Abstract
Introduction:
Percutaneous destruction of cancer cells using a radiofrequency energy source has become an accepted part of the modern armamentarium for managing malignancies. Radiofrequency ablation (RFA) is a relatively novel procedure for treating recurrent and metastatic tumors. It is used for debulking tumors and as adjuvant therapy for palliative care apart from its role as a pain management tool. Its use in the third world countries is limited by various factors such as cost and expertise. In the remotest parts of India, where economic development has been slow, abject poverty with poor health care facilities advanced malignancies present a challenge to health care providers. We undertook this study to assess the safety of the percutaneous RFA tumor ablation as a therapeutic or palliative measure in patients where surgery was not possible. We observed that RFA may be an effective, alternative therapeutic modality for some inoperable tumors where other therapeutic modalities cannot be considered.
Context:
Palliative and therapeutic image-guided RFAs of tumors may be the only treatment option in patients who are inoperable for a variety of reasons. To assess the safety and complications of RFA in such a patient population is important before embarking upon any interventions given their physically, mentally, and socially compromised status in a country such as India.
Aims:
To assess the safety of percutaneous image-guided radiofrequency tumor ablation and to note the various immediate and early complications of the intervention.
Settings and Design:
This was a prospective, observational study conducted in Tata Main Hospital, Jamshedpur, Jharkhand, India.
Subjects and Methods:
After approval by the Hospital Approval Committee all patients who consented for percutaneous RFA of their tumor admitted in the hospital were included after taking fully informed consent from patient/close relative keeping the following criteria in view.
Inclusion Criteria:
Patients who were likely to derive a direct benefit in the survival or as a palliative measure for relief in their symptoms and patients who were inoperable because of any of the following reasons: (1) Exhausted conventional treatment options, (2) technical and anatomical contraindications to conventional treatment, (3) medical comorbidities precluding surgery, (4) patient refusal, (5) recurrent tumors, and (6) advanced tumor stage. Conventional Treatment has been defined as surgical resection, radiotherapy, and/or chemotherapy, although the patient eligibility for each treatment may vary.
Exclusion Criteria:
Patients with the following were excluded: (1) Severe coagulopathy, (2) heart, renal, or liver failure, (3) lesions within 1 cm of gall bladder, hilum, bowel wall, and major blood vessels, (4) patient with any metal implant, (5) patients in sepsis, and (6) tumor adjacent to structures at risk (main bile ducts, pericardium, stomach, or bowel).
Results:
The duration of procedure as well as ablation of tumor free margin was significantly related to the size of the tumor. As the size of tumor increased, duration of procedure increased significantly. A good tumor-free margin also needs to be ablated for optimum results as it prevents residual tumors and recurrences in the future. We observed that tumors sized <3.1 cm were optimal in this regard. Most common adverse event in postprocedure period was pain in and around ablation site. Post-RFA syndrome is also a common and benign self-limiting side effect. Patient counseling and proper selection of patients in the early stages of malignancy can enhance the efficacy of the procedure and patient satisfaction.
Conclusions:
Percutaneous image-guided RFA is an option in patients where most other tumor management modalities have been exhausted or rejected. RFA may not be free from side effects such as postablation syndrome, pain, and there may be other serious complications such as bleeding, but based on our observations, percutaneous image-guided RFA of tumors is a safe palliative and therapeutic treatment option.
Keywords: Computed tomography guided, Percutaneous radiofrequency ablation tumor, Ultrasound guided tumor ablation
INTRODUCTION
Radiofrequency ablation (RFA) technology is an evolving modality for tumor management. Percutaneous RFA treatment has several advantages over other cancer management approaches in being the least invasive and producing minimal morbidity.[1] It is relatively safe and can be repeated as necessary to treat recurrent tumors. Percutaneous RFA may also expand surgical options such that it may convert an inoperable patient into a surgical candidate by treating small liver lesions that are too difficult or too spread out to surgically resect.[1]
RFA destroys the tumor by inducing local hyperthermia accompanied by intravascular thrombosis, micro vessel rupture, endothelial apoptosis, and an inhibition of angiogenesis.[2]
Modern medicine encompasses combined diagnostic and therapeutic applications that involve high technology. There are unresolved problems and very importantly there is a lack of relevant data to establish the clinical efficacy of minimally invasive techniques under trial. In our part of the world, due to inadequate health care services, incidence of advanced tumors is very high. The skepticism among doctors regarding efficacy and safety of such procedures, especially in advanced cancers precludes the use of new modalities. We undertook this study to note down the complications during and after performance of tumor ablations by RFA in our hospital which caters to some of the poorest patients in India.
Objectives
To note the cardiovascular complications during procedure such as vasovagal attack, hypotension, or cardiac arrest
Note, complications due to imaging-guided electrode placement, that is, complications due to deleterious heating encountered, other observations, pain and post-RFA syndrome, and assessment of the postablation tumor necrosis.
SUBJECTS AND METHODS
This was a prospective, observational study conducted in TMH, Jamshedpur, Jharkhand, India. After approval by the Hospital Approval Committee, all patients who consented for percutaneous RFA of their tumor admitted in the hospital were included after taking fully informed consent from patient/close relative keeping the following criteria in view.
Inclusion criteria
Patients who were likely to derive a direct benefit in the survival or symptoms and patients who were inoperable because of any of the following reasons:
Poor pulmonary function
Medical comorbidities precluding surgery
Patient refusal
Technical and anatomical contraindications to conventional treatment
Exhausted conventional treatment options
Recurrent tumors
Advanced tumor stage.
Conventional treatment has been defined as surgical resection, radiotherapy, and/or chemotherapy, although the patient eligibility for each treatment may vary.
Exclusion criteria
Patients with the following were excluded:
Severe coagulopathy
Heart, renal, or liver failure
Lesions within 1 cm of gall bladder, hilum, bowel wall, and major blood vessels
Patient with any metal implant
Current infection
Extrahepatic spread with tumor adjacent to structures at risk (main bile ducts, pericardium, stomach, or bowel).
RESULTS
A total of 14 patients, eight males and six females aged above 11 years agreed to undergo RFA for their tumors. Most of these patients, however, were older than 50 years (nine patients). All patients underwent radiological assessment of their tumors. The imaging modality chosen in ten patients was computed tomography (CT) scan and in four patients, ultrasonography (USG). All radiological findings were subsequently confirmed by pathological diagnosis as shown in Table 1. Tabulation of the procedures done is depicted in Table 2.
Table 1.
Confirmation of radiological diagnosis with pathological diagnosis
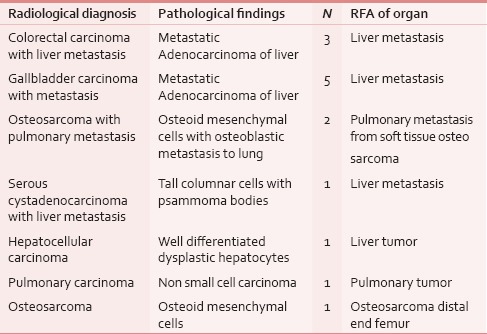
Table 2.
Tabulation of the procedures done
DISCUSSION AND REVIEW OF LITERATURE
We monitored various parameters to assess safety and efficacy of RFA and each of these shall be discussed separately:
Cardiovascular complications during procedure such as vasovagal attack, hypotension, or cardiac arrest
-
Complications due to imaging-guided electrode placement, that is,
-
Bleeding and hemorrhage:
- Damage to adjacent structures, for example,
- Pneumothorax
- Colonic perforation in hepatic ablation
- Bile duct injury.
-
Complications due to deleterious heating encountered at grounding pad sites, that is, electrode pad burns
-
Other observations:
- Pain
- Post-RFA syndrome.
Postablation tumor necrosis.
Cardiovascular complications during procedure
In our study of 14 patients, there was no significant cardiac event requiring intervention of any kind. Meta-analysis of studies done earlier have noted an incidence of 0.13–0.46% of cardiac complications,[3] though no mortality has been attributed directly to these complications in any previous study.
Complications due to imaging-guided electrode placement
Bleeding is an important complication that can occur during or immediately after ablation. We encountered bleeding in only one patient where ablation for debulking of an osteosarcoma of right femur was being done. This accident was due to a procedural error on the part of the operator during track ablation following ablation of the tumor. In this case, all prongs were not withdrawn before removing the electrode and this caused tissue damage with profuse bleeding from the track site. Direct pressure was applied over the site for 15 min, complete hemostasis was assured, and then a pressure bandage was applied. Hemodynamic parameters were constantly monitored and maintained. Dangerous bleeding during procedure has been noted in various studies,[3] and in one case, it has proved to be fatal where retroperitoneal hemorrhage could not be resuscitated.
In 2003, a Korean study group of RFA which surveyed 11 institutions reported that incidence of major bleeding was around 0.46%.[3] These results, however, cannot be compared with our results as we have monitored other tumor ablations apart from the liver ablations. In our series of nine ablations of inoperable liver tumors, we did not come across any major hemorrhage.
Damage to adjacent structures such as lung parenchyma with resultant pneumothorax,[3,4] diaphragmatic injury or thoracic duct injury during lung tumor ablation, or injury to bowel and bile duct during ablation of liver tumors can be a danger.
An international survey from seven institutions reported that the most common adverse event after percutaneous lung RFA was pneumothorax (30%) and that approximately 10% of cases required a chest drain.[5]
One out of three of our patients undergoing lung metastasis ablation originating from an osteosarcoma of right distal femur immediately after tumor ablation developed pneumothorax, which was minimal and asymptomatic, so we put the patient on 100% oxygen via a nonrebreathing mask and obtained an immediate, 2 h and 6 h post-procedure chest X-ray. There was no increase in size of the pneumothorax and so decision to put a chest tube was deferred. Serial radiographs were done for the case and the pneumothorax resolved without further intervention. Belfiore et al.[6] had observed minor pneumothorax in 9% of cases undergoing RFA for lung tumors and also observed reactionary minor pleural effusion in 9% cases.
We observed pleural effusion in two out of the three patients (66%) who had undergone the procedure. Thoracocentesis was not done in either case. The important consideration in cases of unresolving pleural effusions is the possibility of a thermal injury to adjacent diaphragm resulting in a diaphragmatic fistula.[7] Thorough follow-up and evaluation are required in such cases, a simple examination of patient's sputum could be an important clue. Surgical repair with prosthetic mesh reconstruction of the diaphragm may be required in severe cases.[7] Right-sided pleural effusions are also common after liver tumor ablations, though most of them resolve spontaneously.
Gastrointestinal perforation was not encountered in any of our patients, however, Livraghi et al. observed this complication in 0.7% of his cases undergoing liver ablations.[8] Subcapsular lesions carry a high risk of colonic perforation.[9] Colon is a relatively thin-walled and fixed structure which predisposes it to perforation, especially in cases where previous laparotomy has been done and where adhesions prevent any further movement of the gut.
Bile duct injury was not immediately evident in any of the patients we ablated. Rhim et al. had observed 0.07–1% incidence of bile duct injury during ablation of hepatic tumors in their study.[3] Long-term follow-up, however, was not done in our study to assess for presence of any symptoms suggestive of the same. Bile duct injury can result in biliary strictures[3,10] or biliomas[3,11] and they can present late in post-procedure period. Close proximity of tumor mass to the major bile ducts is a major reason for bile duct injuries. Prevention of the heat sink effect[12] described later, might be another cause where, to avoid a major blood vessel, one might compromise on the distance of the ablation electrode[13,14] from the bile duct. Percutaneous biliary drainage procedures might be required in severe cases of strictures.
Pad burn
We had intended to monitor any adverse effects of heating during the procedure. In one of our cases of osteosarcoma distal right femur, the pad was placed on the right calf covering the circumference. Patient suffered second degree pad burn on the right calf, which appeared as blister on first post-procedure day at the site of the electrode pad. This pad burn was probably because distance of the active electrode from pad was <25 cm and not enough surface area was available for heat dispersion. Pad burns may present as hyperemia at the site immediate post-procedure.
In another patient, an innovation with respect to pad placement was necessitated where ablation was done for pulmonary metastases secondary to an osteosarcoma of the right distal end femur. This patient had mid-thigh amputation for the primary lesion. We did not have this thigh for application of pad, so gluteal region was chosen as the site of application of pad avoiding any bony prominences, for example, anterior superior iliac spine. The pad covered the entire gluteal region as shown in Figure 1. The large pad area relative to the thigh resulted in the pad losing its contact with the skin surface, which is not recommended.[15] We thus tied the pad with a long sheet of gauge for proper contact with the surface as shown in Figure 1. This indigenous adaptation proved to be safe and effective as no burn was seen post-procedure, although there was a variation from vendor protocol[16] as both the thigh pads could not be placed at the same level.
Figure 1.
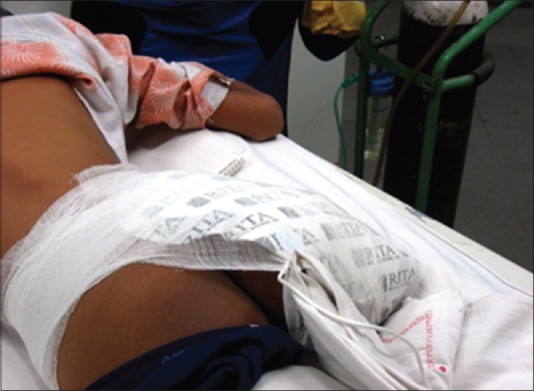
Variation of dispersive electrode placement in amputated right leg
Other observations
Pain
Pain was the most common complaint of patients during the study [Table 3]. Pain assessment was done post-procedure based on the Common Toxicity Criteria of the National Cancer Institute's[17] grading of pain. Pain killers were administered during and immediately afterward by the attending anesthesiologist. After discharge, analgesics were given as per individual needs and physiology. All but one of the 14 patients complained of pain around the site of ablation once they fully recovered from anesthesia and was alert and oriented. Pain was again assessed at 4 h post-procedure. Rescue analgesics were ordered once patient complained of grade 2 or more pain. Regular pain assessment was done thereafter. Pain scores in our patients ranged from mild to moderate, that is, grade 1 to grade 2 pain. Ten patients had moderate pain and three had mild grade 1 pain [Table 4] at the time of recovery. Further follow-up telephonically confirmed that pain at site, even though mild, lasted for more than a week in liver ablation cases and was the most common reason for the patients getting in contact with us in the post-procedure period. Since we did not measure preoperative pain scores, a comparison between the pre- and post-procedure pain scores could not be done.
Table 3.
Common complications noted in the peri-procedure period

Table 4.
Pain scoring noted after RFA of tumors

Postablation syndrome
The most common minor complication of RFA is postablation syndrome and it was our objective to monitor the incidence of this complication. Post-RFA syndrome was defined as the presence of either fever or flu-like symptoms or both any time after the procedure.
There were six cases (42.86%) that developed fever, four patients (28.6%) developed flu-like symptoms of myalgia, nausea, malaise, or light-headedness. In our study, all patients who developed flu-like symptoms also had fever. Six patients had only fever without other symptoms. The total incidence in our study was, therefore, roughly 71%. Four patients (28.6%) were asymptomatic.
Follow-up of patients was done telephonically once they were discharged from hospital on days 3, 5, 7, and 10 post-procedure. All the patients were advised to maintain an 8 hourly check of temperature. Onset of fever was typically seen within the first 36 h in most patients, though in one patient fever onset was noted after three days of procedure. Fever persisted till the 4th to 6th post-procedure day and resolved in all cases by the 6th day. Oral paracetamol was advised if temperature reached 100 F. Four patients presented with flu-like symptoms commencing around 36–48 h after procedure and these symptoms resolved completely by the 8th post-procedure day in three patients. One patient with flu-like symptoms could not be traced as he requested discharge 2 days after procedure and was subsequently lost to follow-up after 7th post-procedure day. In literature, incidence of this syndrome has been observed in approximately one-third patients. The results that we observed are quite similar to these observations by Wah et al.[18]
Patients should be informed that these symptoms are self-limiting after RFA and most patients should be able to resume near-complete pre procedural levels of activity within 10 days after the procedure.
Postablation tumor necrosis
In our study, assessment of postablation necrosis could be done for 10 cases which were ablated with CT guidance. We performed noncontrast CT and contrast-enhanced CT at the end of procedure to determine the extent of coagulation. Goal of tumor ablation is to obtain a well-defined smooth hypodense area which is at least as large as tumor and ideally a few millimeter larger. We noted that tumors which were <3.1 cm had an area of necrosis larger than the parent tumor dimensions as in Figure 2. Tumors that are more than this diameter revealed inconsistent ablation of tumor-free margin. This is consistent with the findings of Dodd et al., who have done extensive work on this aspect.[19] We also attempted ablation of a large osteosarcoma of femur.
Figure 2.
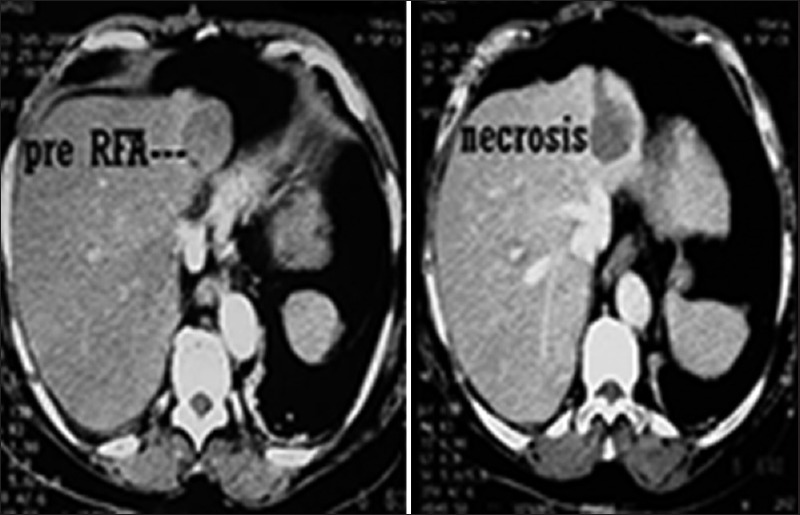
Pre- and immediate post-radiofrequency ablation necrosis of small tumor (approximately 3 cm)
USG guidance was used for RFA of some lesions in the liver, but it was not used for postablation tissue necrosis size estimation because of the bubble effect and poor delineation of the margin.[17] Gray scale ultrasound alone cannot differentiate between viable tumor and necrotic tissue because of variable echogenicity due to overlap of shape and volume of necrosis.[20] Incidentally, no patient who had undergone tumor ablation using ultrasound guidance turned up for follow-up CT imaging which was advised after 4 weeks.
Immediate post-procedure imaging estimation of necrosis size does not ensure the efficacy of treatment.[21,22] Follow-up imaging is necessary to rule out any recurrence and residual tumor which can be possible in some large tumors as well as those ablations where tumor-free margins were not ablated. In postcontrast CT, there is a thin rim of hyperdense[21,23] area visible due to hyperemia in the immediate post-procedure period and this can mask a residual tumor. We observed a hyperdense area in one liver, lung, and osteosarcoma of femur ablation each. Constraints of money and poor general condition of the patients that we encountered precluded multiple ablation sittings and imaging follow-up in most cases. For most patients and even medical fraternity members, this modality of treatment is new and relatively unknown and untested in our part of the country. Most of the patients agreed to RFA as a last resort and we observed that if the relief of symptoms were not as per expectations, it deterred many patients from returning for follow-up imaging.
CONCLUSION
The duration of procedure was significantly related to the size of the tumor based upon the Spearman's correlation test at the 0.01 level (two-tailed.)
Ablation of tumor-free margin is significantly related to the size of tumor. As size of tumor increased, size of tumor free margin ablated decreased.
Tumors <3.1 cm were optimal for ablation as they afforded ablation of a safe margin of tumor-free tissue around the tumor which is necessary to prevent residual tumors and to prevent recurrences in the future.
Most common adverse event in post-procedure period was pain in and around ablation site. Patient counseling and proper selection of patients in early stages of malignancy can enhance the efficacy of the procedure and patient satisfaction. Awareness regarding the procedure, especially among the health care professionals is, therefore, important.
SUMMARY
RFA as a minimally invasive procedure may be a safe treatment option in patients suffering from life-threatening malignancies. We observed that even in terminally ill patients, we did not encounter any complication which could put life of our patient in immediate jeopardy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bilchik AJ, Faries M. Radiofrequency ablation of hepatic malignancies: Inexpensive and minimally invasive but should it replace resection? Ann Surg Oncol. 2003;10:1002–4. doi: 10.1245/aso.2003.09.915. [DOI] [PubMed] [Google Scholar]
- 2.Lencioni R, Crocetti L. Radiofrequency Ablation. In: Van Sonnenberg E, MacMullen W, Solbiati L, editors. Tumor ablation: Principles and practice. New York, NY: Springer-Verlag; 2005. pp. 205–15. [Google Scholar]
- 3.Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: Spectrum of imaging findings. Radiographics. 2003;23:123–34. doi: 10.1148/rg.231025054. [DOI] [PubMed] [Google Scholar]
- 4.Helmberger TK. Radiofrequency Ablation Thermal Ablative techniques. In: Vogl TJ, Helmberger TK, Mack MG, Resier MF, editors. Percutaneous tumor ablation in medical radiology. Berlin, Germany: Springer; 2008. pp. 7–21. [Google Scholar]
- 5.Steinke K, Sewell PE, Dupuy D, Lencioni R, Helmberger T, Kee ST, et al. Pulmonary radiofrequency ablation – An international study survey. Anticancer Res. 2004;24:339–43. [PubMed] [Google Scholar]
- 6.Belfiore G, Moggio G, Tedeschi E, Greco M, Cioffi R, Cincotti F, et al. CT-guided radiofrequency ablation: A potential complementary therapy for patients with unresectable primary lung cancer – A preliminary report of 33 patients. AJR Am J Roentgenol. 2004;183:1003–11. doi: 10.2214/ajr.183.4.1831003. [DOI] [PubMed] [Google Scholar]
- 7.Head HW, Dodd GD, 3rd, Dalrymple NC, Prasad SR, El-Merhi FM, Freckleton MW, et al. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: Frequency of diaphragmatic injury. Radiology. 2007;243:877–84. doi: 10.1148/radiol.2433060157. [DOI] [PubMed] [Google Scholar]
- 8.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: Radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–8. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 9.Rhim H, Goldberg SN, Dodd GD, 3rd, Solbiati L, Lim HK, Tonolini M, et al. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics. 2001;21:S17–35. doi: 10.1148/radiographics.21.suppl_1.g01oc11s17. [DOI] [PubMed] [Google Scholar]
- 10.Rhim H, Dodd GD, 3rd, Chintapalli KN, Wood BJ, Dupuy DE, Hvizda JL, et al. Radiofrequency thermal ablation of abdominal tumors: Lessons learned from complications. Radiographics. 2004;24:41–52. doi: 10.1148/rg.241025144. [DOI] [PubMed] [Google Scholar]
- 11.Cheung L, Livraghi T, Solbiati L, Dodd CD., 3rd . Complications of Tumor Ablation. In: Van Sonnenberg E, MacMullen W, Solbiati L, editors. Tumor ablation: Principles and practice. New York, NY: Springer-Verlag; 2005. pp. 440–51. [Google Scholar]
- 12.Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267–74. doi: 10.1097/01.rvi.0000092666.72261.6b. [DOI] [PubMed] [Google Scholar]
- 13.Ogan K, Jacomides L, Dolmatch BL, Rivera FJ, Dellaria MF, Josephs SC, et al. Percutaneous radiofrequency ablation of renal tumors: Technique, limitations, and morbidity. Urology. 2002;60:954–8. doi: 10.1016/s0090-4295(02)02096-4. [DOI] [PubMed] [Google Scholar]
- 14.Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206–22. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg SN, Solbiati L, Halpern EF, Gazelle GS. Variables affecting proper system grounding for radiofrequency ablation in an animal model. J Vasc Interv Radiol. 2000;11:1069–75. doi: 10.1016/s1051-0443(07)61341-4. [DOI] [PubMed] [Google Scholar]
- 16.Rita Medical System; Radiofrequency Ablation Procedure Guide. 2006:1–100. [Google Scholar]
- 17.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, 3rd, Dupuy DE, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria. Radiology. 2005;235:728–39. doi: 10.1097/01.RVI.0000170858.46668.65. [DOI] [PubMed] [Google Scholar]
- 18.Tze M. Wah, Ronald S. Arellano, Debra A, Gervais, et al. Image-guided Percutaneous Radiofrequency Ablation and Incidence of Post–Radiofrequency Ablation Syndrome: Prospective Survey Radiology. 2005;237:1097–11021. doi: 10.1148/radiol.2373042008. [DOI] [PubMed] [Google Scholar]
- 19.Dodd GD, 3rd, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: Computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001;177:777–82. doi: 10.2214/ajr.177.4.1770777. [DOI] [PubMed] [Google Scholar]
- 20.Leyendecker JR, Dodd GD, 3rd, Halff GA, McCoy VA, Napier DH, Hubbard LG, et al. Sonographically observed echogenic response during intraoperative radiofrequency ablation of cirrhotic livers: Pathologic correlation. AJR Am J Roentgenol. 2002;178:1147–51. doi: 10.2214/ajr.178.5.1781147. [DOI] [PubMed] [Google Scholar]
- 21.Dromain C, de Baere T, Elias D, Kuoch V, Ducreux M, Boige V, et al. Hepatic tumors treated with percutaneous radio-frequency ablation: CT and MR imaging follow-up. Radiology. 2002;223:255–62. doi: 10.1148/radiol.2231010780. [DOI] [PubMed] [Google Scholar]
- 22.Kim SK, Lim HK, Kim YH, Lee WJ, Lee SJ, Kim SH, et al. Hepatocellular carcinoma treated with radio-frequency ablation: Spectrum of imaging findings. Radiographics. 2003;23:107–21. doi: 10.1148/rg.231025055. [DOI] [PubMed] [Google Scholar]
- 23.Choi H, Loyer EM, DuBrow RA, Kaur H, David CL, Huang S, et al. Radio-frequency ablation of liver tumors: Assessment of therapeutic response and complications. Radiographics. 2001;21:S41–54. doi: 10.1148/radiographics.21.suppl_1.g01oc08s41. [DOI] [PubMed] [Google Scholar]


