgery patients n = 25,898 |
gender, race, weight, pulmonary disease, CHF, diabetes, hypertension, type of surgery, previous cardiac surgery, emergency surgery, eGFR, albumin, bicarbonate, sodium, BUN, hemoglobin, platelet count, bilirubin, BMI, potassium, CPB time, intrasurgical transfusion or vasopressor, intrasurgical UOP | AKI requiring dialysis |
| Forni LG et al. 2013 [39] | Patients admitted to an acute medical unit n = 3707 | Age, alertness scale, chronic kidney disease, congestive cardiac failure, diabetes, liver disease | AKI defined per KDIGO guidelines |
| Gao et al. 2014 [96] |
Coronary angiography intervention n = 3945 | Age, hypertension, acute MI, heart failure, use of intra-aortic balloon pump, decreased glomerular filtration rate, contrast volume | Increase in serum creatinine level |
| Grimm JC et al 2015 [97] | Lung transplant n = 10,693 |
Race, sarcoidosis, diabetes, weight, baseline renal function, Kanofsky performance score, previous ICU stay, ECMO, days on list, double transplant | AKI requiring dialysis |
| Gurm HS et al. 2013 [98] | Patients undergoing a percutaneous coronary intervention n = 68,753 | age, weight, height, percutaneous coronary intervention status and indication, coronary artery disease presentation, cardiogenic shock, heart failure, ejection fraction, diabetes, CKMB, creatinine, hemoglobin, troponin I and T | ≥0.5 mg/dl increase in serum creatinine level from baseline, RRT receipt |
| Ho J et al, 2012 [99] | Cardiac surgery patients n = 350 | Bypass time, baseline eGFR, euroSCORE, postoperative serum creatinine | AKI by AKIN criteria |
| Hong SH et al. 2012 [100] | Living donor transplant recipients n = 429 | age, MELD, hypertension, platelet count, surgical time, packed red blood cell transfusion, lactate, furosemide dose, calcium chloride dose, phosphate level | Renal failure was defined according to RIFLE |
| Kane-Gill et al. 2015 [38] | Elderly, critically ill n = 25,230 |
age, gender, race, eGFR, heart failure, diabetes, hypertension, admission type (medical vs surgical), requirement for vasopressors or mechanical ventilation, sepsis, hypotension, nephrotoxic drugs. | AKI by KDIGO criteria |
| Kim JM et al. 2014 [46] | Liver transplant recipients n = 153 |
Hepatic encephalopathy, deceased donor liver donations, MELD score, intraoperative blood loss, and indication for liver transplantation |
Patients who needed renal replacement therapy |
| Kim MY et al, 2011 [101] | Isolated off-pump CABG patients n = 448 |
High systolic blood pressure, low baseline eGFR, coronary angiography less than 7 days prior to surgery | AKI by AKIN criteria |
| Kim WH et al. 2013 [102] | Aortic surgery with cardiopulmonary bypass n = 737 | Age, preoperative glomerular filtration, ejection fraction, operation time, intraoperative urine output, intraoperative furosemide use | AKI defined by RIFLE |
| Kristovic D et al. 2015 [26] | Cardiac surgery patients n = 1056 |
age, atrial fibrillation, CHF classification, previous cardiac surgery, creatinine, endocarditis, weight, gender, COPD, bypass | AKI stage by KDIGO AKI requiring dialysis |
| Legrand M et al. 2013 [103] | Patients with endocarditis/ cardiac surgery with cardiopulmonary bypass n = 202 | Age, gender, pre-existing comorbidities, presence of shock, systemic emboli, NYHA classification, hemoglobin, baseline creatinine, need for mechanical ventilation, characteristics of infection/surgery, use of nephrotoxic agents | Development or progression of AKI in the 7 days following surgery. AKI defined per AKIN |
| McMahon GM et al. 2013 [104] | Rhabdomyolysis within 3 days of admission n = 2371 | Age, female sex, cause of rhabdomyolysis, initial creatinine, creatinine phosphokinase, phosphate, calcium, and bicarbonate | Composite endpoint: Renal replacement therapy or mortality |
| Medha et al. 2013 [41] | Trauma patients n = 4396 |
hepatic dysfunction, urea, glucose, pulmonary dysfunction, severity of injury | serum creatinine level >2.0 mg/dL during the hospital stay |
| Meersch M et al. 2014 [42] | Patients undergoing cardiac surgery with bypass n = 50 | Diabetes, severity of illness, ejection fraction, baseline serum creatinine, cross-clamp time, chronic obstructive pulmonary disease | AKI defined by RIFLE or AKIN together |
| Mehran R et al, 2004 [22] | Patients undergoing percutaneous coronary interventions n = 8357 | Hypotension, intra-aortic balloon pump, congestive heart failure, age >75 years, anemia, diabetes, contrast volume, baseline creatinine or eGFR | Increase of ≥25 % or ≥0.5 mg/dL in pre-PCI serum creatinine at 48 h after PCI |
| Mehta RH et al, 2006 [32] | CABG and/or valve surgery patients n = 449,524 | Preoperative creatinine, age, race, type of surgery, diabetes, shock, NYHA class, lung disease, recent myocardial infarction, prior cardiovascular surgery | AKI requiring dialysis |
| Ng SY et al. 2014 [105] | Cardiac surgery patients n = 28,422 | obesity, infective endocarditis, cardiac procedure, preop creatinine, diabetes, urgency status, eGFR, CHF, age, cardiogenic shock, IABP use, bypass time, non-RBC blood product use, gender, reoperation for bleeding, hypercholesterolemia, hypertension, and respiratory disease | Increased creatinine > 200 mmol/L (2.26 mg/dL), ≥ 2x increase in creatinine over baseline, a new receipt for RRT |
| Palomba H et al. 2007 [18] | Cardiac surgery patients n = 603 |
age, serum creatinine, glucose, heart failure, combined surgeries, cardiopulmonary bypass time, cardiac output, central venous pressure | creatinine > 2.0 mg/dl or increase of 50 % above baseline |
| Park MH et al. 2015 [106] | Living-donor liver transplant n = 538 |
weight; diabetes, alcoholic liver disease, albumin <3.5 mg/dL, model for end-stage liver disease score, child-turcotte-pugh- estimated graft to recipient body weight ratio, operation details, calcineurin inhibitor use without mycophenolate | AKI as defined by RIFLE |
| Rahmanian PB et al, 2011 [107] | Cardiac surgery patients n = 2511 |
Pulmonary hypertension, preoperative renal dysfunction, bypass time, peripheral vascular disease, recent MI, atrial fibrillation, age, CHF, diabetes | AKI requiring dialysis |
| Rodriguez et al. 2013 [108] | Severe Rhabdomyolysis n = 126 | Albumin, metabolic acidosis, prothrombin time, peak creatinine phosphokinase | RIFLE category |
| Romano TG et al. 2013 [109] | Orthotopic liver transplant patients n = 114 | MELD | increase ≥ 0.3 mg/dL in serum creatinine |
| Schneider DF et al, 2012 [110] | Critically ill burn patients n = 309 |
age, sex, race, % body surface area burned, burn mechanism, intubation, inhalation injury, NROF, fraction of predicted Parkland resuscitation, early transfusion, weight, Charlson Score, drug abuse, smoker, number of preadmission medications, ACEI/ARB, diuretic, NSAIDs, methamphetamine, lowest hematocrit, potassium, sodium, pH, glucose, base deficit, lowest mean arterial pressure, temperature | AKI defined using RIFLE classification |
| Simonini M et al. 2014 [111] | Elective cardiac surgery n = 802 | Age, gender, ejection fraction, hypertension, diabetes, renal function, reoperation cardiac surgery, surgery type | AKIN stage II/III AKI |
| Slankamenac K et al. 2013 [112] | Liver surgery n = 549 |
Need for blood transfusion, cirrhosis, oliguria, hepaticojejunostomy, use of colloids, use of diuretics, use of a bolus of catecholamines | R of RIFLE |
| Soto K et al. 2013 [40] | Patients admitted from the emergency department n = 616 | Age, kidney susceptibility stage, chronic heart failure, hypertension, cardiovascular disease, and diabetes mellitus | New onset AKI per RIFLE |
| Thakar CV et al, 2005 [17] | Cardiac surgery patients n = 31,677 |
gender, CHF, ejection fraction, preop intra-aortic balloon pump, COPD, diabetes, previous cardiac surgery, emergency surgery, type of surgery, creatinine >1.2 | AKI requiring dialysis |
| Tsai TT et al. 2014 [113] | Percutaneous coronary intervention n = 947,012 | Age, CKD, prior cardiovascular disease, acute coronary syndrome, cardiac arrest, anemia, CHF, intra-aortic balloon pump prior to procedure, cardiogenic shock | AKI defined by AKIN and AKI requiring dialysis |
| Wang M et al. 2013 [114] | Patients with hemorrhagic fever (Hantann virus) n = 112 | age, gender, presence of shock, proteinuria, hematuria, platelet count, leukocyte | Required dialysis or increased serum creatinine ≥354 mmol/L |
| Wang Y et al. 2013 [115] | Patients hospitalized with acute heart failure n = 1709 | Age, ≥ 3 previous hospital admissions for acute heart failure, systolic blood pressure <90 mmHg, serum sodium <130 mmol/L, heart functional class IV, proteinuria, SCr ≥ 104 mmol/L, intravenous furosemide dose ≥ 80 mg/day | increase in serum creatinine (SCr) of ≥26.4 mmol/L or ≥ 50 % within 48 h. |
| Wijeysundera DN et al, 2007 [19] | Cardiac surgery patients n = 20,131 |
Preoperative eGFR, diabetes, ejection fraction, previous cardiac surgery, procedures other than isolated CABG or ASD repair, non-elective procedure, preoperative intra-aortic balloon pump | AKI requiring dialysis |
| Wong B et al. 2015 [116] | Cardiac surgery patients who developed AKI n = 2316 | Age, weight, preoperative creatinine, gender, preoperative intra-aortic balloon pump, ejection fraction, type of surgery, previous cardiac surgery, diabetes, COPD, cardiopulmonary bypass time, clamp time, pump time, number of bypass grafts | AKI Stage 1, stage 2, stage 3 |
| Xu X et al, 2010 [117] | Liver transplant recipients n = 102 |
age, MELD score, preoperative creatinine, BUN, sodium, and potassium, intraoperative UOP, intraoperative hypotension, intraoperative noradrenaline | Serum creatinine >1.5 mg/dl with an increase of 50 % above baseline and/or RRT |
As stated, ideal variables would be associated with both the development of AKI and patient centered, clinically important outcomes following AKI. Notably, many of the same risk factors described in Table 2 as predicting AKI occurrence have also been shown to predict AKI-associated mortality [36, 45–51]. In addition to these factors, positive fluid balance has been associated with increased mortality in both pediatric and adult patients with AKI [52–56]. Receipt of renal replacement therapy (RRT) is another outcome worth forecasting after AKI has occurred. Although most of the published clinical scores predicting receipt of RRT have focused on post-cardiac surgery patients, they have identified many of the same predictors for AKI occurrence in broader populations [17, 19, 32, 34]. AKI is known to be associated with the development of CKD and ESRD, therefore, prediction of these long-term outcomes among AKI survivors should also be targeted; archetype variables associated with these outcomes are shown in Table 2 [8, 57–68].
While the group believed it was imperative that previously identified AKI predictors be reviewed, to truly harness the power of the EHR a de novo approach which considers the entirety of the dataset is required (Fig. 2). There are a number of potential data-mining, machine learning approaches to this sort of feature selection which could be used alone or in combination (Table 3). One method, neural networks, employ non-linear models that feed a set of predictors (inputs) into a hidden layer of units which then use a non-linear transformation to send a value to an output; the prediction is a summation of all the values coming from the hidden layer [69]. This non-linear technique is sometimes described as a “black box” method since it’s difficult to determine the form of the final predictive model. However, this is of little concern in feature selection, as identifying the group of the most influential variables is of interest. A second potential method is that of random forests which is an extension of the binary split classification tree approach [70, 71]. Multiple trees are created by allowing a random number of the predictor variables to be considered at each split of each tree. This results in trees that cover a larger solution space, potentially increasing accuracy. Another set of methods is cluster analysis, a group of unsupervised learning techniques. Observations are grouped according to their similarities in a multidimensional space, based on a distance measure [72]. The resultant clusters can then be further explored to see which ones have a very high or a very low incidence of the outcome measure. A similar method is a technique known as self-organizing maps, in which unsupervised neural networks map a highly dimensional space onto a two-dimensional map [73, 74]. Principal components analysis and support vector machines are two similar feature selection methods which could be employed in this space [75, 76]. Although this is not a comprehensive list of the methods that might be considered, these are excellent exemplar techniques which can be used to identifying novel features and transform known risk factors.
Fig. 2.
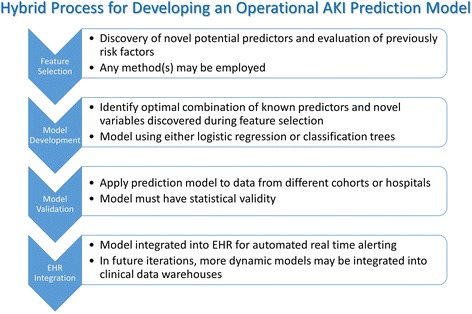
Development of AKI Prediction Algorithm. The first step in the development of an AKI prediction model is feature selection. This process would evaluate known risk factors identified from the literature and would use machine learning techniques to identify novel risk factors from amongst the EHR dataset. All appropriate features would be considered for inclusion in the actual prediction model which would weight individual variables to create a generalizable model. This model would be validated using a different (or subset of existing) dataset. Once validated, the model could then be integrated directly into the EHR to allow real time AKI alerting. Reproduced with permission from ADQI
Table 3.
Big data modeling techniques
| Method | Advantages | Disadvantages |
|---|---|---|
| Neural Networks | Discover non-linear relationships. Can assess multi-level interactions | “Black Box” to clinicians; hard to implement into a DSS* |
| Random Forests | Finds most probable solution set; robust against scaling influences | Not always best in terms of prediction; hard to implement into a DSS |
| Cluster Analysis | Finds groups of very similar patients; exploratory analysis | Unsupervised technique |
| Principal Components Analysis | Uncovers the variables contributing the most to outcome variation | Not amenable to binary outcomes; assumes additive relationship |
| Support Vector Machines | Robust against statistical assumptions | Difficult to implement into a DSS |
In summary, the suggested approach highlights our belief that accurate prediction of AKI takes precedence over finding putative variables, though the suggested approaches do not preclude discovery of new risk factors for AKI. Additionally, while it is useful to review previously established variables associated with AKI from existing studies, application of high content, machine learning techniques to the complete EHR dataset will be the driving force behind variable selection. The ability to dynamically identify and integrate variables from amongst innumerable patient-level data elements represents a marked departure from classically developed model building approaches.
Consensus Statement
Variables included in prototype AKI prediction models should be identified using a hybrid approach; risk factors which are well established in the literature should be considered along with novel risk factors identified via machine learning techniques. Application of these unsupervised approaches should take precedence as it allows feature selection to be dynamic, thereby generating the strongest prediction from existing data elements.
Question 3: What is the optimal approach for model building and EHR integration?
Once the aforementioned hybrid variable selection process was complete, previously identified risk factors and potential predictors discovered via big data techniques could be considered for inclusion in a model. Inclusion criteria could include:
Evidence over multiple studies that the risk factor was a powerful predictor of AKI
Identification by machine learning techniques to be predictive of AKI and outcomes
Available discretely within the EHR to allow easy integration
Reliably/accurately recorded within the EHR
Variables need not necessarily be universal. For example, pediatric or ICU specific variables could be considered; the model could be dynamic with certain features active/inactive in certain locations/populations. Additionally, it is possible that effect modification of the variables could vary between patients or populations; the presence or absence of certain variables might change the weighting of the residual variables.
While we advocate for a big data approach to identify novel predictive features, initially we would recommend that the predictive model itself be built through more standard statistical modelling. This is primarily due to the inherent limitations of current EHR architecture. EHRs are built to optimize patient level data review and display; they are not necessarily organized to optimize cohort level analysis [77]. This makes implementation of a resource-intense machine learning algorithm into the EHR itself technically and operationally problematic. Therefore, once the variables were identified by literature search and machine learning methodology, it is likely that a logistic regression model, discriminant analysis, or decision tree algorithm would be employed to predict the development of AKI [71, 78, 79]. Data could accumulate on a “rolling window” concept and a prediction could be generated at a pre-specified interval (hourly, every two hours, every shift); alternatively, the model could generate a score in real time as each new data value is received. One conceptual approach would allow this model to generate a risk score ranging from 0 to 100; low scores would be indicative of minimal AKI risk and high scores would be indicative of significant AKI risk. Scoring on a continuous scale would allow both low and high thresholds to be set. In many ways, the ability to identify patients at negligible AKI risk could be as valuable as identifying patients at great AKI risk. An algorithm such as this could be active up until the time the patient develops AKI. At that inflection point, a final, one-time score could be generated which would be reflective of the patients AKI-related outcome risk, thereby allowing practitioners to identify patients at great risk for poorer outcomes.
It is important to note that while the EHR has operational and structural limitations to the application of big data techniques, alternatives should be available in the future. For example, many clinical data warehouse (CDW) solutions have become available for analytic purposes [80–83]. These CDWs represent “shadow” EHRs in which data has been manipulated, linked, and stored in a fashion conducive to high-content, high-throughput analytics [82, 83]. Once such CDWs become as ubiquitous as EHRs, big data approaches could be applied directly to the CDW environment. However, to truly exploit the full capacity of the EHR and EHR data, a more progressive approach is necessary. The EHR has transcended its original purpose; although it is currently a care monitoring and delivery tool, it has the potential to revolutionize clinical care paradigms. To achieve this, data architecture must become as important as data entry and analytics must be prioritized. The creation of a true “learning EHR” could be the key to higher quality, lower cost care delivered with greater efficacy and efficiency.
Consensus Statement
While machine learning techniques should be used to identify novel AKI risk factors, prototype AKI prediction models should be built using more standard statistical weighing techniques to allow effective EHR integration. However, analytics should attain higher priority and the operational limitations of the EHR should be addressed. Consequently, subsequent predictive iterations should progress towards full EHR-integration of high content analytic techniques.
Question 4: What is the optimal output of an archetype predictive model?
After the rigorous steps undertaken to select variables and develop a predictive model, we propose that any prototypes be directly integrated into the EHR for automated real time usage. The increasingly widespread use of EHRs across hospitals has substantially increased the amount of data available to providers [84]. However, while EHRs purportedly improve patient outcomes, studies that have validated these benefits are lacking [85–87]. Several potential EHR-related barriers to improving outcomes have been identified and include information overload, ineffective data display, and poor implementation processes [88–90] Therefore, it is imperative that an AKI prediction model not only harness the power of the EHR data set, but also that it effectively conform to the strengths and limitations of EHR processes. Ideally, AKI risk prediction tools should directly extract relevant data predictors in real-time, deliver a relevant “renal risk score,” and provide feedback to practitioners regarding potential actionable items. One potential a concept would be to create a “renal dashboard” (Fig. 3a and b).
Fig. 3.

a and b Renal Dashboard. Once the risk prediction model is developed and validated, it is important to determine how to deliver the information to providers. One possible output might be a “Renal Dashboard” (a). The display would visually display the time trend of AKI as well as a numeric value (with confidence intervals) for the current risk. For any patients who develop AKI, information about outcome risk would be provided; in this example, the outcomes of interest are need for RRT, mortality, development of ESRD, and likelihood of renal recovery. The dashboard could be dynamic, allowing providers to drill into the risk score. In the patient level display (b), information would be available about how the risk had trended over the past 24 h as well as what factors were affecting the current risk score most significantly. In this example, AKI risk information is provided in a visually stimulating manner with a dynamic component capable of driving care modification. Reproduced with permission from ADQI
The main objective of the renal dashboard would be to provide feedback on the absolute risk of developing moderate to severe AKI within the next 48–72 h as well as to present information about the clinical features contributing to these risks. The electronic dashboard format could be tailored for a particular provider, service, or unit. Each patient could have a risk score (in percentage) with an accompanying confidence interval (Fig. 3a); a confidence interval component would give practitioners an idea of how certain the AKI risk was at any given time. In addition to absolute risk scores, the dashboard could be configured to display time trends in risk scores which might give a better sense of evolving AKI risk. Time trends should be displayed in a visually stimulating fashion (i.e., sparklines) to demonstrate the dynamic nature of real-time AKI-risk. A fully optimized dashboard might allow providers to “drill into” the risk score (Fig. 3b), revealing a magnified view as well as more detailed data on the most recent predictors that contributed to a significant increase in risk score. The identification of specific vital sign indicators, laboratory parameters, medication administration data, or other clinical factors that contributed directly to a rise in AKI risk will help guide providers toward implementing risk reduction actions.
A secondary objective of the dashboard might be to provide updated feedback on the risk of adverse outcomes associated with AKI once it actually develops. Early iterations of this sort of prototype may be limited to one-time scores for AKI-related outcomes. However, at the inflection of AKI development, separate risk scores for mortaltiy, receipt of RRT, CKD, and renal recovery could be provided. As an example, the ability to predict receipt of RRT may help providers plan for appropriate patient disposition (i.e., transfer to ICU for CRRT) and timely procedures (i.e., placement of dialysis catheter). Prediction of long-term renal and cardiovascular outcomes could be especially useful at the time of discharge, facilitating appropriate referrals, vascular access planning, and long-term care goal discussions.
We anticipate that a renal dashboard such as this could be displayed either directly within the system or independently from the EHR platform. Although information would be directly fed to the prediction model from up-to-date EHR data, each healthcare system, service, or unit may tailor the physical setting of the dashboard display to fit their workflows. For example, in an ICU setting where incidence of AKI may be as high as 40 %, the renal dashboard may be displayed on computerized workstations on wheels so that providers can incorporate the real-time information and feedback provided by the renal dashboard into their multi-disciplinary rounds [31]. For other services and locations where incidence of AKI is much lower - for example, the labor and delivery unit - the renal dashboard may serve in a more adjunctive role, to be monitored by a specialized “renal response” team (akin to traditional “rapid response” teams).
The consensus group acknowledges that numerous such dashboards could be created for similar medical conditions to assist with risk stratification. The approach described in this manuscript is designed to underscore the utility of a dashboard scheme. We realize that developing multiple dashboards for individualized diseases is unlikely to be efficient or effective in the long run. Operationally, a superior approach would be to seamlessly integrate a renal dashboard component into existing dashboard which is used to evaluate a range of quality and performance indicators.
Consensus Statement
The output from predictive models should be delivered to practitioners in a fashion that is cognizant of EHR limitations and strengths, minimizes workflow inefficiency, and maximizes utility.
Conclusion
The EHR dataset is a massive collection of clinically relevant data elements generated through the routine provision of patient care. Its size and complexity lend themselves to “big data” techniques; these in turn offer the potential to use the entire EHR dataset to predict AKI and AKI related outcomes. Variable selection should employ high-content, unsupervised analytic techniques. Developing predictive models should focus on EHR integration and optimize the output for clinical utility.
Acknowledgments
ADQI 15 Consensus Meeting Contributors
Sean M. Bagshaw, Division of Critical Care Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB, Canada; Rajit Basu, Division of Critical Care and the Center for Acute Care Nephrology, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Azra Bihorac, Division of Critical Care Medicine, Department of Anesthesiology, University of Florida, Gainesville, FL, USA; Lakhmir S. Chawla, Departments of Medicine and Critical Care, George Washington University Medical Center, Washington, DC, USA; Michael Darmon, Department of Intensive Care Medicine, Saint-Etienne University Hospital, Saint-Priest-En-Jarez, France; R.T. Noel Gibney, Division of Critical Care Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB, Canada; Stuart L. Goldstein, Department of Pediatrics, Division of Nephrology &Hypertension, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Charles E. Hobson, Department of Health Services Research, Management and Policy, University of Florida, Gainesville, FL, USA; Eric Hoste, Department of Intensive Care Medicine, Ghent University Hospital, Ghent University, Ghent, Belgium, and Research Foundation – Flanders, Brussels, Belgium; Darren Hudson, Division of Critical Care Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB, Canada; Raymond K. Hsu, Department of Medicine, Division of Nephrology, University of California San Francisco, San Francisco, CA, USA; Sandra L. Kane-Gill, Departments of Pharmacy, Critical Care Medicine and Clinical Translational Sciences, University of Pittsburgh, Pittsburgh, PA, USA; Kianoush Kashani, Divisions of Nephrology and Hypertension, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Mayo Clinic, Rochester, MN, USA; John A. Kellum, Center for Critical Care Nephrology, Department of Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA, USA; Andrew A. Kramer, Prescient Healthcare Consulting, LLC, Charlottesville, VA, USA; Matthew T. James, Departments of Medicine and Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, Canada; Ravindra Mehta, Department of Medicine, UCSD, San Diego, CA, USA; Sumit Mohan, Department of Medicine, Division of Nephrology, College of Physicians & Surgeons and Department of Epidemiology Mailman School of Public Health, Columbia University, New York, NY, USA; Hude Quan, Department of Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, Canada; Claudio Ronco, Department of Nephrology, Dialysis and Transplantation, International Renal Research Institute of Vicenza, San Bortolo Hospital, Vicenza, Italy; Andrew Shaw, Department of Anesthesia, Division of Cardiothoracic Anesthesiology, Vanderbilt University Medical Center, Nashville, TN, USA; Nicholas Selby, Division of Health Sciences and Graduate Entry Medicine, School of Medicine, University of Nottingham, UK; Edward Siew, Department of Medicine, Division of Nephrology, Vanderbilt University Medical Center, Nashville, TN, USA; Scott M. Sutherland, Department of Pediatrics, Division of Nephrology, Stanford University, Stanford, CA, USA; F. Perry Wilson, Section of Nephrology, Program of Applied Translational Research, Yale University School of Medicine, New Haven, CT, USA; Hannah Wunsch, Department of Critical Care Medicine, Sunnybrook Health Sciences Center and Sunnybrook Research Institute; Department of Anesthesia and Interdepartmental Division of Critical Care, University of Toronto, Toronto, Canada
Continuing medical education
The 15th ADQI Consensus Conference held in Banff, Canada on September 6–8th, 2015 was CME accredited Continuing Medical Education and Professional Development, University of Calgary, Calgary, Canada.
Financial support
Funding for the 15th ADQI Consensus Conference was provided by unrestricted educational support from: Division of Critical Care Medicine, Faculty of Medicine and Dentistry, and the Faculty of Medicine and Dentistry, University of Alberta (Edmonton, AB, Canada); Center for Acute Care Nephrology, Cincinnati Children's Hospital Medical Center (Cincinnati, OH, USA); Astute Medical (San Diego, CA, USA); Baxter Healthcare Corp (Chicago, IL, USA); Fresenius Medical Care Canada (Richmond Hill, ON, Canada); iMDsoft Inc (Tel Aviv, Israel); La Jolla Pharmaceutical (San Diego, CA, USA); NxStage Medical (Lawrence, MA, USA); Premier Inc (Charlotte, NC, USA); Philips (Andover, MA, USA); Spectral Medical (Toronto, ON, Canada).
Abbreviations
- AKI
acute kidney injury
- KDIGO
Kidney Disease: Improving Global Outcomes
- RIFLE
Risk, Injury, Failure, Loss, ESRD
- AKIN
Acute kidney injury network
- ADQI
Acute Dialysis Quality Initiative
- EHR
Electronic health record
Footnotes
Competing interests
The authors declare that they have no competing interests
Authors’ contributions
SS, LC, SK, RH, and AK performed the initial comprehensive literature search, formulated the key questions and concepts, presented their findings to the 15th ADQI Consensus Group and drafted the initial manuscript. SG, JK, CR, and SB guided and chaired the pre-, intra-, and post-conference sessions, critiqued and oversaw the conference debate and refinement process, and revised the final manuscript. The 15th ADQI group participated in the conference, debate, and refinement process which led to the final core concepts and manuscript output. All authors read, revised, and approved the final manuscript.
References
- 1.Schadt EE, Linderman MD, Sorenson J, Lee L, Nolan GP. Computational solutions to large-scale data management and analysis. Nature reviews Genetics. 2010;11(9):647–57. doi: 10.1038/nrg2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger B, Peng J, Singh M. Computational solutions for omics data. Nature reviews Genetics. 2013;14(5):333–46. doi: 10.1038/nrg3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baro E, Degoul S, Beuscart R, Chazard E. Toward a Literature-Driven Definition of Big Data in Healthcare. BioMed research international. 2015;2015:639021. doi: 10.1155/2015/639021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Krishnan E. Big data and clinicians: a review on the state of the science. JMIR medical informatics. 2014;2(1):e1. doi: 10.2196/medinform.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney international. 2012;2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 6.Sutherland SM, Byrnes JJ, Kothari M, Longhurst CA, Dutta S, Garcia P, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10(4):554–61. doi: 10.2215/CJN.01900214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutherland SM, Ji J, Sheikhi FH, Widen E, Tian L, Alexander SR, et al. AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol. 2013;8(10):1661–9. doi: 10.2215/CJN.00270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59(4):523–30. doi: 10.1053/j.ajkd.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 9.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006;69(1):184–9. doi: 10.1038/sj.ki.5000032. [DOI] [PubMed] [Google Scholar]
- 10.Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, et al. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care. 2011;15(3):R146. doi: 10.1186/cc10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. Journal of the American Society of Nephrology : JASN. 2005;16(11):3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 12.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khwaja A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin Pract. 2012;120(4):179–84. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 14.Ftouh S, Lewington A. Prevention, detection and management of acute kidney injury: concise guideline. Clinical medicine (London, England) 2014;14(1):61–5. doi: 10.7861/clinmedicine.14-1-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellum JA, Bellomo R, Ronco C. Acute Dialysis Quality Initiative (ADQI): methodology. The International journal of artificial organs. 2008;31(2):90–3. doi: 10.1177/039139880803100202. [DOI] [PubMed] [Google Scholar]
- 16.Mehta RH, Grab JD, O'Brien SM, Bridges CR, Gammie JS, Haan CK, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114(21):2208–16. doi: 10.1161/CIRCULATIONAHA.106.635573. [DOI] [PubMed] [Google Scholar]
- 17.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. Journal of the American Society of Nephrology : JASN. 2005;16(1):162–8. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 18.Palomba H, de Castro I, Neto AL, Lage S, Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS Score. Kidney Int. 2007;72(5):624–31. doi: 10.1038/sj.ki.5002419. [DOI] [PubMed] [Google Scholar]
- 19.Wijeysundera DN, Karkouti K, Dupuis JY, Rao V, Chan CT, Granton JT, et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. Jama. 2007;297(16):1801–9. doi: 10.1001/jama.297.16.1801. [DOI] [PubMed] [Google Scholar]
- 20.Brown JR, Cochran RP, Leavitt BJ, Dacey LJ, Ross CS, MacKenzie TA, et al. Multivariable prediction of renal insufficiency developing after cardiac surgery. Circulation. 2007;116(11 Suppl):I139–43. doi: 10.1161/CIRCULATIONAHA.106.677070. [DOI] [PubMed] [Google Scholar]
- 21.Aronson S, Fontes ML, Miao Y, Mangano DT. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation. 2007;115(6):733–42. doi: 10.1161/CIRCULATIONAHA.106.623538. [DOI] [PubMed] [Google Scholar]
- 22.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. Journal of the American College of Cardiology. 2004;44(7):1393–9. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 23.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative w. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–35. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 26.Kristovic D, Horvatic I, Husedzinovic I, Sutlic Z, Rudez I, Baric D, et al. Cardiac surgery-associated acute kidney injury: risk factors analysis and comparison of prediction models. Interactive cardiovascular and thoracic surgery. 2015;21(3):366–73. doi: 10.1093/icvts/ivv162. [DOI] [PubMed] [Google Scholar]
- 27.Leedahl DD, Frazee EN, Schramm GE, Dierkhising RA, Bergstralh EJ, Chawla LS, et al. Derivation of urine output thresholds that identify a very high risk of AKI in patients with septic shock. Clin J Am Soc Nephrol. 2014;9(7):1168–74. doi: 10.2215/CJN.09360913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12–20. doi: 10.2215/CJN.02730313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basu RK, Zappitelli M, Brunner L, Wang Y, Wong HR, Chawla LS, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85(3):659–67. doi: 10.1038/ki.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kandler K, Jensen ME, Nilsson JC, Moller CH, Steinbruchel DA. Acute kidney injury is independently associated with higher mortality after cardiac surgery. Journal of cardiothoracic and vascular anesthesia. 2014;28(6):1448–52. doi: 10.1053/j.jvca.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–23. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 32.Mehta RH, Grab JD, O'Brien SM, Bridges CR, Gammie JS, Haan CK, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114(21):2208–16. doi: 10.1161/CIRCULATIONAHA.106.635573. [DOI] [PubMed] [Google Scholar]
- 33.Fortescue EB, Bates DW, Chertow GM. Predicting acute renal failure after coronary bypass surgery: cross-validation of two risk-stratification algorithms. Kidney Int. 2000;57(6):2594–602. doi: 10.1046/j.1523-1755.2000.00119.x. [DOI] [PubMed] [Google Scholar]
- 34.Englberger L, Suri RM, Li Z, Dearani JA, Park SJ, Sundt TM, et al. Validation of clinical scores predicting severe acute kidney injury after cardiac surgery. Am J Kidney Dis. 2010;56(4):623–31. doi: 10.1053/j.ajkd.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Kiers HD, van den Boogaard M, Schoenmakers MC, van der Hoeven JG, van Swieten HA, Heemskerk S, et al. Comparison and clinical suitability of eight prediction models for cardiac surgery-related acute kidney injury. Nephrol Dial Transplant. 2013;28(2):345–51. doi: 10.1093/ndt/gfs518. [DOI] [PubMed] [Google Scholar]
- 36.Uchino S, Kellum J, Bellomo R, Doig G, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. Jama. 2005;294(7):813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 37.Chawla LS, Davison DL, Brasha-Mitchell E, Koyner JL, Arthur JM, Shaw AD, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17(5):R207. doi: 10.1186/cc13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kane-Gill SL, Sileanu FE, Murugan R, Trietley GS, Handler SM, Kellum JA. Risk factors for acute kidney injury in older adults with critical illness: a retrospective cohort study. Am J Kidney Dis. 2015;65(6):860–9. doi: 10.1053/j.ajkd.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forni LG, Dawes T, Sinclair H, Cheek E, Bewick V, Dennis M, et al. Identifying the patient at risk of acute kidney injury: a predictive scoring system for the development of acute kidney injury in acute medical patients. Nephron Clin Pract. 2013;123(3–4):143–50. doi: 10.1159/000351509. [DOI] [PubMed] [Google Scholar]
- 40.Soto K, Papoila AL, Coelho S, Bennett M, Ma Q, Rodrigues B, et al. Plasma NGAL for the diagnosis of AKI in patients admitted from the emergency department setting. Clin J Am Soc Nephrol. 2013;8(12):2053–63. doi: 10.2215/CJN.12181212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medha S. A, Pandey RM, Sawhney C, Upadhayay AD, Albert V. Incidence, clinical predictors and outcome of acute renal failure among North Indian trauma patients. J Emerg Trauma Shock. 2013;6(1):21–8. doi: 10.4103/0974-2700.106321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0093460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JM, Jo YY, Na SW, Kim SI, Choi YS, Kim NO, et al. The predictors for continuous renal replacement therapy in liver transplant recipients. Transplant Proc. 2014;46(1):184–91. doi: 10.1016/j.transproceed.2013.07.075. [DOI] [PubMed] [Google Scholar]
- 44.Demirjian S, Schold JD, Navia J, Mastracci TM, Paganini EP, Yared JP, et al. Predictive models for acute kidney injury following cardiac surgery. Am J Kidney Dis. 2012;59(3):382–9. doi: 10.1053/j.ajkd.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 45.Demirjian S, Chertow GM, Zhang JH, O'Connor TZ, Vitale J, Paganini EP, et al. Model to predict mortality in critically ill adults with acute kidney injury. Clin J Am Soc Nephrol. 2011;6(9):2114–20. doi: 10.2215/CJN.02900311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA, et al. Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int. 2006;70(6):1120–6. doi: 10.1038/sj.ki.5001579. [DOI] [PubMed] [Google Scholar]
- 47.Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM. Refining predictive models in critically ill patients with acute renal failure. Journal of the American Society of Nephrology : JASN. 2002;13(5):1350–7. doi: 10.1097/01.ASN.0000014692.19351.52. [DOI] [PubMed] [Google Scholar]
- 48.Lins RL, Elseviers M, Daelemans R, Zachée P, Gheuens E, Lens S, et al. Prognostic value of a new scoring system for hospital mortality in acute renal failure. Clin Nephrol. 2000;53(1):10–7. [PubMed] [Google Scholar]
- 49.Lins RL, Elseviers MM, Daelemans R, Arnouts P, Billiouw JM, Couttenye M, et al. Re-evaluation and modification of the Stuivenberg Hospital Acute Renal Failure (SHARF) scoring system for the prognosis of acute renal failure: an independent multicentre, prospective study. Nephrol Dial Transplant. 2004;19(9):2282–8. doi: 10.1093/ndt/gfh364. [DOI] [PubMed] [Google Scholar]
- 50.Chang JW, Jeng MJ, Yang LY, Chen TJ, Chiang SC, Soong WJ, et al. The epidemiology and prognostic factors of mortality in critically ill children with acute kidney injury in Taiwan. Kidney Int. 2015;87(3):632–9. doi: 10.1038/ki.2014.299. [DOI] [PubMed] [Google Scholar]
- 51.Poukkanen M, Vaara ST, Reinikainen M, Selander T, Nisula S, Karlsson S, et al. Predicting one-year mortality of critically ill patients with early acute kidney injury: data from the prospective multicenter FINNAKI study. Crit Care. 2015;19:125. doi: 10.1186/s13054-015-0848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foland JA, Fortenberry JD, Warshaw BL, Pettignano R, Merritt RK, Heard ML, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32(8):1771–6. doi: 10.1097/01.CCM.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 53.Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55(2):316–25. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 54.Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD. National Heart Ln, and Blood Institute Acute Respiratory Distress Syndrome Network. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6(5):966–73. doi: 10.2215/CJN.08781010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76(4):422–7. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 56.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lo L, Go A, Chertow G, McCulloch C, Fan D, Ordoñez J, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76(8):893–9. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009;76(10):1089–97. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- 59.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–8. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE, Perkins RM. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81(5):477–85. doi: 10.1038/ki.2011.405. [DOI] [PubMed] [Google Scholar]
- 61.Ishani A, Nelson D, Clothier B, Schult T, Nugent S, Greer N, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171(3):226–33. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 62.Ishani A, Xue J, Himmelfarb J, Eggers P, Kimmel P, Molitoris B, et al. Acute kidney injury increases risk of ESRD among elderly. Journal of the American Society of Nephrology : JASN. 2009;20(1):223–8. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wald R, Quinn R, Luo J, Li P, Scales D, Mamdani M, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. Jama. 2009;302(11):1179–85. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 64.Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891–8. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79(12):1361–9. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cerdá J, Liu KD, Cruz DN, Jaber BL, Koyner JL, Heung M, et al. Promoting Kidney Function Recovery in Patients with AKI Requiring RRT. Clin J Am Soc Nephrol. 2015;10(10):1859–67. doi: 10.2215/CJN.01170215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heung M, Chawla LS. Predicting progression to chronic kidney disease after recovery from acute kidney injury. Curr Opin Nephrol Hypertens. 2012;21(6):628–34. doi: 10.1097/MNH.0b013e3283588f24. [DOI] [PubMed] [Google Scholar]
- 68.Hickson LJ, Chaudhary S, Williams AW, Dillon JJ, Norby SM, Gregoire JR, et al. Predictors of Outpatient Kidney Function Recovery Among Patients Who Initiate Hemodialysis in the Hospital. Am J Kidney Dis. 2014;65(4):592–602. doi: 10.1053/j.ajkd.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Widrow B, Lehr M. Perceptron, Madaline, and back propagation. Proceedings of IEEE. 1990;78(9):1415–42. doi: 10.1109/5.58323. [DOI] [Google Scholar]
- 70.Brieman L. Random Forests. Machine Learning. 2001;45(1):5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 71.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Monterey, CA: Wadsworth & Brooks; 1984. [Google Scholar]
- 72.Bailey K. Typologies and Taxonomies: An Introduction to Classification Techniques. Thousand Oaks, CA: Sage University Paper. Sage Publications; 1994. [Google Scholar]
- 73.Kohonen T. Self-organized formation of topologically correct feature maps. Biol Cybern. 1982;43(1):59–69. doi: 10.1007/BF00337288. [DOI] [Google Scholar]
- 74.Kramer A, Lee D, Axelrod R. Use of a Kohonen Neural Network to Characterize Respiratory Patients for Medical Intervention. In: Malmgren H, Borga M, Niklasson L, editors. Artificial Neural Networks in Medicine and Biology. Perspectives in Neural Computing. London: Springer; 2000. pp. 192–6. [Google Scholar]
- 75.Abdi H, Williams LJ. Principal component analysis. Wiley Interdisciplinary Reviews: Computational Statistics. 2010;2(4):433–59. doi: 10.1002/wics.101. [DOI] [Google Scholar]
- 76.Drucker H, Chris, Kaufman B, Smola A, Vapnik V (eds). Support vector regression machines. Advances in Neural Information Processing Systems 9; 1997.
- 77.Hoffman M, Williams M. Electronic medical records and personalized medicine. Hum Genet. 2011;130(1):33–9. doi: 10.1007/s00439-011-0992-y. [DOI] [PubMed] [Google Scholar]
- 78.Hosmer DW, Lemeshow S. Applied logistic regression. Statistics in Medicine, vol 7. New York: Wiley; 1989. [Google Scholar]
- 79.Fisher RA. The use of multiple measurements in taxonomic problems. Annals of Eugenics. 1936;7(2):179–88. doi: 10.1111/j.1469-1809.1936.tb02137.x. [DOI] [Google Scholar]
- 80.Frankovich J, Longhurst CA, Sutherland SM. Evidence-Based Medicine in the EMR Era. New England Journal of Medicine. 2011;365(19):1758–9. doi: 10.1056/NEJMp1108726. [DOI] [PubMed] [Google Scholar]
- 81.Odgers DJ, Dumontier M. Mining Electronic Health Records using Linked Data. AMIA Summits on Translational Science Proceedings. 2015;2015:217–21. [PMC free article] [PubMed] [Google Scholar]
- 82.Lowe HJ, Ferris TA, Hernandez PM, Weber SC. STRIDE – An Integrated Standards-Based Translational Research Informatics Platform. AMIA Annual Symposium Proceedings. 2009;2009:391–5. [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy SN, Mendis ME, Berkowitz DA, Kohane I, Chueh HC. Integration of Clinical and Genetic Data in the i2b2 Architecture. AMIA Annual Symposium Proceedings. 2006;2006:1040. [PMC free article] [PubMed] [Google Scholar]
- 84.Jha AK, DesRoches CM, Campbell EG, Donelan K, Rao SR, Ferris TG, et al. Use of electronic health records in U.S. hospitals. N Engl J Med. 2009;360(16):1628–38. doi: 10.1056/NEJMsa0900592. [DOI] [PubMed] [Google Scholar]
- 85.Han YY, Carcillo JA, Venkataraman ST, Clark RS, Watson RS, Nguyen TC, et al. Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system. Pediatrics. 2005;116(6):1506–12. doi: 10.1542/peds.2005-1287. [DOI] [PubMed] [Google Scholar]
- 86.Chaudhry B, Wang J, Wu S, Maglione M, Mojica W, Roth E, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144(10):742–52. doi: 10.7326/0003-4819-144-10-200605160-00125. [DOI] [PubMed] [Google Scholar]
- 87.Ash JS, Berg M, Coiera E. Some unintended consequences of information technology in health care: the nature of patient care information system-related errors. J Am Med Inform Assoc. 2004;11(2):104–12. doi: 10.1197/jamia.M1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hemp P. Death by information overload. Harv Bus Rev. 2009;87(9):82–9. [PubMed] [Google Scholar]
- 89.Pickering BW, Dong Y, Ahmed A, Giri J, Kilickaya O, Gupta A, et al. The implementation of clinician designed, human-centered electronic medical record viewer in the intensive care unit: a pilot step-wedge cluster randomized trial. Int J Med Inform. 2015;84(5):299–307. doi: 10.1016/j.ijmedinf.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 90.Sittig DF, Ash JS, Zhang J, Osheroff JA, Shabot MM. Lessons from "Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system". Pediatrics. 2006;118(2):797–801. doi: 10.1542/peds.2005-3132. [DOI] [PubMed] [Google Scholar]
- 91.Aronson S, Fontes ML, Miao Y, Mangano DT. Group IotMSoPIR, Foundation IRaE. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation. 2007;115(6):733–42. doi: 10.1161/CIRCULATIONAHA.106.623538. [DOI] [PubMed] [Google Scholar]
- 92.Brown JR, Cochran RP, Leavitt BJ, Dacey LJ, Ross CS, MacKenzie TA, et al. Multivariable prediction of renal insufficiency developing after cardiac surgery. Circulation. 2007;116(11 Suppl):I139–43. doi: 10.1161/CIRCULATIONAHA.106.677070. [DOI] [PubMed] [Google Scholar]
- 93.Koyner JL, Davison DL, Brasha-Mitchell E, Chalikonda DM, Arthur JM, Shaw AD, et al. Furosemide Stress Test and Biomarkers for the Prediction of AKI Severity. Journal of the American Society of Nephrology : JASN. 2015;26(8):2023–31. doi: 10.1681/ASN.2014060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chong E, Shen L, Poh KK, Tan HC. Risk scoring system for prediction of contrast-induced nephropathy in patients with pre-existing renal impairment undergoing percutaneous coronary intervention. Singapore Med J. 2012;53(3):164–9. [PubMed] [Google Scholar]
- 95.Cruz DN, Ferrer-Nadal A, Piccinni P, Goldstein SL, Chawla LS, Alessandri E, et al. Utilization of small changes in serum creatinine with clinical risk factors to assess the risk of AKI in critically lll adults. Clin J Am Soc Nephrol. 2014;9(4):663–72. doi: 10.2215/CJN.05190513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao YM, Li D, Cheng H, Chen YP. Derivation and validation of a risk score for contrast-induced nephropathy after cardiac catheterization in Chinese patients. Clin Exp Nephrol. 2014;18(6):892–8. doi: 10.1007/s10157-014-0942-9. [DOI] [PubMed] [Google Scholar]
- 97.Grimm JC, Lui C, Kilic A, Valero V, Sciortino CM, Whitman GJ, et al. A risk score to predict acute renal failure in adult patients after lung transplantation. Ann Thorac Surg. 2015;99(1):251–7. doi: 10.1016/j.athoracsur.2014.07.073. [DOI] [PubMed] [Google Scholar]
- 98.Gurm HS, Seth M, Kooiman J, Share D. A novel tool for reliable and accurate prediction of renal complications in patients undergoing percutaneous coronary intervention. Journal of the American College of Cardiology. 2013;61(22):2242–8. doi: 10.1016/j.jacc.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 99.Ho J, Reslerova M, Gali B, Nickerson PW, Rush DN, Sood MM, et al. Serum creatinine measurement immediately after cardiac surgery and prediction of acute kidney injury. Am J Kidney Dis. 2012;59(2):196–201. doi: 10.1053/j.ajkd.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 100.Hong SH, Park CO, Park CS. Prediction of newly developed acute renal failure using serum phosphorus concentrations after living-donor liver transplantation. J Int Med Res. 2012;40(6):2199–212. doi: 10.1177/030006051204000618. [DOI] [PubMed] [Google Scholar]
- 101.Kim MY, Jang HR, Huh W, Kim YG, Kim DJ, Lee YT, et al. Incidence, risk factors, and prediction of acute kidney injury after off-pump coronary artery bypass grafting. Ren Fail. 2011;33(3):316–22. doi: 10.3109/0886022X.2011.560406. [DOI] [PubMed] [Google Scholar]
- 102.Kim WH, Lee SM, Choi JW, Kim EH, Lee JH, Jung JW, et al. Simplified clinical risk score to predict acute kidney injury after aortic surgery. Journal of cardiothoracic and vascular anesthesia. 2013;27(6):1158–66. doi: 10.1053/j.jvca.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 103.Legrand M, Pirracchio R, Rosa A, Petersen ML, Van der Laan M, Fabiani JN, et al. Incidence, risk factors and prediction of post-operative acute kidney injury following cardiac surgery for active infective endocarditis: an observational study. Crit Care. 2013;17(5):R220. doi: 10.1186/cc13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McMahon GM, Zeng X, Waikar SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821–8. doi: 10.1001/jamainternmed.2013.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ng SY, Sanagou M, Wolfe R, Cochrane A, Smith JA, Reid CM. Prediction of acute kidney injury within 30 days of cardiac surgery. J Thorac Cardiovasc Surg. 2014;147(6):1875–83. doi: 10.1016/j.jtcvs.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 106.Park MH, Shim HS, Kim WH, Kim HJ, Kim DJ, Lee SH, et al. Clinical Risk Scoring Models for Prediction of Acute Kidney Injury after Living Donor Liver Transplantation: A Retrospective Observational Study. PLoS One. 2015;10(8):e0136230. doi: 10.1371/journal.pone.0136230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rahmanian PB, Kwiecien G, Langebartels G, Madershahian N, Wittwer T, Wahlers T. Logistic risk model predicting postoperative renal failure requiring dialysis in cardiac surgery patients. Eur J Cardiothorac Surg. 2011;40(3):701–7. doi: 10.1016/j.ejcts.2010.12.051. [DOI] [PubMed] [Google Scholar]
- 108.Rodríguez E, Soler MJ, Rap O, Barrios C, Orfila MA, Pascual J. Risk factors for acute kidney injury in severe rhabdomyolysis. PLoS One. 2013;8(12):e82992. doi: 10.1371/journal.pone.0082992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Romano TG, Schmidtbauer I, Silva FM, Pompilio CE, D'Albuquerque LA, Macedo E. Role of MELD score and serum creatinine as prognostic tools for the development of acute kidney injury after liver transplantation. PLoS One. 2013;8(5):e64089. doi: 10.1371/journal.pone.0064089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schneider DF, Dobrowolsky A, Shakir IA, Sinacore JM, Mosier MJ, Gamelli RL. Predicting acute kidney injury among burn patients in the 21st century: a classification and regression tree analysis. J Burn Care Res. 2012;33(2):242–51. doi: 10.1097/BCR.0b013e318239cc24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Simonini M, Lanzani C, Bignami E, Casamassima N, Frati E, Meroni R, et al. A new clinical multivariable model that predicts postoperative acute kidney injury: impact of endogenous ouabain. Nephrol Dial Transplant. 2014;29(9):1696–701. doi: 10.1093/ndt/gfu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Slankamenac K, Beck-Schimmer B, Breitenstein S, Puhan MA, Clavien PA. Novel prediction score including pre- and intraoperative parameters best predicts acute kidney injury after liver surgery. World J Surg. 2013;37(11):2618–28. doi: 10.1007/s00268-013-2159-6. [DOI] [PubMed] [Google Scholar]
- 113.Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath-PCI Registry. J Am Heart Assoc. 2014;3(6):e001380. doi: 10.1161/JAHA.114.001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang M, Wang J, Wang T, Li J, Hui L, Ha X. Thrombocytopenia as a predictor of severe acute kidney injury in patients with Hantaan virus infections. PLoS One. 2013;8(1):e53236. doi: 10.1371/journal.pone.0053236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang YN, Cheng H, Yue T, Chen YP. Derivation and validation of a prediction score for acute kidney injury in patients hospitalized with acute heart failure in a Chinese cohort. Nephrology (Carlton) 2013;18(7):489–96. doi: 10.1111/nep.12092. [DOI] [PubMed] [Google Scholar]
- 116.Wong B, St Onge J, Korkola S, Prasad B. Validating a scoring tool to predict acute kidney injury (AKI) following cardiac surgery. Can J Kidney Health Dis. 2015;2:3. doi: 10.1186/s40697-015-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu X, Ling Q, Wei Q, Wu J, Gao F, He ZL, et al. An effective model for predicting acute kidney injury after liver transplantation. Hepatobiliary Pancreat Dis Int. 2010;9(3):259–63. [PubMed] [Google Scholar]


