To the editor:
During recent years, variants in iron metabolism genes have been identified as molecular etiologic factors of hereditary microcytic anemias, such as iron-refractory iron deficiency anemia caused by TMPRSS6 mutations, hypotransferrinemia resulting from TF mutations, congenital sideroblastic anemia (CSA) due to mutations in SLC25A38, and so on.1-3 Recently, the STEAP3 gene, also known as TSAP6, was identified as one of the potential candidate for CSA, because it encodes an endosomal ferrireductase required for efficient transferrin-dependent iron uptake in developing erythroblasts.4-6 Steap3/Tsap6 null mice display moderately severe microcytic anemia due to reduced activity of ferrireductase and abnormal erythroid maturation.5,7-9 Recently, the first, and thus far only, human STEAP3 mutation causing transfusion-dependent severe hypochromic anemia was reported in 1 family due to a combination of a mutant allele (p.Cys100Ter) and a hypomorphic allele.10
Our study investigated the prevalence of STEAP3 mutations in humans and their physiologic consequences. We used large cohorts of normal individuals and α-thalassemia subjects from China to study the phenotypic effect of STEAP3 mutations on normal and abnormal erythrocyte indices. Surprisingly, we found a relatively high prevalence of potentially harmful recessive alleles. However, although the identified STEAP3 mutations exhibited impaired ferrireductase activity in vitro, they had little or no effect on erythrocyte phenotypes.
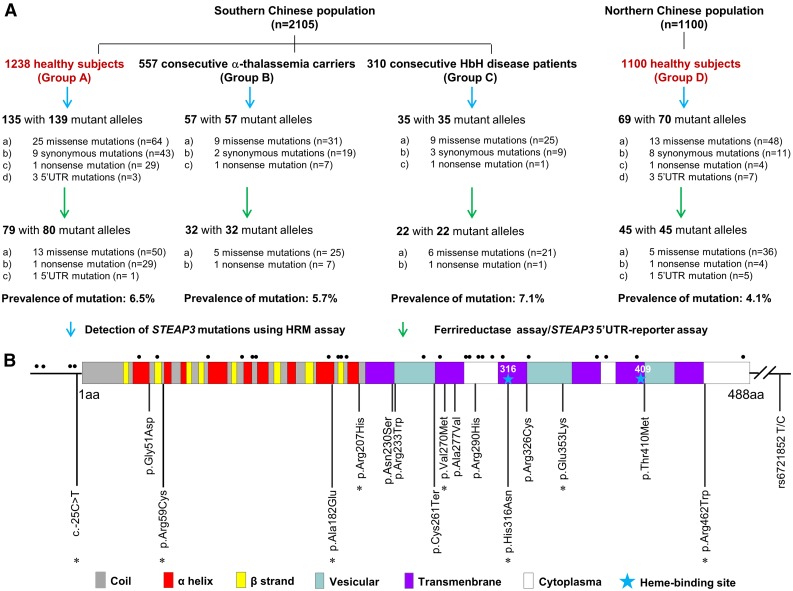
Details regarding the study design and methods used are available in the supplemental Data available on the Blood Web site. In the present population-based study, using a molecular screening approach (supplemental Figure 1) and functional analysis, we found multiple mutations in STEAP3 with a surprisingly high prevalence of 5.3% in a total of 2338 Chinese healthy subjects, with slightly higher rates in southern China (6.5%) than in northern China (4.1%, P = .011; Figure 1A). A total of 179 mutant alleles involving 16 different loss-of-function STEAP3 mutations (14 missense mutations, 1 nonsense mutation, and 1 mutation in the 5′ untranslated region [UTR]) were identified in these 2 geographically distinct study populations (Figure 1B), each with their own characteristic spectrum of mutations. The 3 most common mutations, p.Cys261Ter, p.Arg290His, and p.Gly51Asp, accounted for 72.6% of the total (supplemental Table 1). As shown in Figure 1B and supplemental Figure 2, the affected residues within the key domains of the protein are highly conserved evolutionarily and may be critical for enzyme activity.
Figure 1.
The prevalence and spectrum of STEAP3 mutations in the Chinese population. (A) Study design and study outcomes. A total of 3205 individuals from 2 representative regions (southern and northern China) were studied to document the incidence of STEAP3 mutations. Southern Chinese population: 3 groups of 2105 subjects from Guangxi where thalassemias are endemic included 1238 normal subjects (group A), 557 α-thalassemia carriers (group B), and 310 individuals with hemoglobin H (HbH) disease samples (group C). STEAP3 mutations occurred at similar frequencies among these 3 groups (P = .720), confirming a high prevalence of STEAP3 mutations in southern China. The high prevalence of STEAP3 mutations was also noted in 1100 normal individuals recruited from the Shandong Province in northern China. All 179 mutant alleles detected in these 2 populations were used to assess the spectrum of STEAP3 mutations. (B) Domains in the gene structure of STEAP3 are indicated by the different color boxes and the untranslated regions are delineated by the solid black line. Sixteen functional STEAP3 mutations are shown below the map and 8 novel mutations are marked with an asterisk. Twenty-eight neutral mutations (4 variants in the 5′UTR and 23 missense mutations in the coding regions) are shown with black dots above the map and are presented in supplemental Table 1. The eSNP (rs6721852) in the STEAP3-C2orf76 intergenic region is also indicated at the right.
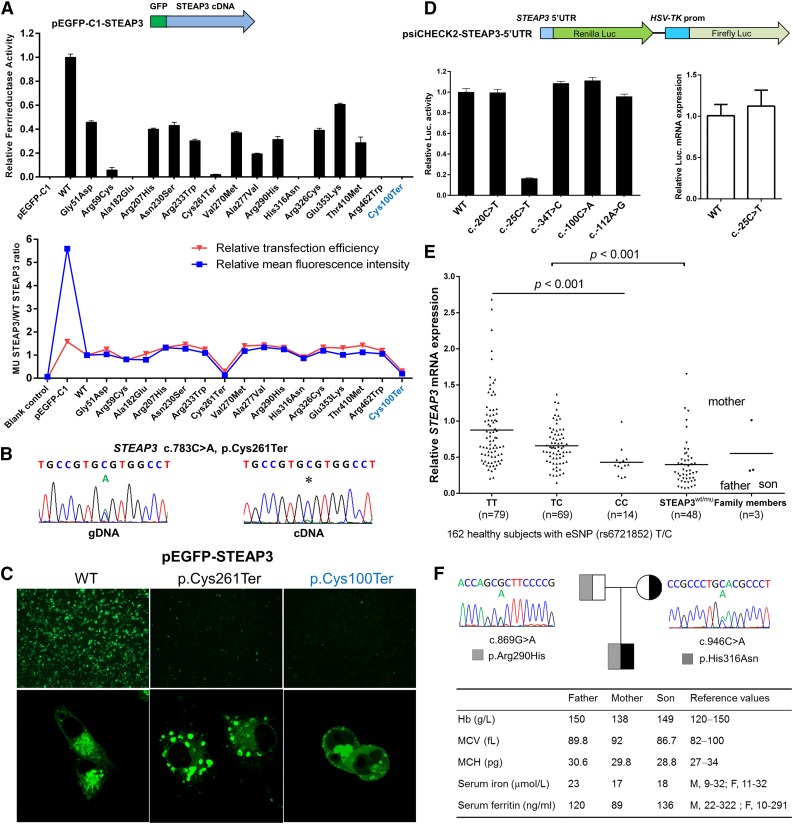
We systematically performed molecular and functional analysis of all variants on STEAP3 and identified 16 different loss-of-function STEAP3 mutations. As shown in Figure 2A, the various mutant STEAP3 proteins exhibited impaired ferrireductase activity (5 severe and 10 moderately reduced). Notably, the p.Arg59Cys and the p.His316Asn mutations completely abrogated iron reduction, as mutations at these 2 sites are implicated in NAD(P)H-binding motif (Ser58 and Arg59) or in heme-binding sites (His316 and His409) of STEAP3.5,6,11,12 Additionally, 2 other human STEAP3 mutants (p.Ala182Glu and p.Arg462Trp) also severely impaired protein function, demonstrating the functional importance of these 2 residues. Surprisingly, the mutant messenger RNA (mRNA) (p.Cys261Ter), while massively degraded, could still be detected by complementary DNA (cDNA) sequencing (Figure 2B). It is likely that either incomplete nonsense-mediated decay or other mechanisms resulted in extremely low ferrireductase activity (Figure 2A) and protein expression in vitro (Figure 2A,C). Subcellular localization of this mutant green fluorescent protein (GFP)-tagged truncated protein revealed abnormal aggregates in the endosomes and on the plasma membrane (Figure 2C). However, all 14 missense mutations had no effect on protein expression (Figure 2A) and exhibited normal cellular localization (data not shown). Figure 2D demonstrates that the luciferase activity was significantly reduced by >80% for the mutant (c.-25C>T), whereas the mRNA level remained relatively stable, suggesting that the mutation in the STEAP3 5′UTR disrupts posttranscriptional regulation. We further measured STEAP3 mRNA levels in human peripheral blood cells by quantitative reverse transcriptase-polymerase chain reaction (RT-PCR). As shown in Figure 2E, considerable variability and a wide range of STEAP3 mRNA expression were observed in the 162 normal individuals, and significantly lower expression levels of STEAP3 were noted in normal samples containing the C allele compared with the T allele at rs6721852 (P < .001), which is the expressed single nucleotide polymorphism (eSNP) in the STEAP3-C2orf76 intergenic region (http://www.gtexportal.org/home/gene/STEAP3).13 The expression levels were lower in all 48 subjects with STEAP3 mutations compared with the 162 normal samples (P < .001). These findings confirm a previous report that STEAP3 is expressed as a quantitative trait locus.10,13,14
Figure 2.
Effects of mutations on STEAP3 function. (A) Transient transfection of HeLa cells with GFP-tagged STEAP3 expression constructs of wild-type (WT) and mutant cDNA to determine relative ferrireductase activity by iron reductase assay. Five mutants completely abrogated this activity, whereas 10 expressed moderately decreased activity compared with the wild-type STEAP3. Similarly transfection efficiency and stable STEAP3 protein expression were noted for all mutant STEAP3s except for 2 nonsense mutants (p.Cys261Ter and p.Cys100Ter) by flow cytometry analysis. (B) Sequence analysis of genomic DNA and cDNA from a case with the STEAP3 nonsense mutation (c.783C>A, p.Cys261Ter) indicated that the mutant allele was massively degraded but still could be detected at the cDNA level. (C) Subcellular localization of GFP-tagged wild-type STEAP3 and the 2 mutants in COS7 cells in images taken at ×4 and ×180 magnification. Confocal microscopy confirmed the endosomal and the plasma membrane localization of the wild-type protein, whereas the p.Cys261Ter mutant formed unusual aggregates, and the p.Cys100Ter mutant localized in both the nucleus and the cytoplasm of COS7 cells. Only a small number of cells exhibited these changes (bottom center/right figures), and most of cultured cells were not detected due to lack of fluorescence. (D) The Renilla luciferase gene was used as a reporter driven by the STEAP3 5′UTR with firefly luciferase as the endogenous control. The −25C>T mutant decreased STEAP3 expression at the protein level, whereas the mRNA levels remained relatively stable compared with the wild type. (E) Quantitative RT-PCR analysis of the STEAP3 mRNA from peripheral blood cells. A cohort of 210 samples randomly selected from southern China was used for mRNA quantification, which included the healthy group (n = 162), consisting of 3 su-groups according to the eSNP allele at rs6721852 (n = 79 in T/T, n = 69 in T/C, and n = 14 in C/C) and the STEAP3 mutant group (n = 48). Three members from a family with compound heterozygous mutations (p.His316Asn and p.Arg290His) are shown in the last column. (F) Study of a family with compound heterozygous STEAP3 mutations. The hematologic parameters (Hb, mean cell volume [MCV], and mean cell hemoglobin [MCH]), serum iron, and serum ferritin are indicated in the table. The p.His316Asn is a severe mutation, whereas the p.Arg290His is a moderate mutation. Data in A, D, and E represent mean ± standard deviation of 3 independent experiments.
Unexpectedly, we observed that there were no significant alterations in erythrocyte hematologic phenotypes in all subjects carrying those STEAP3 heterozygous mutations (79 in group A and 45 in group D; Figure 1A), including 33 individuals with p.Cys261Ter variants (29 in group A and 4 in group D; Figure 1A), as shown in supplemental Table 2. Hypochromic anemia was not found in subjects with STEAP3 mutations or normal individuals with low-level STEAP3 mRNA expression due to the hypomorphic C allele. In addition to this, we also confirmed no changes in the 2 iron metabolic indices, serum iron and serum ferritin, in the individuals with 8 representative heterozygous mutations in STEAP3 (supplemental Table 3). Interestingly, we identified a family (Figure 2F) in which the child carried compound heterozygous mutations (p.His316Asn, a null mutation from the father and p.Arg290His from the mother). There was decreased expression of STEAP3 expression in both the father and the child (Figure 2E). Importantly, the hematologic parameters and iron status were essentially normal despite the compound heterozygosity (Figure 2F). This finding is in marked contrast to a recent report that described a transfusion-dependent severe hypochromic anemia in a family due to a combination of a mutant allele (p.Cys100Ter) and a hypomorphic allele.10 Taking together, these findings suggest that only a very large reduction in enzyme activity or complete absence of activity could lead to the manifestation of the hypochromic erythrocyte phenotypes as suggested by Grandchamp et al.10 Homozygous null STEAP3 mutations such as the most common STEAP3 null variant, p.Cys261Ter, would be sufficient to cause moderately severe congenital hypochromic anemia arising from lack of heme synthesis. However, we were unable to identify such cases in our study. Another possibility is that other pathways of reducing iron in the transferrin-cycle endosome are present in erythroid cells, as evidenced by the previous findings that Steap3−/− reticulocytes in mice still retain some ferrireductase activity.5,15 The mRNA for Steap2 and Steap4, which express ferrireductase activity,16 are indeed consistently and significantly upregulated in bone marrow–derived macrophages and hepatocytes in the absence of Steap3.17
As α-thalassemia is a common inherited hemolytic disorder globally and can cause phenotypic alterations of erythrocytes,18,19 we examined whether STEAP3 mutations have synergetic effects on hematologic indices and clinical severity in α-thalassemia. Unexpectedly, we were unable to document the adverse effects of heterozygous STEAP3 mutations on hematologic phenotypes of the α-thalassemia carriers (supplemental Figure 3). Furthermore, we identified STEAP3 mutations in 22 subjects among the 310 HbH disease patients studied (supplemental Table 4). Also, no adverse effects were noted as a consequence of STEAP3 mutation on the severity of HbH disease (odds ratio = 1.365, P = .499; supplemental Table 5).
Taken together, our findings imply that heterozygous STEAP3 mutations are relatively common in humans and that their deleterious effect in humans remains to be confirmed. The present study, to our knowledge, represents the first comprehensive mapping of genetic alterations in the human STEAP3 gene and shows that reduced ferrireductase activity resulting from STEAP3 heterozygous mutations in humans fails to have a significant effect on heme synthesis.
Footnotes
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank all of the members who participated in this study; Prof Zilong Wen for review of this paper and valuable suggestions; Qiji Liu for assistance in sample collection in northern China; and Yihong Li, Huajie Huang, and Fei He for experiment assistance.
This study was supported by National Natural Science Foundation of China (NSFC)-Guangdong Joint Fund grant NO.U1201222, NSFC grant 81260093, the National Key Technology R&D Program grant 2012BAI09B01, and National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (grant DK26263).
Contribution: Xinhua Zhang, C.Z., R.C., Y.Z., L.L., and F.C. collected samples and clinical data; Xinhua Zhang and X.X. performed clinical classifications, diagnosis, and management of thalassemia; D.L., S.Y., P.F., L.L. Y.L., and F.C. performed laboratory and DNA analysis; Y.Y. and D.L. carried out statistical analyses; Xuelian Zhang and W.Z. did molecular diagnosis of thalassemia; N.M. and X.A. participated in the study design and interpretation of the data and edited the paper; and D.L. and X.X. designed the study, analyzed and interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiangmin Xu, Department of Medical Genetics, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, Guangdong, People's Republic of China; e-mail: gzxuxm@pub.guangzhou.gd.cn.
References
- 1.DeLoughery TG. Microcytic anemia. N Engl J Med. 2014;371(14):1324–1331. doi: 10.1056/NEJMra1215361. [DOI] [PubMed] [Google Scholar]
- 2.Bottomley SS, Fleming MD. Sideroblastic anemia: diagnosis and management. Hematol Oncol Clin North Am. 2014;28(4):653–670. doi: 10.1016/j.hoc.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Donker AE, Raymakers RA, Vlasveld LT, et al. Practice guidelines for the diagnosis and management of microcytic anemias due to genetic disorders of iron metabolism or heme synthesis. Blood. 2014;123(25):3873–3886, quiz 4005. doi: 10.1182/blood-2014-01-548776. [DOI] [PubMed] [Google Scholar]
- 4.Grunewald TG, Bach H, Cossarizza A, Matsumoto I. The STEAP protein family: versatile oxidoreductases and targets for cancer immunotherapy with overlapping and distinct cellular functions. Biol Cell. 2012;104(11):641–657. doi: 10.1111/boc.201200027. [DOI] [PubMed] [Google Scholar]
- 5.Ohgami RS, Campagna DR, Greer EL, et al. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet. 2005;37(11):1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sendamarai AK, Ohgami RS, Fleming MD, Lawrence CM. Structure of the membrane proximal oxidoreductase domain of human Steap3, the dominant ferrireductase of the erythroid transferrin cycle. Proc Natl Acad Sci USA. 2008;105(21):7410–7415. doi: 10.1073/pnas.0801318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lespagnol A, Duflaut D, Beekman C, et al. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008;15(11):1723–1733. doi: 10.1038/cdd.2008.104. [DOI] [PubMed] [Google Scholar]
- 8.Lambe T, Simpson RJ, Dawson S, et al. Identification of a Steap3 endosomal targeting motif essential for normal iron metabolism. Blood. 2009;113(8):1805–1808. doi: 10.1182/blood-2007-11-120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanc L, Papoin J, Debnath G, et al. Abnormal erythroid maturation leads to microcytic anemia in the TSAP6/Steap3 null mouse model. Am J Hematol. 2015;90(3):235–241. doi: 10.1002/ajh.23920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grandchamp B, Hetet G, Kannengiesser C, et al. A novel type of congenital hypochromic anemia associated with a nonsense mutation in the STEAP3/TSAP6 gene. Blood. 2011;118(25):6660–6666. doi: 10.1182/blood-2011-01-329011. [DOI] [PubMed] [Google Scholar]
- 11.Bateman A, Coin L, Durbin R, et al. The Pfam protein families database. Nucleic Acids Res. 2004;32(Database issue):D138–D141. doi: 10.1093/nar/gkh121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleven MD, Dlakić M, Lawrence CM. Characterization of a single b-type heme, FAD, and metal binding sites in the transmembrane domain of six-transmembrane epithelial antigen of the prostate (STEAP) family proteins. J Biol Chem. 2015;290(37):22558–22569. doi: 10.1074/jbc.M115.664565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. GTEx Consortium. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648-660. [DOI] [PMC free article] [PubMed]
- 14.Emilsson V, Thorleifsson G, Zhang B, et al. Genetics of gene expression and its effect on disease. Nature. 2008;452(7186):423–428. doi: 10.1038/nature06758. [DOI] [PubMed] [Google Scholar]
- 15.Ohgami RS, Campagna DR, Antiochos B, et al. nm1054: a spontaneous, recessive, hypochromic, microcytic anemia mutation in the mouse. Blood. 2005;106(10):3625–3631. doi: 10.1182/blood-2005-01-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood. 2006;108(4):1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang F, Tao Y, Zhang Z, et al. Metalloreductase Steap3 coordinates the regulation of iron homeostasis and inflammatory responses. Haematologica. 2012;97(12):1826–1835. doi: 10.3324/haematol.2012.063974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong F, Sun M, Zhang X, et al. Molecular epidemiological survey of haemoglobinopathies in the Guangxi Zhuang Autonomous Region of southern China. Clin Genet. 2010;78(2):139–148. doi: 10.1111/j.1399-0004.2010.01430.x. [DOI] [PubMed] [Google Scholar]
- 19.Piel FB, Weatherall DJ. The α-thalassemias. N Engl J Med. 2014;371(20):1908–1916. doi: 10.1056/NEJMra1404415. [DOI] [PubMed] [Google Scholar]