Abstract
Double-unit cord blood (DCB) grafts are a rapidly available stem cell source for adults with high-risk leukemias. However, how disease-free survival (DFS) after DCB transplantation (DCBT) compares to that of unrelated donor transplantation (URDT) is not fully established. We analyzed 166 allograft recipients (66 8/8 HLA-matched URDT, 45 7/8 HLA-matched URDT, 55 DCBT) aged 16–60 years with high-risk acute leukemia or chronic myelogenous leukemia (CML). URDT and DCBT recipients were similar except DCBT recipients were more likely to have lower weight, non-European ancestry, and receive intermediate intensity conditioning. All URDT recipients received a CD34+ cell selected (T-cell depleted) graft. Overall, differences between the 3-year transplant-related mortality were not significant (8/8 URDT 18%, 7/8 URDT 39%, DCBT 24%, p = 0.108) whereas the 3-year relapse risk was decreased after DCBT (8/8 URDT 23%, 7/8 URDT 20%, DCBT 9%, p = 0.037). Three-year DFS was 57% in 8/8 URDT, 41% in 7/8 URDT, and 68% in DCBT recipients (p = 0.068), and the 3-year DFS in DCBT recipients was higher than that of 7/8 URDT recipients (p = 0.021). In multivariate analysis in acute leukemia patients, factors adversely associated with DFS were female gender (HR 1.68, p = 0.031), diagnosis of acute lymphoblastic leukemia (HR 2.09, p = 0.004), and 7/8 T-cell depleted URDT (HR 1.91, p = 0.037). High DFS can be achieved in adults with acute leukemia and CML with low relapse rates after DCBT. Our findings support performing DCBT in adults in preference to HLA-mismatched T-cell depleted URDT and suggest DCBT is a readily available alternative to T-cell depleted 8/8 URDT especially in patients requiring urgent transplantation.
Keywords: unrelated donor transplantation, cord blood transplantation, acute leukemia, HLA-match
Introduction
Unrelated donor cord blood (CB) is now routinely used as a source of allogeneic hematopoietic stem cells (HSC) for the transplantation of patients with hematologic malignancies. The reduced stringency of required human leukocyte antigen (HLA)-match in CB transplantation (CBT) has successfully extended transplant access to racial and ethnic minorities1–3. This is in contrast to unrelated donor transplantation (URDT) in which the requirement for a closely HLA-allele matched volunteer greatly restricts its application in non-European patients and those with mixed origins1–3. CBT also has the advantages of rapid availability and flexibility of patient admission whereas URDT admissions are determined by donor availability.
Double-unit CBT (DCBT) has achieved high rates of sustained donor engraftment partially mitigating the adverse effect of low total nucleated cell (TNC) dose in adults2,4–8. Moreover, retrospective analyses and one randomized study have demonstrated low rates of relapse after DCBT predominantly in adult patients5,9–12. Adult DCBT has also been associated with a low rate of late post-transplant mortality6,13. However, it is associated with prolonged hospitalization early after transplantation, the high cost of purchasing 2 units, and an increased risk of early transplant-related mortality (TRM)13 limiting its wide application. Consequently, most transplant centers select an 8 HLA-allele matched URD as the standard HSC source in the absence of a matched sibling donor. Moreover, some centers prefer a 7/8 HLA-matched URD over a CB graft especially in view of the rapid count recovery associated with peripheral blood HSC. The validity of this donor algorithm is not established, however, especially in centers with a high degree of expertise in the practice of both URD and CB transplantation. Therefore, we compared DFS in 66 8/8 HLA-matched URDT, 45 7/8 HLA-matched URDT, and 55 DCBT recipients aged 16–60 years with high-risk acute leukemia and advanced chronic myelogenous leukemia (CML) transplanted over the same time period with similar supportive care. Our hypothesis was that the 3-year DFS after DCBT is higher than that of 7/8 HLA-matched URDT.
Methods
Patients Characteristics
Patients were transplanted at Memorial Sloan Kettering Cancer Center (MSKCC) between 10/2005–11/2012. During the study period, 8/8 HLA-matched URD were given priority as the optimal donor. Otherwise, a 7/8 URD (mismatched at either the antigen or allele level) or double-unit CB grafts were selected according to transplant urgency, speed of donor availability, and physician preference. All patients provided written informed consent for transplantation, outcome analysis was approved by the MSKCC Institutional Review/ Privacy Board, and patients included in this analysis were transplanted on ClinicalTrials.gov NCT00587054, NCT00582933, NCT00597519, NCT00629798, NCT00739141, NCT01119066, and NCT00387959 trials. Eligible patients for this analysis included all consecutive patients aged 16–60 years who received their first myeloablative allograft for the treatment of acute leukemia, including acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), undifferentiated or acute biphenotypic leukemia in morphologic remission or aplasia, or patients who had advanced CML. CB units were 4–6/6 HLA-A,-B antigen, -DRB1 allele matched to the recipient and had a cryopreserved TNC dose of at least 1.5 × 107/ kilogram (kg)/ unit. The bank of origin was considered in unit selection whereas the unit-unit HLA-match was not8,14. Comorbidity scores were assigned according to Sorror et al15.
Conditioning Regimens and Immunosuppression
Pre-transplant conditioning varied according to patient’s age, diagnosis, remission status, and co-morbidities, and consisted of high-dose or intermediate intensity regimens. All intermediate intensity regimens were functionally myeloablative. In URDT, the conditioning was either total body irradiation (TBI)-based with Thiotepa/ Fludarabine/ TBI (1375–1500 cGy), or Thiotepa/ Cyclophosphamide/ TBI (1320–1500 cGy), or was chemotherapy-based (Clofarabine/ Thiotepa/ Melphalan). The intermediate intensity regimen consisted of Busulfan/ Fludarabine/ Melphalan as previously described16–20, and all patients received anti-thymocyte globulin (ATG). In DCBT, high-dose conditioning consisted of TBI-based regimens (1320–1375 cGy) with high dose Cyclophosphamide and Fludarabine, or less commonly a chemotherapy-based regimen of Clofarabine/ Thiotepa/ Melphalan. The intermediate intensity DCBT regimen was 400 cGy of TBI combined with Cyclophosphamide/ Fludarabine/ Thiotepa as previously described8,13,21. URD recipients underwent ex-vivo T-cell depletion (TCD) using CD34+ cell selection of peripheral blood HSC with either the Isolex 300i Magnetic Cell Separator (Baxter, Deefield, Illinois) and subsequent sheep red blood cell rosette depletion, or the CliniMACS CD34 reagent System (Miltenyi Biotech, Gladbach, Germany)20. Bone marrow was used in a small minority of patients and was T-cell depleted using soybean lectin agglutination and sheep red blood cell-rosette depletion16. All DCBT recipients received a calcineurin inhibitor (CNI) (predominantly cyclosporine-A) and mycophenolate mofetil starting on day −3 intravenously and none received ATG13,22. Granulocyte-colony-stimulating factor was given to all URDT and DCBT recipients post-transplant to promote neutrophil recovery. All patients received similar supportive care.
Study Definitions and Statistical Analysis
Time to neutrophil recovery was defined as the first of 3 consecutive days with a sustained absolute neutrophil count (ANC) ≥ 0.5 × 109/l. Time to platelet recovery was defined as the first of 3 consecutive days at ≥ 20 × 109/l and at least 7 days without platelet transfusion support. Primary graft failure was the lack of donor-derived neutrophil recovery by day 45, death as from day 28 but prior to day 45 without neutrophil recovery, or requirement for either a boost from the same URD or a second transplant for lack of count recovery. Secondary graft failure was defined as a fall in ANC to < 0.5 × 109/l for ≥ 14 consecutive days after donor-derived neutrophil recovery, or requirement for a HSC boost from the same URD, or a second transplant as therapy for severe cytopenias after initial engraftment.
GVHD was diagnosed clinically with histologic confirmation when appropriate. Acute and chronic GVHD were graded according to International Bone Marrow Transplant Registry (grades A-D)23 and the National Institutes of Health consensus criteria24, respectively. Acute and chronic GVHD in URDT was analyzed according to graft manipulation (CD34+ selected versus unmodified grafts). Relapse was defined as recurrence of leukemia post-transplant whereas TRM was defined as death from any cause in continued remission except for de-novo or recurrence of solid tumor malignancies post-allograft (n = 3). Overall survival (OS) and DFS were defined according to standard criteria. The primary cause of death was defined according to the algorithm of Copelan et al25.
Patient and graft characteristics were compared using Chi-square or Fisher’s exact test for categorical variables as appropriate and the Wilcoxon rank-sum test for continuous variables. Cumulative incidence functions were used to estimate neutrophil and platelet engraftment, GVHD, relapse, and TRM. The competing risks for each outcome were death for engraftment, death or relapse for GVHD, death in the absence of relapse for relapse, and relapse for TRM. Gray’s test compared the cumulative incidence across patient and treatment characteristics. OS and DFS were calculated using Kaplan-Meier methodology and were compared using a logrank test. Tests for a difference in OS and DFS between URDT and DCBT reflect testing for a specific difference in survival probabilities at a fixed time point26. All multivariate models for DFS were fit using weighted Cox regression to account for potential violations in proportional hazards27. Covariates in the model included patient or disease characteristics with a significant or trending association in either URDT and/or DCBT. All analyses were done using the R statistical package version 3.1.1 (R Development Core Team, 2011, Vienna, Austria).
Results
Patient and Graft Characteristics
Table 1 summarizes patient and graft characteristics. URDT and DCBT recipients had similar age, gender, recipient with cytomegalovirus (CMV) sero-positivity, and HCT-CI scores. DCBT recipients were more likely to have lower weight, be of non-European ancestry and receive intermediate intensity conditioning. The median times from diagnosis to HSC transplantation for patients in first complete remission (CR1) were similar at 5 months in URDT and 5.1 months in DCBT as were the times to transplantation from relapse for patients in CR2 (URDT 4.2 months and DCBT 3.4 months), and in ≥ CR3 (URDT 3.4 months and DCBT 3.9 months).
Table 1.
Patient and graft characteristics in URDT and DCBT recipients.
| 8/8 URDT (N = 66) |
7/8 URDT (N = 45) |
DCBT (N = 55) |
P-value | |
|---|---|---|---|---|
| Median age (range) | 50 years (16–60) | 42 years (16–60) | 42 years (16–60) | 0.087 |
| N (%) male | 40 (61%) | 23 (51%) | 26 (47%) | 0.317 |
| Median weight (kg, range) | 77 (40–144) | 82 (40–119) | 69 (45–96) | 0.010 |
| N (%) recipient CMV+ | 37 (56%) | 24 (53%) | 36 (66%) | 0.417 |
| N (%) recipient ancestry | ||||
| European | 54 (82%) | 29 (64%) | 25 (46%) | < 0.001 |
| Non-European | 12 (18%) | 16 (36%) | 30 (55%) | |
| N (%) HCT-CI score | 0–1: 22 (33%) | 0–1: 16 (36%) | 0–1: 15 (27%) | 0.933 |
| 2: 14 (21%) | 2: 8 (18%) | 2: 9 (16%) | ||
| 3: 14 (21%) | 3: 10 (22%) | 3: 14 26%) | ||
| ≥ 4: 16 (24%) | ≥ 4: 11 (24%) | ≥ 4: 17 (31%) | ||
| Median 2 (range 0–8) | Median 2 (range 0–7) | Median 3 (range 0–8) | ||
| N (%) diagnosis | - | |||
| AML* | 44 (67%) | 29 (64%) | 36 (65%) | |
| CR1 | 31 | 20 | 24 | |
| CR2–3 | 13 | 9 | 12 | |
| ALL | 17 (26%) | 13 (29%) | 17 (31%) | |
| CR1 | 14 | 6 | 7 | |
| CR2–4 | 3 | 7 | 10 | |
| CML | 5 (8%) | 3 (7%) | 2 (4%) | |
| N (%) conditioning | ||||
| High dose | 48 (73%) | 31 (69%) | 26 (47%) | 0.010 |
| Intermediate intensity | 18 (27%) | 14 (31%) | 29 (53%) | |
| N (%) GVHD prophylaxis | ||||
| T-cell depletion | 66 (100%) | 45 (100%) | - | - |
| CNI / MMF | - | - | 55 (100%) | |
|
Median infused CD34+ dose (range) |
5.7 × 106/kg (range 0.7–17.5) |
6.3 × 106/kg (range 1.2–14.2) |
Larger unit: 1.3 × 105/kg (0.3–4.2) Smaller unit: 0.7 × 105/kg (0.2–1.4) |
- |
N indicates number; URDT, unrelated donor transplantation; DCBT, double-unit cord blood transplantation; Kg, kilogram; CMV, cytomegalovirus; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; GVHD, graft-versus-host disease; CNI, calcineurin inhibitor; TNC, total nucleated cell.
Biphenotypic and undifferentiated acute leukemias were included with AML.
Diagnoses and disease risk were also comparable in URDT and DCBT recipients. Of patients with previously detected cytogenetic and/or molecular abnormalities at diagnosis, 14/57 (25%) 8/8 HLA-matched URDT, 11/35 (31%) 7/8 HLA-matched URDT, and 13/40 (33%) DCBT recipients in morphologic remission had measurable minimal residual disease pre-transplant. Overall, AML was the most common diagnosis. Similar percentages of AML URDT and DCBT recipients transplanted in CR1 (35/51, 69% versus 16/24, 67%, respectively) had high-risk diagnoses. Specifically, the 35 high risk URDT AML CR1 patients included 14 prior myelodysplastic syndromes or myeloproliferative diseases, 5 therapy-related disease, 6 FLT3-ITD mutations, 10 high-risk chromosomal abnormalities. High risk CR1 AML DCBT recipients included 6 prior myelodysplastic syndromes or myeloproliferative diseases, 2 therapy-related diseases, 6 FLT3-ITD mutations, and 2 high-risk chromosomal abnormalities.
URDT and DCBT patients with ALL were also high risk. URDT patients included 9 Philadelphia chromosome positive patients, 5 had complex karyotype or t(4;11), and 6 had intermediate or normal cytogenetics. Five DCBT recipients were Philadelphia chromosome positive, 1 had high-risk cytogenetics, and 1 was previously refractory to multiple chemotherapy cycles. Twelve URD-T and DCB-T recipients with CML had preceding accelerated or blast crisis (n = 6), or had failed (n = 4) or were intolerant to multiple tyrosine kinase inhibitors (n = 2). Eleven of these patients were positive for the Philadelphia chromosome by karyotype or fluorescent in-situ hybridization or BCR/ABL positive by molecular studies immediately pre-transplant.
High-dose conditioning was more common in URDT recipients whereas over half of the patients in the DCBT group received a preparative regimen of intermediate intensity (Table 1). The majority of URDT recipients received peripheral blood HSC (107/111, 96%), and CD34+ cell selection was done by using Isolex (n = 60) or Miltenyi (n = 43) columns, or soybean lectin agglutination and sheep red blood cell–rosette depletion (n = 8). DCBT recipients received CNI-based prophylaxis.
Approximately two-thirds of the URDT recipients received an 8/8 HLA-matched graft. DCBT recipients received grafts with a high degree of HLA disparity and the units had greater than a log less CD34+ cells. Of the 110 CB units transplanted 4 (4%) were 6/6, 51 (46%) were 5/6, and 55 (50%) were 4/6 HLA-A,-B antigen, -DRB1 allele HLA-matched to the recipient. At 8 HLA-alleles, over one-third of CB units were only 2–4/8 HLA-allele matched to the recipient [(7–8/8: 12 (11%), 5–6/8: 53 (48%), 2–4/8: 45 (41%)], and the median donor-recipient HLA-allele match was 5/8 (range 2–8/8). The median infused total nucleated cell dose in DCBT recipients was 2.62 × 107/kg and 1.93 × 107/kg in the larger and smaller units, respectively. DCB grafts had greater than a log less CD34+ cells than URD grafts (Table 1).
The median follow-up of survivors was 3 years 11 months (range 14–98 months) in 8/8 HLA-matched URDT recipients, 4 years 10 months (17–98 months) in 7/8 HLA-matched URDT recipients, and 3 years 10 months (range 15–92 months) in DCBT recipients.
Neutrophil and Platelet Engraftment
The cumulative incidence of primary neutrophil engraftment was 100% in 8/8 URDT, 100% in 7/8 URDT, and 95% in DCBT recipients whereas the speed of neutrophil recovery was faster in 8/8 and 7/8 URDT when compared to DCBT recipients (p < 0.001, Table 2). Primary graft failure was seen in 2 DCBT recipients. These patients had 100% bone marrow donor chimerism but failed to recover counts prior to their deaths on days 30 and 35 post-allograft in the setting of early onset multi-organ failure. While no URDT recipients had primary graft failure, secondary graft failure was observed in 5 (4%) URDT recipients (1 8/8 HLA-matched, 4 7/8 HLA-matched). Four received CD34+ cell selected boosts and 1 received anti-thymocyte globulin to facilitate count recovery. In DCBT recipients, sustained engraftment was mediated by a single unit in nearly all patients as previously described7,8 with the engrafting unit having a median viable infused CD34+ cell dose of 1.3 × 105/kg (range 0.3–4.2). The cumulative incidence of platelet engraftment at day 180 was 99%, 96% and 86% in 8/8 URDT, 7/8 URDT and DCBT recipients, respectively, with more rapid recovery in URDT than in DCBT recipients (p = < 0.001, Table 2). Of the 51 DCBT recipients alive at day 100 all but one had platelet recovery prior to day 180.
Table 2.
Cumulative incidence of engraftment and GVHD.
| 8/8 URDT (N = 66) (95%CI) |
7/8 URDT (N = 45) (95%CI) |
DCBT (N = 55) (95%CI) |
P-value | |
|---|---|---|---|---|
|
Neutrophil Engraftment (Day 45) |
100% (95–100) Median 11 days (range 8–15) |
100% (92–100) Median 11 days (range 9–19) |
95% (80–99) Median 24 days (range 13–40) |
< 0.001 |
|
N (%) Secondary graft failure |
1 (2%) | 4 (9%) | - | - |
|
Platelet Engraftment (Day 180) |
99% (69–100) Median 18 days (range 10–48) |
96% (79–99) Median 19 days (range 14–43) |
86% (71–93) Median 48 days (range 21–162) |
< 0.001 |
|
Day 100 grade II-IV aGVHD |
14% (7–23) | 18% (8–30) | 55% (40–67) | 0.001 |
|
Day 100 grade III-IV aGVHD |
6% (2–14) | 7% (2–17) | 13% (6–23) | 0.832 |
|
3 year cGVHD* |
8% (3–16) | 7% (2–17) | 11% (4–21) | 0.707 |
URDT indicates unrelated donor transplantation; DCBT, double-unit cord blood transplantation; ANC, absolute neutrophil count; GVHD, graft-versus-host disease; cGVHD, chronic graft-versus-host disease.
The severity of chronic GVHD after TCD URDT was mild (n = 5) or moderate (n = 3). DCBT recipients had mild (n = 2) or moderate (n = 4) disease.
Acute and Chronic GVHD
Recipients of 8/8 and 7/8 T-cell depleted URD grafts had a lower incidence of grade II-IV (grades B-D) acute GVHD at day 100 of 14% and 18%, respectively, whereas DCBT recipients had an incidence of 55% (p = 0.001, Table 2). The day 100 incidence of grade III-IV acute GVHD, however, was similar among the groups (6% 8/8 URDT, 7% 7/8 URDT, 13% DCBT), p = 0.832. The 3-year cumulative incidence of chronic GVHD was also similar in the 3 groups (p = 0.707, Table 2).
Transplant-Related Mortality
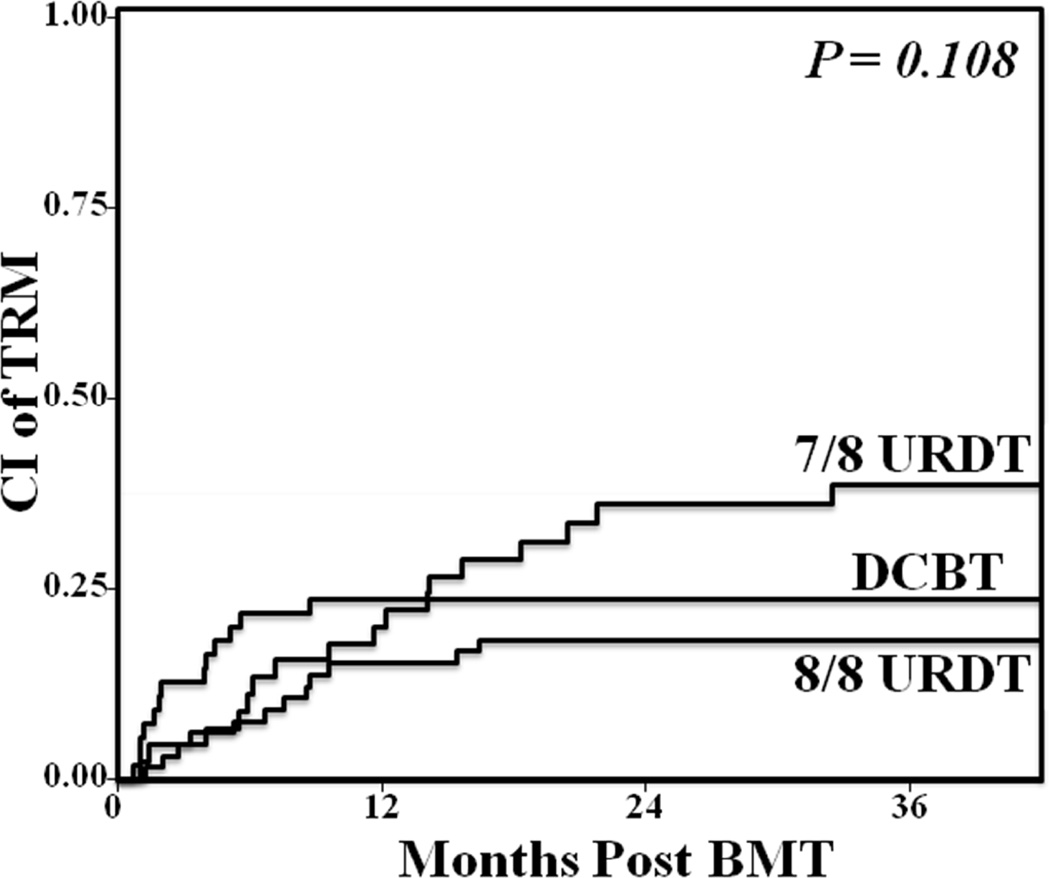
The 3-year TRM incidences after 8/8 URDT, 7/8 URDT, and DCBT are compared in Figure 1. The day 180 TRM was 8% (95%CI:3–16) in 8/8 URDT, 11% (95%CI:4–22) in 7/8 URDT, and 22% (95%CI: 12–34) in DCBT recipients. Overall, differences between the 3-year transplant-related mortality were not significant [8/8 URDT 18% (95%CI:10–29), 7/8 URDT 39% (95%CI:24–53), DCBT 24% (95%CI:13–36), (p = 0.108)].
Figure 1. Comparison of the cumulative incidence of 3-year TRM in adult 8/8 HLA-matched URDT, 7/8 HLA-matched URDT, and DCBT recipients.
Overall, differences between the 3-year transplant-related mortality were not significant (8/8 URDT 18%, 7/8 URDT 39%, DCBT 24%, p = 0.108)
The most common cause of TRM in URDT recipients was GVHD (3 had grade II, 3 grade III-IV, and 7 late acute), Table 3. Four of the patients received 8/8 HLA-matched URD and 9 received 7/8 HLA-matched URD grafts and most deaths occurred after day 180. The second most common cause of death was infection in 12 URDT recipients (4 bacterial, 1 CMV, 3 adenovirus, 1 BK virus, 1 progressive multifocal leukoencephalopathy, and 2 with Epstein-Barr virus post-transplant lymphoproliferative disease).
Table 3.
Comparison of early and late causes of treatment failure after URDT and DCBT.
| Events | 8/8 URDT (28/ 66, 42%) |
7/8 URDT (26/ 45, 58%) |
DCBT (17/ 55, 31%) |
|---|---|---|---|
| Before Day 180 | |||
| TRM | 5 (8%) | 5 (11%) | 12 (22%) |
| Graft Failure | - | - | 2 |
| GVHD | - | 1 | 4 |
| Organ Failure | 1 | - | 4 |
| Infection | 4 | 3 | 2 |
| Other* | - | 1 | - |
| Relapse | 1 (2%) | 3 (7%) | 1 (2%) |
| After Day 180 | |||
| TRM | 9 (14%) | 12 (27%) | 1 (2%) |
| Graft Failure | - | 1 | - |
| GVHD | 4 | 8 | 1 |
| Organ Failure | 1 | - | - |
| Infection | 2 | 3 | - |
| Other* | 2 | - | - |
| Relapse | 13 (20%) | 6 (13%) | 3 (5%) |
URDT indicates unrelated donor transplantation; DCBT, double-unit cord blood transplantation; TRM, transplant-related mortality; GVHD, graft-versus-host disease.
Other included one patient who died prior to day 180 of leukoencephalopathy of unknown etiology, one with recurrent breast cancer and one who died of secondary malignancy after day 180.
GVHD was also the most common cause of death in 5 DCBT recipients with the second most common cause of death being organ failure (3 pulmonary, 1 cardiac). Death due to primary graft failure occurred in 2 patients and infection was relatively uncommon as a primary cause of death after DCBT (n = 2). No DCBT recipient developed or died of CMV pneumonia although all patients who died of GVHD also had CMV viremia or CMV gastrointestinal disease.
Relapse
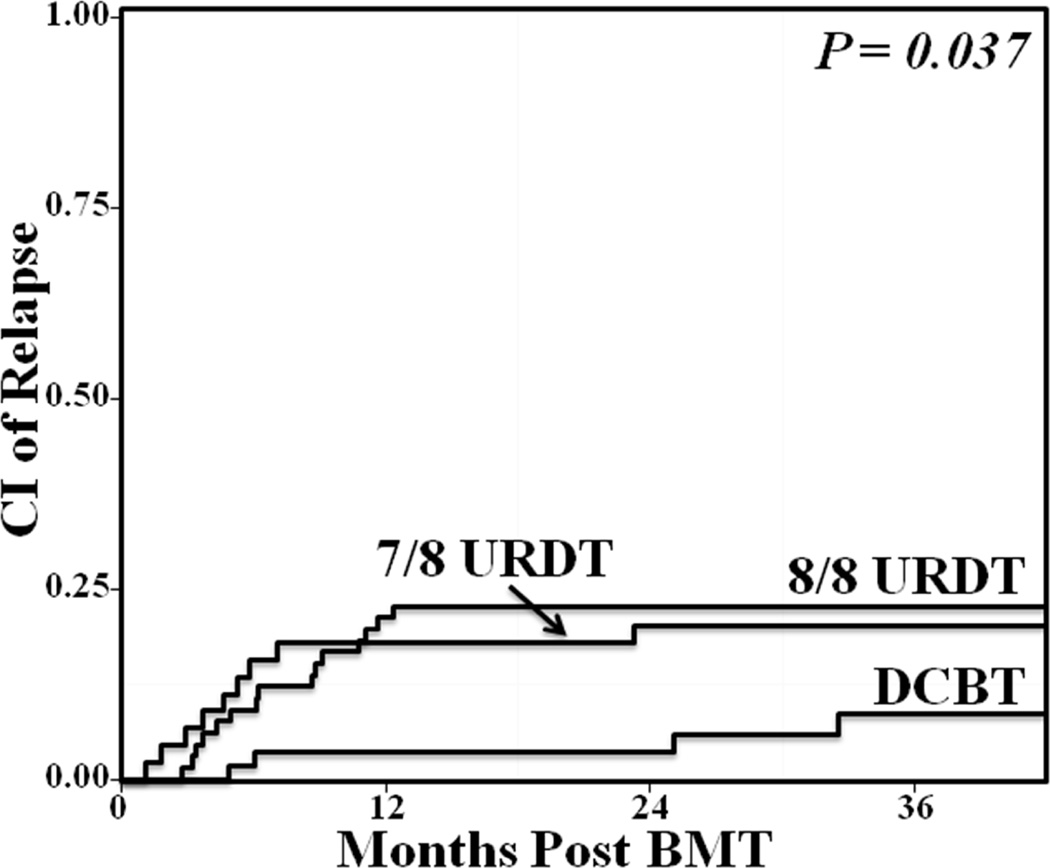
The cumulative incidence of relapse at 3-years was decreased after DCBT [8/8 URDT 23% (95%CI:12–32), 7/8 URDT 20% (95%CI:8–30), 9% (95%CI:3–19) in DCBT, (p = 0.037)] (Figure 2 and Table 3). Of 26 relapsing URDT recipients, 16 had AML (11 CR1, 5 CR2), 9 ALL (5 CR1, 1 CR2, 3 CR3), and 1 CML. The majority (22/26, 85%) received high-dose conditioning. Three URDT recipients received donor lymphocyte infusions for the treatment of relapse. In DCBT, 4 patients relapsed [2 CR1 AML (one FLT3 mutation and one secondary AML), and 2 ALL (1 CR1, 1 CR3)], and all had received intermediate intensity conditioning.
Figure 2. Comparison of the cumulative incidence of 3-year relapse in adult 8/8 HLA-matched URDT, 7/8 HLA-matched URDT, and DCBT recipients.
The 3-year relapse risk was decreased after DCBT (8/8 URDT 23%, 7/8 URDT 20%, DCBT 9%, p = 0.037).
3-Year OS and DFS
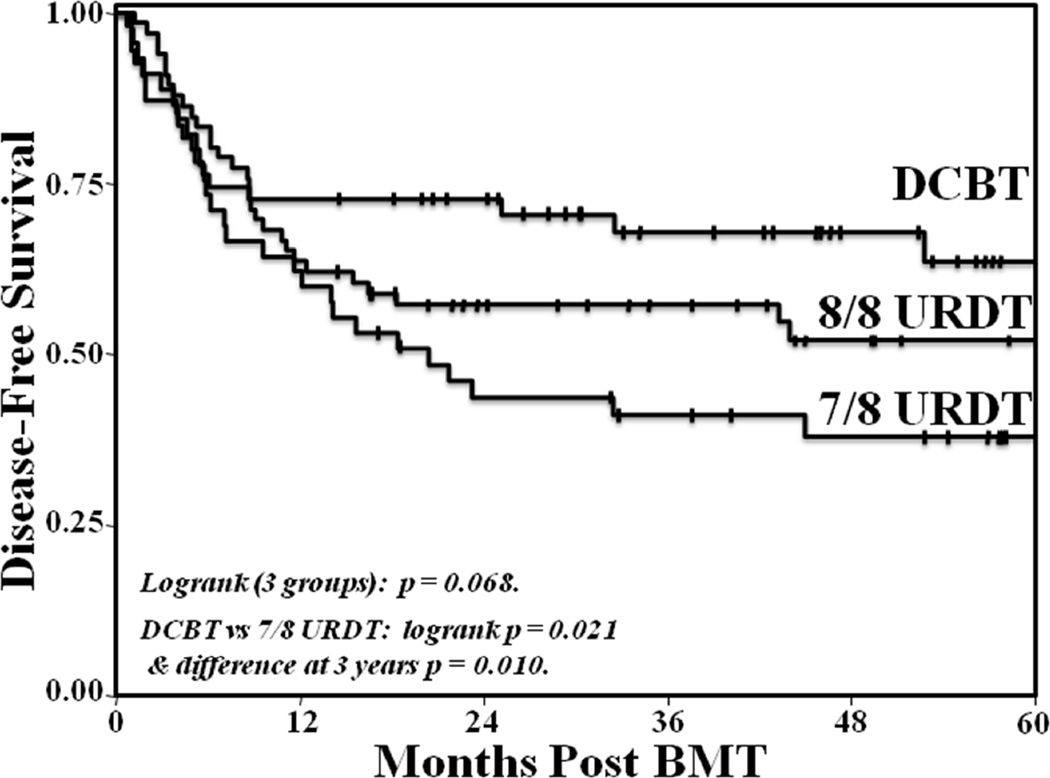
The 3-year OS was 57% (95%CI:44–69) in 8/8 URDT, 44% (95%CI:29–56), and 73% (95%CI:59–83) in DCBT recipients (p = 0.067 by logrank). The 3-year DFS in 8/8 URDT [57% (95%CI:45–68)], 7/8 URDT [41% (95%CI:27–55)], and DCBT recipients [68% (95%CI:53–79)] is shown in Figure 3. The overall comparison of the 3 groups by logrank was p = 0.068. However, DCBT recipients had a higher 3-year DFS as compared to 7/8 URDT recipients when compared by logrank (p = 0.021). The difference between these 2 groups at 3-years post-transplant was also significant (p = 0.010, Figure 3) whereas there was no difference between the 3-year DFS in 8/8 URDT and DCBT recipients (p = 0.259). There was no difference in DFS between recipients of Isolex-selected versus Miltenyi-selected URD grafts (data not shown).
Figure 3. Comparison of the Kaplan-Meier estimate of 3-year DFS in adult 8/8 HLA-matched URDT, 7/8 HLA-matched URDT, and DCBT recipients.
Three-year DFS was 57% in 8/8 URDT, 41% in 7/8 URDT, and 68% in DCBT recipients (p = 0.068) overall whereas the 3-year DFS in DCBT recipients was higher than that of 7/8 URDT recipients (p = 0.021).
Univariate and Multivariate Analyses of Determinants of DFS by HSC Source and All Patients Combined
Further analysis was performed to evaluate risk factors of DFS individually by HSC source (Table 4A and 4B), and in the entire patient population (Table 5). Three-year DFS estimates, univariate DFS analyses, and multivariate DFS analyses according to patient and graft variables by source are shown (Tables 4A and 4B). In multivariate analyses, CML patients were excluded to enable a better understanding of the variables affecting DFS in patients with acute leukemia, the bulk of the patient cohort in each group. In URDT recipients (Table 4A), a diagnosis of ALL had a significantly higher risk of treatment failure. In DCBT recipients (Table 4B), multivariate analysis revealed female gender and ALL diagnosis were associated with a higher risk of treatment failure. As conditioning intensity was not significant in the univariate analysis in either URT or DCBT it was not included in the multivariate model. To confirm the validity this finding when the multivariate model incorporated conditioning intensity it remained non-significant (data not shown).
Table 4.
Univariate and multivariate analysis of variables potentially associated with DFS in URDT (4A) and DCBT (4B) recipients with acute leukemia.
| 4A: URDT recipients | |||||
|---|---|---|---|---|---|
| Variable | 3-year DFS (95%CI) |
Univariate HR (95%CI) |
P- value |
Multivariate HR* (95%CI) |
P- value |
| Recipient age | |||||
| < 45 years (n = 54) | 51% (37–64) | Reference | 0.962 | - | |
| ≥ 45 years (n = 57) | 50% (36–62) | 1.01 (0.99–1.66) | |||
| Recipient gender | |||||
| Male (n = 63) | 56% (43–68) | Reference | 0.135 | Reference | 0.332 |
| Female (n = 48) | 43% (29–57) | 1.49 (0.88–2.51) | 1.31 (0.76–2.27) | ||
| Recipient CMV status | |||||
| Negative (n = 50) | 47% (32–60) | Reference | 0.862 | Reference | 0.597 |
| Positive (n = 61) | 54% (41–65) | 0.96 (0.57–1.61) | 1.17 (0.66–2.06) | ||
| HCT-CI score | |||||
| 0–2 (n = 60) | 58% (44–69) | Reference | 0.120 | Reference | 0.165 |
| ≥ 3 (n = 51) | 43% (29–56) | 1.51 (0.90–2.55) | 1.48 (0.85–2.57) | ||
| Diagnosis | |||||
| AML (n = 73) | 57% (45–67) | Reference | 0.011 | Reference | 0.020 |
| ALL (n = 30) | 30% (15–46) | 2.01 (1.17–3.45) | 2.00 (1.12–3.58) | ||
| Conditioning | |||||
| High dose (n = 79) | 48% (37–59) | Reference | 0.604 | - | |
| Intermediate (n = 32) | 56% (37–71) | 0.86 (0.48–1.53) | |||
| HLA-match | |||||
| 8/8 (n = 66) | 57% (45–68) | Reference | 0.197 | Reference | 0.275 |
| 7/8 (n = 45) | 41% (27–55) | 1.41 (0.84–2.37) | 1.35 (0.79–2.30) | ||
| Transplant year | |||||
| 2005–2008 (n = 48) | 46% (31–59) | 1.30 (0.77–2.18) | 0.332 | - | |
| 2009–2012 (n = 63) | 54% (40–66) | Reference | |||
| 4B: DCBT recipients | |||||
|---|---|---|---|---|---|
| Variable | 3-year DFS (95%CI) |
Univariate HR (95% CI) |
P-value | Multivariate HR* (95% CI) |
P-value |
| Recipient age | |||||
| < 45 years (n = 31) | 74% (54–86) | Reference | 0.454 | - | |
| ≥ 45 years (n = 24) | 61% (37–78) | 1.42 (0.57–3.59) | |||
| Recipient gender | |||||
| Male (n = 26) | 77% (53–90) | Reference | 0.058 | Reference | 0.024 |
| Female (n = 29) | 59% (39–74) | 2.72 (0.97–7.63) | 3.86 (1.19–12.49) | ||
| Recipient CMV status | |||||
| Negative (n = 19) | 81% (52–94) | Reference | 0.146 | Reference | 0.399 |
| Positive (n = 36) | 61% (43–75) | 2.28 (0.75–6.95) | 1.61 (0.53–4.89) | ||
| Recipient ancestry | |||||
| European (n = 25) | 71% (48–85) | Reference | 0.833 | - | |
| Non-European (n = 30) | 65% (45–80) | 1.11 (0.44–2.81) | |||
| HCT-CI score | |||||
| 0–2 (n = 24) | 75% (53–88) | Reference | 0.340 | - | |
| ≥ 3 (n = 31) | 62% (42–77) | 1.61 (0.61–4.30) | |||
| Diagnosis | |||||
| AML (n = 36) | 72% (54–84) | Reference | 0.312 | Reference | 0.028 |
| ALL (n = 17) | 54% (25–76) | 1.64 (0.63–4.24) | 3.16 (1.13–8.80) | ||
| Conditioning | |||||
| High dose (n = 26) | 69% (48–83) | Reference | 0.819 | - | |
| Intermediate (n = 29) | 66% (43–81) | 0.90 (0.35–2.27) | |||
|
HLA-match (engrafting unit)** |
|||||
| 7/8 (n = 7) | 57% (17–84) | Reference | 0.845 | - | |
| 5–6/8 (n = 31) | 71% (51–84) | 0.70 (0.19–2.54) | |||
| ≤ 4/8 (n = 17) | 67% (36–85) | 0.68 (0.16–2.88) | |||
|
Median infused CD34+ cell dose (dominant unit)** |
0.458 | - | |||
| < 0.86 (n = 27) | 70% (49–84) | Reference | |||
| ≥ 0.86 (n = 28) | 65% (42–81) | 1.42 (0.56–3.61) | |||
| Transplant year | |||||
| 2005–2008 (n = 16) | 63% (35–81) | 1.56 (0.59–4.08) | 0.368 | - | |
| 2009–2012 (n = 39) | 69% (51–82) | Reference | |||
DFS indicates disease-free survival; URDT, unrelated donor transplantation; DCBT, double-unit cord blood transplantation; HR, hazard ratio; CI, confidence interval; CMV, cytomegalovirus; HCI-CI, hematopoietic cell transplant-co-morbidity index; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; HLA, human leukocyte antigen.
Patients diagnosed with chronic myelogeneous leukemia (n = 8) were excluded from the multivariate analysis.
DCBT indicates double-unit cord blood transplantation; DFS; disease-free survival; HR, hazard ratio; CI, confidence interval; CMV, cytomegalovirus; HCI-CI, hematopoietic cell transplant-comorbidity index; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; HLA, human leukocyte antigen.
Patients diagnosed with chronic myelogeneous leukemia (n = 2) were excluded from the multivariate analysis.
In patients with clinical graft failure the dominant unit based on bone marrow chimerism was used.
Table 5.
Multivariate analysis of variables potentially associated with DFS of AML and ALL patients (n = 156).
| Variable | Multivariate HR (95% CI) |
P-value |
|---|---|---|
| Recipient gender | ||
| Male | Reference | 0.031 |
| Female | 1.68 (1.05–2.68) | |
| Recipient CMV serology | ||
| Negative | Reference | 0.217 |
| Positive | 1.35 (0.84–2.17) | |
| HCT-CI score | ||
| 0–2 | Reference | 0.092 |
| ≥ 3 | 1.51 (0.94–2.45) | |
| Diagnosis | ||
| AML | Reference | 0.004 |
| ALL | 2.09 (1.26–3.46) | |
| Graft Type | ||
| DCB | Reference | |
| 8/8 HLA-matched URD | 1.43 (0.77–2.63) | 0.257 |
| 7/8 HLA-matched URD | 1.91 (1.04–3.50) | 0.037 |
DFS indicates disease-free survival; HR, hazard ratio; CI, confidence interval; CMV, cytomegalovirus; HCI-CI, hematopoietic cell transplant-co-morbidity index; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; DCBT, double-unit cord blood transplantation; HLA, human leukocyte antigen; URDT, unrelated donor transplantation.
Multivariate analysis of acute leukemia patients (n = 156) is shown in Table 5. When adjusting for gender, recipient CMV status, diagnosis, and HCT-CI, 7/8 HLA-matched URDT was associated with inferior DFS (HR 1.91, p = 0.037) compared to DCBT. There was no difference between 8/8 HLA-matched URDT and the DCBT reference group (HR 1.43, p = 0.257). In the same model, diagnosis of ALL (HR 2.09, p = 0.004) and female gender (HR 1.68, p = 0.031) had worse DFS whereas an HCT-CI score of ≥ 3 approached significance (p = 0.092).
Discussion
The standard HSC source for adult patients with high-risk hematologic malignancies who require an allograft and lack an HLA-matched related donor is an 8/8 HLA-matched unrelated volunteer donor and many centers will consider a 7/8 HLA-matched donor as the next best alternative. Multiple approaches to prevent GVHD in URDT recipients are available. TCD, a strategy that has achieved DFS rates similar to those of unmodified grafts,16–20,28,29 and currently under investigation in the Bone Marrow Transplant Clinical Trials Network, has been the priority at our institution. In this analysis, we demonstrate that DCBT achieved a 3-year DFS comparable to that of recipients of an 8/8 HLA-matched URDT and higher than a 7/8 HLA-matched URDT when the volunteer donor grafts are T-cell depleted.
Each transplant modality had both advantages and disadvantages. Engraftment speed was a major benefit of URDT utilizing peripheral blood HSC. Additionally, TCD was associated with low rates of grade II-IV acute GVHD16–20,28,29. The lethality of GVHD when it occurred after URDT was high, however, especially when the grafts were HLA-mismatched. In DBCT recipients, the incidence of sustained donor engraftment was high by CBT standards likely reflecting the unit selection, unit handling, and conditioning employed by an experienced center. However, while there were no secondary graft failures the speed of neutrophil recovery was substantially delayed likely contributing to the increased early TRM risk. Platelet recovery was also delayed when compared to URDT recipients, although in patients alive at day 100 all but one recovered platelets indicating that failed platelet engraftment is a manifestation of early TRM. In this ATG-free platform, while rates of chronic GVHD were low as previously reported22,30–32, the acute GVHD incidence was 55% supporting the current investigation of measures to mitigate severe acute GVHD after DCBT22,33–36.
While the 3-year incidences of TRM were similar in recipients of T-cell depleted URDT and DCBT, the patterns of TRM were different. URDT recipients had a delayed and prolonged TRM risk that was worse with mismatched grafts. By contrast, nearly all of the TRM after DCBT was early after transplantation. Moreover, despite DCBT recipients having similar disease risk, percentages of minimal residual disease pre-transplant, and a lower percentage of patients receiving high-dose conditioning, the likelihood of relapse was significantly reduced suggesting CB has a robust graft-versus-leukemia (GVL) effect. This is consistent with previous comparisons of unmodified URDT and DCBT6 and reinforces the observation that relapse protection is a major advantage of DCBT in adults. Whether this GVL effect requires a double-unit graft or is inherent in CB as a HSC source remains to be established, however, especially in the setting of intermediate or reduced intensity conditioning.
Multivariate analysis of factors associated with treatment failure by HSC source demonstrated that the most significant adverse patient or graft characteristic in URDT recipients was a diagnosis of ALL. In recipients of DCBT, despite marked HLA-mismatch and low cell dose, neither the dominant unit donor-recipient HLA-match nor its infused CD34+ cell dose influenced DFS whereas multivariate analysis revealed a worse outcome in female patients and those with ALL. In the multivariate analysis of DFS in all acute leukemia patients, 7/8 HLA-matched URDT was associated with inferior DFS as compared to DCBT. Female gender and a diagnosis of ALL also had a higher risk of treatment failure. There was a trend worse DFS with high comorbidity scores. The finding that female gender was associated with inferior DFS was neither expected or explained and requires further investigation.
Overall, this analysis serves to emphasize the major obstacles to success in T-cell depleted URDT and DCBT. In URDT recipients undergoing TCD, mortality from infection and relapse suggests the lack of effective immune reconstitution is the major challenge. It is likely that the small number of patients who do develop GVHD in this setting have increased mortality due to delayed immune recovery being exacerbated by the need for additional immunosuppression. Strategies to augment immune recovery are under investigation37,38. In contrast, DCBT recipients are most compromised by early TRM due to delayed engraftment, organ toxicity, and acute GVHD. Numerous strategies to mitigate these complications such as improved CB graft selection with consideration of CD34+ cell dose7,8 and high resolution HLA-match22,39,40, augmentation of engraftment (improved homing, ex vivo expansion or co-infusion of third-party progenitors)41–47, reduced toxicity preparative regimens21,31,48–50, augmented GVHD prophylaxis35,36,51, and intensive supportive care52–54 are being investigated, and will be assisted by an increase in the size of the global CB inventory.
This analysis has the limitations of a retrospective study and that the URDT population is comprised exclusively of TCD grafts. These findings, therefore, require confirmation in a larger prospective study of DCB and URD transplants including URD grafts that are unmodified. Nonetheless, in the interim, that CB grafts extend transplant access to minorities is evident from this study, and that non-European DCBT recipients had comparable DFS to those with European origins is an important additional finding that warrants a larger analysis. With a follow-up of approximately 4 years, our analysis has implications for donor algorithms and the allocation of resources to URD registries versus public CB banks. DCB grafts can be rapidly secured especially if CB is pursued at the outset of the search. Our data, combined with similar previous studies comparing DCBT with unmodified URDT recipients6, would support DCBT being considered as a readily available therapy for high-risk acute leukemia in adult patients ≤ 60 years especially in those without a readily available 8/8 allele HLA-matched volunteer donor.
Highlights.
The 3-year TRM incidences after the transplantation of 8/8 URD T-cell depleted, 7/8 URD T-cell depleted, and double-unit CB grafts in adults with acute leukemia and CML are similar.
The 3-year relapse incidence after double-unit CB transplantation in adults with acute leukemia and CML is lower than that of 8/8 and 7/8 HLA-matched T-cell depleted URD transplantation recipients.
The 3-year DFS after 8/8 HLA-matched T-cell depleted URD and double-unit CB transplantation in adults with acute leukemia and CML is similar.
The 3-year DFS after double-unit CBT is higher than that of 7/8 HLA-matched T-cell depleted URDT in adults with acute leukemia and CML.
Acknowledgements
This work was supported in part by the Gabrielle’s Angel Foundation for Cancer Research (J.N.B.), the Society of Memorial Sloan-Kettering Cancer Center (J.N.B. and S.G.), the Memorial Sloan Kettering Cancer Center Translational and Integrative Medicine Research Program (J.N.B.), and P01 CA23766 from the National Cancer Institute, National Institutes of Health. We would like to acknowledge the nursing staff, transplant coordinators and Laboratory Medicine staff who greatly contributed to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: D.M.P. and J.N.B. designed the study, interpreted the data, and wrote the manuscript. P.H. and S.M.D. analyzed the data and wrote the manuscript. M.M and M.L. collected the data. H.C.M., P.D., S.G., K.H., A.J., R.J., G.K, E.P., M.A.P., C.S., R.T., M.vdB., J.W.Y, N.A.K., A.S., R.J.O’R. wrote the manuscript.
Conflict of Interest: The authors have no relevant conflicts of interest to declare.
References
- 1.Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant. 2010;16(11):1541–1548. doi: 10.1016/j.bbmt.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scaradavou A, Brunstein CG, Eapen M, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121(5):752–758. doi: 10.1182/blood-2012-08-449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S registry. N Engl J Med. 2014;371(4):339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 5.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematological malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avery S, Shi W, Lubin M, et al. Influence of infused cell dose and HLA match on engraftment after double-unit cord blood allografts. Blood. 2011;117(12):3277–3285. doi: 10.1182/blood-2010-08-300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purtill D, Smith K, Devlin S, et al. Dominant unit CD34+ cell dose predicts engraftment after double-unit cord blood transplantation and is influenced by bank practice. Blood. 2014;124(19):2905–2912. doi: 10.1182/blood-2014-03-566216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114(19):4293–4299. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrigues CA, Sanz G, Brunstein CG, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27(2):256–263. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 11.Kindwall-Keller TL, Hegerfeldt Y, Meyerson HJ, et al. Prospective study of one- vs two-unit umbilical cord blood transplantation following reduced intensity conditioning in adults with hematological malignancies. Bone Marrow Transplant. 2012;47(7):924–933. doi: 10.1038/bmt.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labopin M, Ruggeri A, Gorin NC, et al. Cost-effectiveness and clinical outcomes of double versus single cord blood transplantation in adults with acute leukemia in France. Haematologica. 2014;99(3):535–540. doi: 10.3324/haematol.2013.092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponce DM, Zheng J, Gonzales AM, et al. Reduced late mortality risk contributes to similar survival after double-unit cord blood transplantation compared with related and unrelated donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(9):1316–1326. doi: 10.1016/j.bbmt.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker JN, Byam C, Scaradavou A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood. 2011;117(8):2332–2339. doi: 10.1182/blood-2010-04-280966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91(3):1083–1090. [PubMed] [Google Scholar]
- 17.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110(13):4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakubowski AA, Small TN, Kernan NA, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2011;17(9):1335–1342. doi: 10.1016/j.bbmt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg JD, Linker A, Kuk D, et al. T cell-depleted stem cell transplantation for adults with high-risk acute lymphoblastic leukemia: long-term survival for patients in first complete remission with a decreased risk of graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19(2):208–213. doi: 10.1016/j.bbmt.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19(6):898–903. doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponce DM, Sauter C, Devlin S, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biol Blood Marrow Transplant. 2013;19(5):799–803. doi: 10.1016/j.bbmt.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponce DM, Gonzales A, Lubin M, et al. Graft-versus-host disease after double-unit cord blood transplantation has unique features and an association with engrafting unit-to-recipient HLA match. Biol Blood Marrow Transplant. 2013;19(6):904–911. doi: 10.1016/j.bbmt.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. British Journal of Haematology. 1997;97(4):855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 24.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13(12):1469–1476. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 26.Klein JP, Logan B, Harhoff M, Andersen PK. Analyzing survival curves at a fixed point in time. Stat Med. 2007;26(24):4505–4519. doi: 10.1002/sim.2864. [DOI] [PubMed] [Google Scholar]
- 27.Schemper M, Wakounig S, Heinze G. The estimation of average hazard ratios by weighted Cox regression. Stat Med. 2009;28(19):2473–2489. doi: 10.1002/sim.3623. [DOI] [PubMed] [Google Scholar]
- 28.Wagner JE, Thompson JS, Carter SL, Kernan NA. Unrelated Donor Marrow Transplantation T Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II-III trial. Lancet. 2005;366(9487):733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 29.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30(26):3194–3201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arora M, Nagaraj S, Wagner JE, et al. Chronic graft versus host disease following unrelated donor hematopoietic stem cell transplantation: higher response rate in recipients of unrelated donor umbilical cord blood. Biol Blood Marrow Transplant. 2007;13:1145–1152. doi: 10.1016/j.bbmt.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Ostronoff F, Milano F, Gooley T, et al. Double umbilical cord blood transplantation in patients with hematologic malignancies using a reduced-intensity preparative regimen without antithymocyte globulin. Bone Marrow Transplant. 2013;48(6):782–786. doi: 10.1038/bmt.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutman JA, Myint H, Lee CK, Smith C, Nguyen V, Pollyea DA. Chronic graft versus host disease and immunosuppression burden is significantly lower following adult cord blood transplantation versus matched unrelated donor transplantation. Blood. 2014:35. doi: 10.1038/bmt.2016.186. [DOI] [PubMed] [Google Scholar]
- 33.MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113(11):2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponce DM, Hilden P, Mumaw C, et al. High day 28 ST2 levels predict for acute graft-versus-host disease and transplant-related mortality after cord blood transplantation. Blood. 2015;125(1):199–205. doi: 10.1182/blood-2014-06-584789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harnicar S, Ponce DM, Hilden P, et al. Intensified Mycophenolate Mofetil Dosing and Higher Mycophenolic Acid Trough Levels Reduce Severe Acute Graft-versus-Host Disease after Double-Unit Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21(5):920–925. doi: 10.1016/j.bbmt.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bejanyan N, Rogosheske J, DeFor T, et al. Higher Dose of Mycophenolate Mofetil Reduces Acute Graft-versus-Host Disease in Reduced-Intensity Conditioning Double Umbilical Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21(5):926–933. doi: 10.1016/j.bbmt.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perales MA, Goldberg JD, Yuan J, et al. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood. 2012;120(24):4882–4891. doi: 10.1182/blood-2012-06-437236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velardi E, Dudakov JA, van den Brink MR. Clinical strategies to enhance thymic recovery after allogeneic hematopoietic stem cell transplantation. Immunol Lett. 2013;155(1–2):31–35. doi: 10.1016/j.imlet.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eapen M, Klein JP, Ruggeri A, et al. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123(1):133–140. doi: 10.1182/blood-2013-05-506253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahi PB, Ponce DM, Devlin S, et al. Donor-recipient allele-level HLA matching of unrelated cord blood units reveals high degrees of mismatch and alters graft selection. Bone Marrow Transplant. 2014;49(9):1184–1186. doi: 10.1038/bmt.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bautista G, Cabrera JR, Regidor C, et al. Cord blood transplants supported by co-infusion of mobilized hematopoietic stem cells from a third-party donor. Bone Marrow Transplant. 2009;43(5):365–373. doi: 10.1038/bmt.2008.329. [DOI] [PubMed] [Google Scholar]
- 42.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(2):232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367(24):2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Besien K, Liu H, Jain N, Stock W, Artz A. Umbilical cord blood transplantation supported by third-party donor cells: rationale, results, and applications. Biol Blood Marrow Transplant. 2013;19(5):682–691. doi: 10.1016/j.bbmt.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cutler C, Multani P, Robbins D, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122(17):3074–3081. doi: 10.1182/blood-2013-05-503177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horwitz ME, Chao NJ, Rizzieri DA, et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest. 2014;124(7):3121–3128. doi: 10.1172/JCI74556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broxmeyer HE, Pelus LM. Inhibition of DPP4/CD26 and dmPGE(2) treatment enhances engraftment of mouse bone marrow hematopoietic stem cells. Blood Cells Mol Dis. 2014;53(1–2):34–38. doi: 10.1016/j.bcmd.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciurea SO, Saliba RM, Hamerschlak N, et al. Fludarabine, melphalan, thiotepa and anti-thymocyte globulin conditioning for unrelated cord blood transplant. Leuk Lymphoma. 2012;53(5):901–906. doi: 10.3109/10428194.2011.631159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brunstein CG, Eapen M, Ahn KW, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood. 2012;119(23):5591–5598. doi: 10.1182/blood-2011-12-400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peffault de Latour R, Brunstein CG, Porcher R, et al. Similar overall survival using sibling, unrelated donor, and cord blood grafts after reduced-intensity conditioning for older patients with acute myelogenous leukemia. Biol Blood Marrow Transplant. 2013;19(9):1355–1360. doi: 10.1016/j.bbmt.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Rogosheske JR, Fargen AD, DeFor TE, et al. Higher therapeutic CsA levels early post transplantation reduce risk of acute GVHD and improves survival. Bone Marrow Transplant. 2014;49(1):122–125. doi: 10.1038/bmt.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milano F, Pergam SA, Xie H, et al. Intensive strategy to prevent CMV disease in seropositive umbilical cord blood transplant recipients. Blood. 2011;118(20):5689–5696. doi: 10.1182/blood-2011-06-361618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olson AL, Dahi PB, Zheng J, et al. Frequent human herpesvirus-6 viremia but low incidence of encephalitis in double-unit cord blood recipients transplanted without antithymocyte globulin. Biol Blood Marrow Transplant. 2014;20(6):787–793. doi: 10.1016/j.bbmt.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dahi PB, Perales MA, Devlin SM, et al. Incidence, Nature and Mortality of Cytomegalovirus Infection after Double-Unit Cord Blood Transplantation. Leuk Lymphoma. 2014:1–19. doi: 10.3109/10428194.2014.963079. [DOI] [PMC free article] [PubMed] [Google Scholar]