Abstract
The stable propagation of genetic material during cell division depends on the congression of chromosomes to the spindle equator before the cell initiates anaphase. It is generally assumed that congression requires that chromosomes are connected to the opposite poles of the bipolar spindle (“bioriented”). In mammalian cells, we found that chromosomes can congress before becoming bioriented. By combining the use of reversible chemical inhibitors, live-cell light microscopy, and correlative electron microscopy, we found that monooriented chromosomes could glide toward the spindle equator alongside kinetochore fibers attached to other already bioriented chromosomes. This congression mechanism depended on the kinetochore-associated, plus end–directed microtubule motor CENP-E (kinesin-7).
Successful cell division requires proper “biorientation” of chromosomes, whereby microtubule bundles (K fibers) connect sister kinetochores of each chromosome to opposite spindle poles (1). Biorientation errors are linked to chromosome loss and cancers (2). Formation of sister K fibers occurs asynchronously (3), and once a kinetochore captures microtubules growing from a spindle pole, the chromosome is transported toward this pole and becomes “monooriented” (4). Monooriented chromosomes remain near the spindle pole for variable times (3, 4) until they suddenly “congress” to the spindle equator. Current models of mitotic spindle formation (5, 6) postulate that chromosome congression occurs as the result of biorientation (7).
We followed movements of individual chromosomes in mammalian cells by differential interference contrast (DIC) time-lapse microscopy (8). In addition to the chromosome oscillations that occur toward and away from spindle poles, we frequently observed monooriented chromosomes making direct movements to the metaphase plate as if they were attempting to congress (fig. S1). Centromeres on these congressing chromosomes were frequently stretched, which indicated force generation by the leading kinetochore (Movie S1). However, these movements did not always result in a stable alignment on the metaphase plate, because chromosomes often returned to the spindle pole after a 3- to 4-μm excursion. This chromosome behavior was observed in essentially every cell we imaged and has also been previously reported (9–12). To determine whether these chromosomes were bioriented, we followed mitotic cells by DIC microscopy until one of the chromosomes exhibited an extended linear movement toward the metaphase plate, and we fixed the cell when the chromosome had almost reached the metaphase plate (~5 to 7 μm from the proximal spindle pole) (Fig. 1; Movie S2). Three of five chromosomes analyzed by electron microscopy (EM) (8) were already bioriented, as expected for congressing chromosomes (7). However, in the other two cases, no microtubules emanated from the leading kinetochore plate on the congressing chromosome. Instead, this kinetochore laterally interacted with microtubules of a mature K fiber attached to a kinetochore of another bioriented chromosome positioned on the metaphase plate (Fig. 1D). The trailing kinetochore was attached to the proximal spindle pole via a mature K fiber. This unexpected type of kinetochore-microtubule interaction suggested that chromosomes may not need to be bioriented during congression.
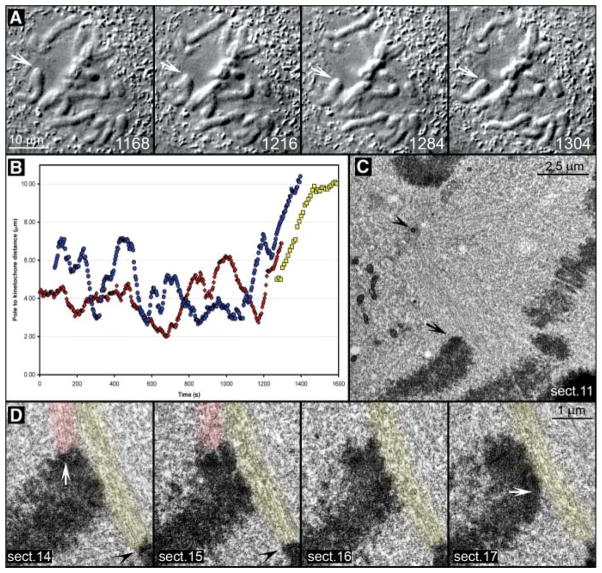
Fig. 1.
Leading kinetochores are not properly attached to microtubules during a chromosome’s attempt to congress. (A) Selected frames from a DIC time-lapse recording (also see Movie S2). The cell was fixed as one chromosome (arrows) moved toward the spindle equator (1304 s). (B) Distance versus time plot confirmed that the chromosome’s movement (red curve) was typical for chromosome congression [compare with blue and yellow curves, which represent movements of chromosomes shown in fig. S1 and Fig. 2, respectively]. (C) Lower-magnification EM image of the cell showing the position of the chromosome of interest (arrow) with respect to the spindle pole (arrowhead). (D) Selected 100-nm EM section from a full series through the centromere region of the chromosome. Note the prominent bundle of microtubules (highlighted red) connecting the trailing kinetochore (white arrow in section 14) to the proximal spindle pole. These microtubules approached the kinetochore at ~90° angle and terminated within the trilaminar kinetochore plate. By contrast, the leading kinetochore (white arrow, section 17) lacked attached microtubules but was laterally associated with a mature kinetochore fiber (highlighted yellow) that was attached to the kinetochore of a bioriented chromosome (black arrowheads in sections 14 and 15) positioned on the metaphase plate.
Because individual K fibers are not resolved by DIC microscopy, we could not correlate the trajectory of an individual chromosome moving toward the spindle equator with the positions of surrounding K fibers. To overcome this limitation, we simultaneously imaged both microtubules and kinetochores by live-cell dual-channel fluorescence microscopy. PtK1 epithelial cells derived from the marsupial rat kangaroo, Potorous tridactylis, were coinjected with a fluorescein-conjugated antibody against the kinetochore protein CENP-F that does not perturb its function (to label kinetochores) and X-rhodamine–conjugated αβ-tubulin (to label microtubules) (13). In 12 of the 49 cells analyzed, we found at least one monooriented chromosome whose trajectory, during the movement toward the spindle equator, precisely followed K fibers of other, already bioriented chromosomes (Fig. 2; Movie S3). This pattern indicated that such congressing chromosomes were not simply ejected away from the pole by the spindle ejection force acting on the entire chromosome (11, 14), but glided on the microtubules of mature K fibers.
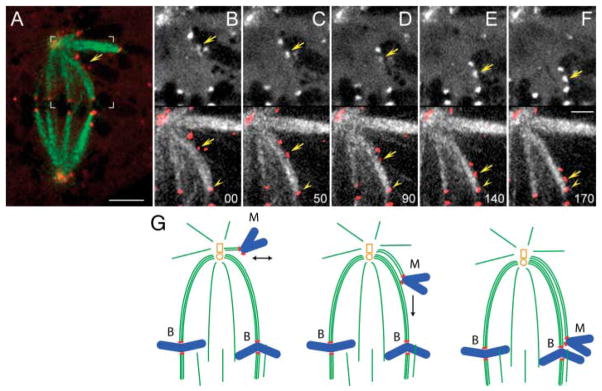
Fig. 2.
Monooriented chromosomes are transported toward the spindle equator along kinetochore fibers of other chromosomes. (A) Two-color fluorescence image of a live PtK1 cell in which kinetochores were labeled with CENP-F/Alexa488 (red) and microtubules with tubulin/rhodamine (green). Area marked with white brackets is enlarged in (B to F). (B to F) selected frames from the two-color time-lapse recording. In each frame, CENP-F/Alexa488 fluorescence (kinetochores) is shown alone (top) and overlaid in red on microtubules (bottom). Arrows mark the kinetochore that moved toward the spindle equator. Note that trajectory of this kinetochore coincided with a prominent kinetochore fiber that extended from the spindle pole to a kinetochore on a bioriented chromosomes already positioned on the metaphase plate (arrowhead). Time in seconds. Scale bars: (A) 5 μm, (F) 2.5 μm. (G) Schematic illustrating the sequence of events presented in (B to F).
The time a monooriented chromosome spends at a spindle pole is variable, and the number of attempts it makes before finally achieving stable positioning on the metaphase plate is unpredictable (15). To examine the state of kinetochore-microtubule interaction during the first congression attempt, we established an experimental system in which several chromosomes congressed in a single cell within a narrow time window (Fig. 3A). We combined high-resolution imaging and chemical inhibitors to manipulate chromosome positions in dividing cells. Cells were treated with monastrol, a small-molecule inhibitor of the kinesin Eg5 (kinesin 5). This treatment blocked cells in monopolar mitosis with high incidence of syntelic (both sister kinetochores attached to the same spindle pole) chromosomes (16). Then, cells were released from monastrol into an Aurora kinase inhibitor. Under these conditions, spindles bipolarized while many chromosomes remained syntelic (17). Relief from Aurora kinase inhibition resulted in the transport of syntelic chromosomes to spindle poles from where they congressed to the metaphase plate (17). This assay allowed us to accumulate monooriented chromosomes whose congression was temporally controlled through washout of cell-permeable chemical inhibitors.
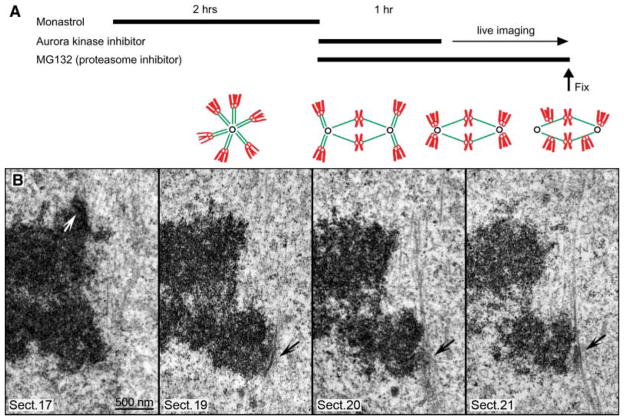
Fig. 3.
Leading kinetochores are laterally associated with kinetochore fibers of other chromosomes during chromosome congression. (A) Protocol for inducing synchronous chromosome congression. Cells were arrested in mitosis with monastrol to accumulate monopolar mitosis with high incidence of syntelic chromosomes (green, microtubules; red, chromosomes). Then, monastrol was removed and Hesperadin was added with MG132 for 1 hour. This resulted in spindle bipolarization, although many chromosomes remained syntelic. After 1 hour, Hesperadin was removed, and cells were imaged live until fixation. Removal of Hesperadin resulted in simultaneous correction of syntelic attachments. Syntelic chromosomes moved to the pole, became monooriented, and then attempted to congress. (B) Selected 100-nm EM sections from a full series through the centromere of a congressing chromosome. Note that, similarly to untreated cells (Fig. 1), chromosomes congressed with their leading kinetochores unattached (black arrows) but slide alongside mature kinetochore fibers of other chromosomes. By contrast, trailing kinetochores (white arrow) were always attached to prominent kinetochore fibers that terminated within the trilaminar plate.
Using this assay, we imaged individual cells by time-lapse DIC and spinning-disk confocal microscopy. Once several monooriented chromosomes initiated their movement toward the metaphase plate, the cell was fixed for correlative serial-section EM analysis. On six out of seven congressing chromosomes analyzed by this approach, the leading kinetochore was laterally associated with a mature K fiber that extended from a different bioriented chromosome toward the proximal spindle pole (Fig. 3B; fig. S2). By contrast, the trailing kinetochore was attached in typical tip-on fashion to a K fiber connected to the proximal spindle pole (Fig. 3B; fig. S2). Thus, ~85% of chromosomes lacked microtubule attachments to the distal spindle pole (that is, remained monooriented) during congression in our experimental system. Importantly, centromeres on the congressing chromosomes were stretched (>2 μm) (Fig. 3B; fig. S2), which indicated a force acting at the leading kinetochore.
Chromosomes fixed before initiating congression were either syntelic [five out of six (fig. S3)] or monooriented [one of six (fig. S4)]. In the latter case, one of the kinetochores was connected to the pole by a K fiber, while its sister was laterally associated with a bundle of microtubules bypassing the kinetochore and extending toward the spindle equator (fig. S4). This configuration, once again, suggests that chromosome congression can be initiated by sliding of the unattached kinetochores alongside mature K fibers.
We next considered the molecular mechanisms responsible for congression of monooriented chromosomes. Because microtubule polarity within a K fiber is uniform (18), this movement is likely to depend on a motor protein that transports cargo toward the microtubule plus ends. Further, this motor must be concentrated at kinetochores during prometaphase. CENP-E (a member of the kinesin-7 family) is the only plus end–directed motor that meets both criteria (19, 20). Depleting CENP-E in human cells results in a mitotic arrest, with significant numbers of monooriented chromosomes positioned very close to the spindle pole (21, 22). In addition, recombinant CENP-E binds the sides of microtubule bundles in vitro (23). We used our chemical inhibitor–based assay and small interfering RNA (siRNA) to determine whether CENP-E was responsible for the congression mechanism observed for monooriented chromosomes. Because the rat kangaroo CENP-E has not yet been cloned, we used human cells for these experiments.
Multiple syntelic chromosomes were observed after spindle bipolarization in the presence of an Aurora kinase inhibitor in control and CENP-E–depleted HeLa cells (Fig. 4, A and B). Thus, kinetochores remained capable of capturing microtubules in the absence of CENP-E. Within 1 hour after Aurora kinase activation by inhibitor removal, all chromosomes were positioned at the metaphase plate in 73 ± 2% of the controls but only in 15 ± 7% of CENP-E–depleted cells (four experiments, >80 cells per experiment; Fig. 4, C to E). At the same time, the number of polar chromosomes in CENP-E–depleted cells increased dramatically (from 3.4 ± 0.7 to 10.5 ± 1.3) after Aurora activation (Fig. 4F). Thus, syntelic chromosomes that reside at a substantial distance from their poles move to the pole and become monooriented after activation of Aurora kinase in CENP-E–depleted cells. However, these monooriented chromosomes are not subsequently transported to the metaphase plate in the absence of CENP-E. Hence, depletion of CENP-E does not affect chromosome attachment to the proximal spindle pole. Instead, it diminishes the probability for monooriented chromosomes to be transported from the spindle pole toward the spindle equator, where they can acquire connections to the distal pole and become bioriented. Our EM data revealed that sliding of kinetochores toward the plus ends of mature K fibers is a major mechanism for aligning monooriented chromosomes positioned near a pole, and this mechanism is missing in the absence of CENP-E. This defect explains why persistent monooriented chromosomes positioned very close to the spindle pole have been found consistently in CENP-E–deficient cells (19, 21, 22, 24).
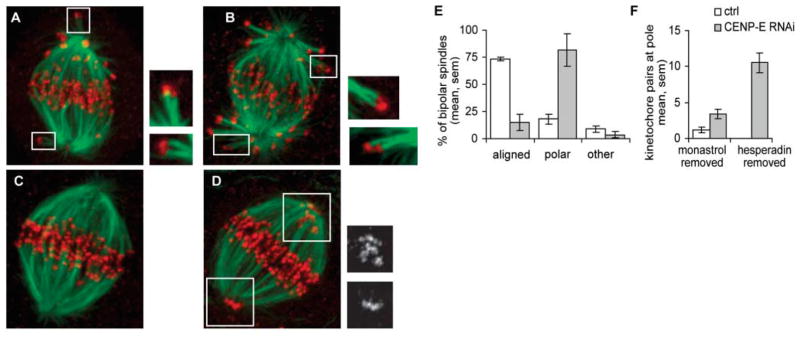
Fig. 4.
CENP-E is required for congression of monooriented chromosomes. Twenty-four hours after transfection, with mock (control) or CENP-E siRNA, synchronous chromosome congression was induced using the chemical inhibition and reactivation approach described in Fig. 3. Cells were fixed either after 1 hour in Hesperadin (A and B) or 1 hour after removal of Hesperadin (C to F) and processed for immunostaining [tubulin, green; kinetochores (CREST), red]. In the presence of Hesperadin, syntelic attachments were observed in both control (A) and CENP-E–depleted cells (B). After removal of Hesperadin, chromosomes congressed to the metaphase plate in control cells (C), but monooriented chromosomes were observed near spindle poles in CENP-E–depleted cells (Insets in D) CREST staining. For cells fixed 1 hour after removal of Hesperadin, bipolar spindles were counted and classified as fully aligned or containing polar chromosome(s) (E) (average of four experiments). To quantify the number of chromosomes at the pole, the number of kinetochore pairs with no detectable K fiber was counted in three-dimensional confocal images [(F), averages from 19 CENP-E–depleted or 7 control cells for each condition, two experiments]. All images presented as maximal-intensity projections. (Insets in A and B) Optical sections at 2× magnification.
Our findings address a long-standing question in cell division. It was unclear how chromosome accumulation at spindle poles in prometaphase and during correction of syntelic attachments leads to biorientation (25). Our data reveal that at spindle poles, monooriented chromosomes are likely to find mature K fibers that are attached to other, already bioriented chromosomes and to congress alongside these K fibers via a CENP-E–dependent mechanism. In this congression mechanism, the probability that a monooriented chromosome will be transported toward the spindle equator progressively increases as more and more chromosomes become bioriented, which increases the density of K fibers in the spindle. Thus, chromosome congression is a cooperative process that depends on chromosome positions relative to the mitotic spindle and is promoted for those chromosomes that remain monooriented as the spindle fully assembles and establishes a metaphase plate. Abrogating this cooperativity would mostly affect chromosomes that congress late during spindle formation, thereby inducing loss of one or two chromosomes, as has been observed after CENP-E depletion in murine cells (26).
Supplementary Material
Acknowledgments
We acknowledge use of Wadsworth Center’s EM core facility. Supported by grants from the NIH (GM59363 to A.K., GM65933 to T.M.K., GM24364 to E.D.S, and GM06627 to B.F.M.) and NSF (MCB 0110821 to B.F.M.).
Footnotes
References and Notes
- 1.Rieder CL, Salmon ED. Trends Cell Biol. 1998;8:310. doi: 10.1016/s0962-8924(98)01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajagopalan H, Lengauer C. Nature. 2004;432:338. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 3.Roos UP. Chromosoma. 1976;54:363. doi: 10.1007/BF00292816. [DOI] [PubMed] [Google Scholar]
- 4.Rieder CL, Alexander SP. J Cell Biol. 1990;110:81. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirschner M, Mitchison T. Cell. 1986;45:329. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- 6.Murray AW, Mitchison TJ. Curr Biol. 1994;4:38. doi: 10.1016/s0960-9822(00)00007-5. [DOI] [PubMed] [Google Scholar]
- 7.McEwen BF, Heagle AB, Cassels GO, Buttle KF, Rieder CL. J Cell Biol. 1997;137:1567. doi: 10.1083/jcb.137.7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Materials and methods are available as supporting material on Science Online.
- 9.Skibbens RV, Rieder CL, Salmon ED. J Cell Biol. 1993;122:859. doi: 10.1083/jcb.122.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waters JC, Skibbens RV, Salmon ED. J Cell Sci. 1996;109:2823. doi: 10.1242/jcs.109.12.2823. [DOI] [PubMed] [Google Scholar]
- 11.Khodjakov A, Rieder CL. J Cell Biol. 1996;135:315. doi: 10.1083/jcb.135.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khodjakov A, Cole RW, McEwen BF, Buttle KF, Rieder CL. J Cell Biol. 1997;136:229. doi: 10.1083/jcb.136.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cimini D, Cameron LA, Salmon ED. Curr Biol. 2004;14:2149. doi: 10.1016/j.cub.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 14.Rieder CL, Salmon ED. J Cell Biol. 1994;124:223. doi: 10.1083/jcb.124.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieder CL, Schultz A, Cole R, Sluder G. J Cell Biol. 1994;127:1301. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ. J Cell Biol. 2000;150:975. doi: 10.1083/jcb.150.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM. Nat Cell Biol. 2004;6:232. doi: 10.1038/ncb1102. [DOI] [PubMed] [Google Scholar]
- 18.Euteneuer U, McIntosh JR. J Cell Biol. 1981;89:338. doi: 10.1083/jcb.89.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood KW, Sakowicz R, Goldstein LSB, Cleveland DW. Cell. 1997;91:357. doi: 10.1016/s0092-8674(00)80419-5. [DOI] [PubMed] [Google Scholar]
- 20.Yao X, Anderson KL, Cleveland DW. J Cell Biol. 1997;139:435. doi: 10.1083/jcb.139.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaar BT, Chan GKT, Maddox P, Salmon ED, Yen TJ. J Cell Biol. 1997;139:1373. doi: 10.1083/jcb.139.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwen BF, et al. Mol Biol Cell. 2001;12:2776. doi: 10.1091/mbc.12.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao Y, Desai A, Cleveland DW. J Cell Biol. 2005;170:873. doi: 10.1083/jcb.200505040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Putkey FR, et al. Dev Cell. 2002;3:351. doi: 10.1016/s1534-5807(02)00255-1. [DOI] [PubMed] [Google Scholar]
- 25.Hauf S, Watanabe D. Cell. 2004;119:317. doi: 10.1016/j.cell.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Weaver BA, et al. J Cell Biol. 2003;162:551. doi: 10.1083/jcb.200303167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.