Abstract
Objectives
To explore current practices and decision-making regarding antimicrobial prescribing among Emergency Department (ED) clinical providers.
Methods
We conducted a survey of ED providers recruited from eight sites in three cities. Using purposeful sampling, we then recruited 21 providers for in-depth interviews. Additionally, we observed ten patient-provider interactions at one of the ED sites. SAS 9.3 was used for descriptive and predictive statistics. Interviews were audio-recorded, transcribed and analyzed using a thematic, constructivist approach with consensus coding using NVivo 10.0. Field and interview notes collected during the observational study were aligned with themes identified through individual interviews.
Results
Of 150 survey respondents, 76% agreed or strongly agreed antibiotics are overused in the ED, while half believed they personally did not overprescribe. Eighty nine percent used a smartphone or tablet in the ED for antibiotic prescribing decisions. Several significant differences were found between attending and resident physicians. Interview analysis identified 42 codes aggregated into the following themes: (1) resource and environmental factors that affect care; (2) access to and quality of care received outside of the ED consult; (3) patient-provider relationships; (4) clinical inertia; and (5) local knowledge generation. The observational study revealed limited patient understanding of antibiotic use. Providers relied heavily upon diagnostics and provided limited education to patients. Most patients denied a priori expectations of being prescribed antibiotics.
Conclusions
Patient, provider, and healthcare system factors should be considered when designing interventions to improve antimicrobial stewardship in the ED setting.
Keywords: Clinical decision-making, antimicrobial use, antimicrobial stewardship
INTRODUCTION
Antibiotic-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) and extended spectrum beta lactamase–producing organisms (ESBL) have emerged and expanded their presence from healthcare settings to the community, leading to increased mortality, morbidity and rising healthcare costs. 1,2 Inappropriate antimicrobial use has been described as the most important preventable cause of drug resistance in both hospital and community settings.3,4,5,6 Antimicrobial stewardship, or the organized optimization of antibiotic utilization, has been demonstrated to reduce unnecessary antibiotic use. At least 15% of ED visits result in antibiotic use,7 with poor compliance to evidence-based guidelines8,9 and overuse of broad-spectrum antibiotics.10,11 Despite the important role of the ED in antimicrobial prescribing, it remains a largely untapped setting for antimicrobial stewardship interventions, with no studies to date on barriers to practice change. To address this gap, a mixed-method approach was chosen to examine provider, patient and environmental factors associated with antimicrobial prescribing in the ED. This approach is optimal for an understudied phenomenon as it allows for an exploratory approach and data triangulation.12
METHODS
This study was approved by institutional review boards at the George Washington University, Johns Hopkins University, MedStar Health, and Olive View-University of California Los Angeles Medical Center.
Provider Survey
From September 2012 to July 2013, we conducted a quantitative survey of ED providers recruited from eight sites in three cities including urban tertiary care academic centers, military treatment facilities, a county facility, and a tertiary pediatric center. Some providers also practiced in community settings. Convenience sampling was used; the 8 EDs are sites for research collaborations on infectious diseases. The survey was modified from previous surveys on antimicrobial stewardship13,14 and administered via RedCap, a secure web application. Eligible providers (435 attending physicians, residents, and midlevel providers with at least 2 years of ED experience) were invited to participate through electronic mailings and distribution of surveys at faculty and resident conferences. Data was collected using Likert scale and multiple choice format including demographics, practice site, types of resources used in the ED when making antibiotic prescribing decisions, and knowledge, attitudes and beliefs regarding antibiotic prescribing.
In-Depth Interviews
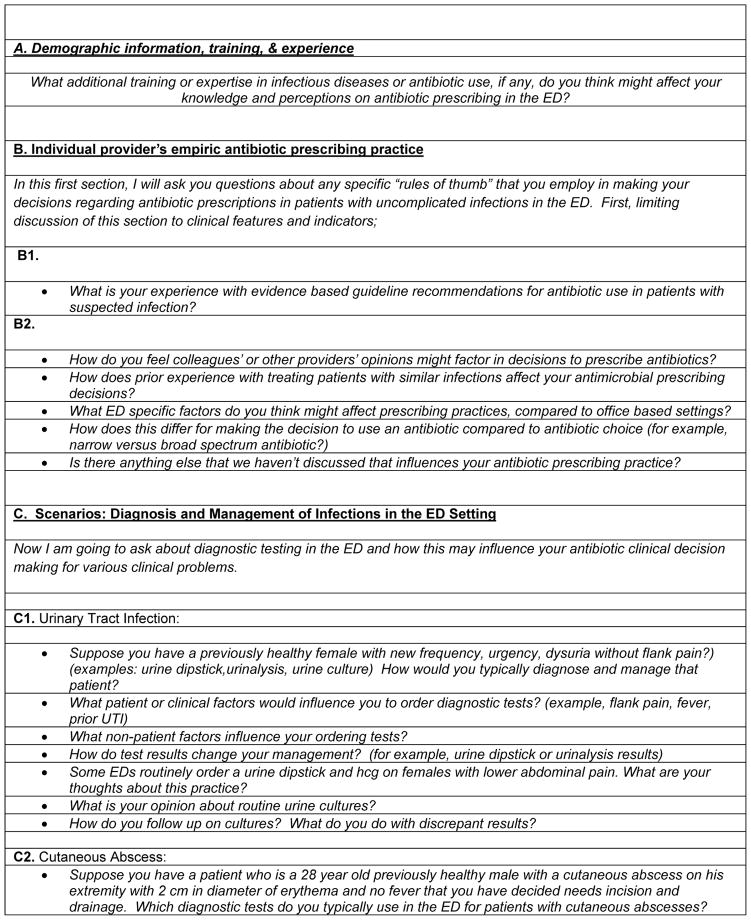
We recruited a convenience subset of 21 survey participants to complete in-depth interviews, balancing provider experience, setting, and gender. We selected this number based on available funding for the 20–25 total required for qualitative analysis. From November 2012 to June 2013, interviews were conducted in person after verbal informed consent using a semi-structured interview guide (Figure 1) by LM, a board certified emergency physician and PA, an emergency medicine resident with two years of experience. The interview contained four primary questions and two clinical scenarios (urinary tract and skin and soft tissue infection) related to antimicrobial prescribing, and lasted 45–60 minutes (Figure 1). Interviews were audio-recorded, and de-identified transcriptions were produced by Daily Transcriptions Inc. Interviewees received a $50 gift card for their participation.
Figure 1.
Semi-Structured Interview Guide Questions
ED Observational Study
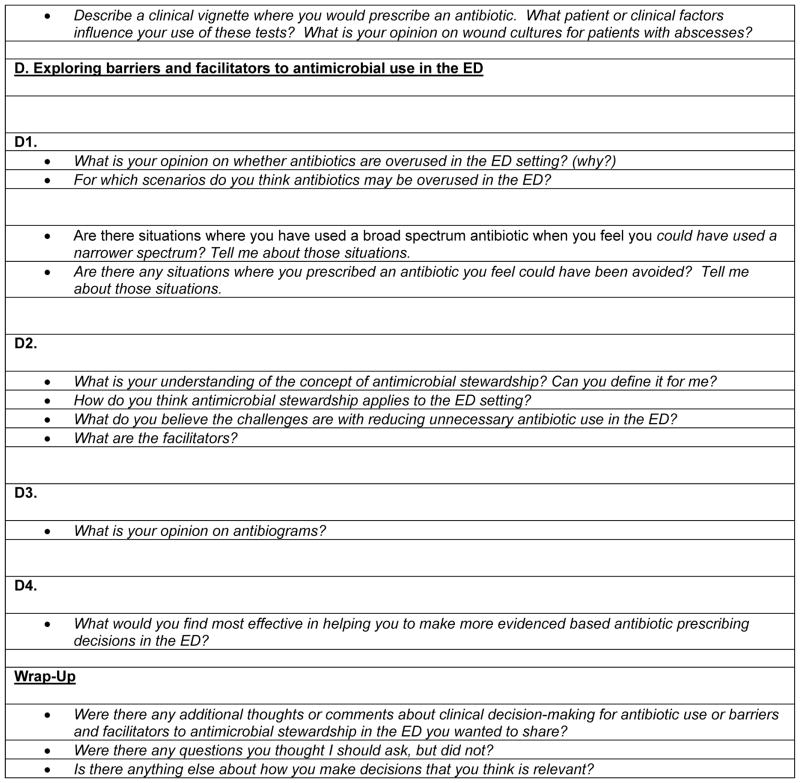
From June 2013 to August 2013, we observed ten patient-provider interactions at one ED site, an urban academic center. Observed interactions had a chief complaint of upper respiratory, urinary tract, or skin and soft tissue infection. Providers had previously completed our in-depth interview. Patients and providers were verbally consented, in-person. GB, a biostatistician, collected data on chief complaint, diagnosis, and antibiotic use and de-identified all records per IRB stipulations. All observations were conducted by GG, a medical anthropologist; notes were taken of the informants’ responses and general observations of the ED visit (Figure 2). Six key indicators of antibiotic clinical-decision making, as informed by the literature on this topic, were selected and monitored for occurrence: (1) patient explicitly or implicitly asked for antibiotics; (2) provider informed patient whether the infection was viral or bacterial; (3) provider explained which types of infections antibiotics successfully treat; (4) patient asked provider questions about his or her treatment plan; (5) provider gave patient a choice of treatment; and (6) patient asked for treatment during their ED visit that had not yet been provided. Follow-up interviews were conducted at the conclusion of the visit with the participant and the provider to assess satisfaction with the outcome. As an incentive, patients and providers were offered a $5 gift card for their participation.
Figure 2.
ED Observational Study Data Collection Tool
Data Analysis
SAS 9.3 was used for survey analysis. Descriptive frequencies and non-parametric Chi-Square tests were performed for quantitative data.
Interviews with providers were audio-recorded, transcribed and coded using a thematic approach based on a constructivist theoretical perspective, which acknowledges the multiple truths and realities of subjectivism and incorporates mutuality between researcher and subjects.15 We created an initial interview codebook from themes identified in the literature, with modifications made during the analysis phase. Codes were grouped according to the knowledge-attitudes-behaviors model and heuristics and biases in medicine.16,17
Qualitative codes were analyzed thematically across interviews to provide detail on the contribution of various factors to antibiotic decision-making. We used a cyclical process of data collection, analysis and provisional coding, with data collated into subthemes during subsequent analysis. Codes were continually added until coders perceived achievement of theme saturation. After the first ten interviews, codes were combined on the basis of similarity of meaning and co-occurrence, and again at the end of 20 interviews. First level codes were collapsed into second level codes in a hierarchical fashion. Analysis of the 21st interview was used as a validation interview. Interviews were coded jointly by LM and GG, with consensus on analysis and interpretation through continual discussion with and arbitration by PA in cases of disagreement. Data analysis was facilitated using NVivo 10.0 software (QSR International, Victoria, Australia).
For the observational study of patient-provider interactions, field and interview notes and frequencies of key indicators of clinical decision making were compiled. We synthesized data for each individual patient and then compared trends and outliers among all informants.
RESULTS
Provider Survey
150 participants (35%) responded, with an even distribution across gender. Of the participants, 59% were attendings, 36% residents, and 5% midlevel providers. The mean number of years in practice for attendings was 16.4 (Table 1). Among the 54 emergency medicine residents, the mean number of years in residency was 2.8. Interns were excluded.
Table 1.
Survey Descriptive Results
| Characteristic (n=150) | Frequency (%) |
|---|---|
|
| |
| Demographics | |
|
| |
| Age | 25% <30 |
| 55% 31–40 | |
| 12% 41–50 | |
| 8% >50 | |
|
| |
| Gender | 50% Female |
| 50% Male | |
|
| |
| Title | 59% Attending |
| 36% Resident | |
| 5% PA/NP | |
|
| |
| Location Setting | 52% Urban Tertiary Academic Centers |
| →19% (GW residents) rotate with a Community Tertiary Hospital | |
| 18% Urban County Hospital | |
| →49% (UCLA residents) rotate with an Urban Tertiary Academic Center | |
| 15% Military Treatment Facility | |
| 15% Urban Academic Pediatric Center | |
|
| |
| Years in Practice | Mean=8.2 years |
| Range: (0.4, 37) | |
|
| |
| Antibiotic Use and Confidence | |
|
| |
| On a typical shift, what % of patients being discharged to home do you prescribe antibiotics? | 36% <10% |
| 51% 10–20% | |
| 8% 21–40% | |
| 2% 41–60% | |
| 1% >60% | |
| 2% Not Sure | |
|
| |
| Mobile Use | |
|
| |
| Currently Use a Smart Phone or ipad | 89% Yes |
| 11% No | |
When comparing attending with resident physicians, there were several significant differences (Table 2). Of the physicians who felt “very” or “somewhat” confident” they were using antibiotics optimally in ED patients being discharged home, significantly more attendings (87%) versus residents (57%) agreed or strongly agreed antibiotics are overused in the ED (p<.0001). However, only 10% and 14%, respectively, believed they over-prescribe antibiotics. Providers used different information sources in their prescribing decisions, with residents relying more on their ED colleagues (15%) than attendings (32%) (p=0.001). The vast majority (89%) reported using a smartphone or tablet, with 44% of attendings versus 72% of residents reporting online decision support via a smart device would be useful for making antibiotic selections (p=0.001).
Table 2.
Analysis of Important Factors and Predictors from Quantitative Survey
| Important Factors and Predictors | Attendings (n=88) | Residents (n=54) | Non-Parametric Chi-Square Tests |
|---|---|---|---|
| p-Value | |||
| Antibiotic Use and Confidence in Prescribing | |||
| How confident are you that antibiotics are used optimally in ED patients being discharged from the hospital? | Very Confident: 30% Somewhat Confident: 61% Somewhat Unconfident: 9% Very Unconfident: 0% |
Very Confident: 4% Somewhat Confident: 81% Somewhat Unconfident:13% Very Unconfident: 2% |
p=0.001 |
| How confident are you that antibiotics are used optimally for ED patients being admitted to the hospital? | Very Confident: 33% Somewhat Confident: 59% Somewhat Unconfident: 7% Very Unconfident: 1% |
Very Confident: 26% Somewhat Confident: 67% Somewhat Unconfident: 7% Very Unconfident: 0% |
p=0.48 |
| Mobile Use/Online Tool Beliefs | |||
| Important Sources of Information | ID Faculty: 14% Other ED Colleagues: 15% Internet: 21% Med Letter/Journals: 8% Sanford Guide: 26% EMRA Guide: 17% Smart Phone/Mobile App:19% Hospital Pharmacist: 12% |
ID Faculty: 13% Other ED Colleagues: 32% Internet: 23% Med Letter/Journals: 4% Sanford Guide: 21% EMRA Guide: 44% Smart Phone/Mobile App: 30% Hospital Pharmacist: 31% |
p=0.19 p=.001 p=0.36 p=0.30 p=0.07 p=0.002 p=0.31 p=0.003 |
| If it was provided to you via smart phone or iPad, how useful would you find an online decision support tool for antibiotic selection in your ED practice? | Extremely Useful: 44% Somewhat Useful: 44% Not Very Useful: 5% Not Useful At All: 2% Don’t Know: 5% |
Extremely Useful: 72% Somewhat Useful: 26% Not Very Useful: 0% Not Useful At All: 0% Don’t Know: 2% |
p=0.001 |
| If antibiotic recommendations were embedded in the electronic medical record, how useful would you find an on-line decision support tool for antibiotic selection in your ED practice? | Extremely Useful: 51% Somewhat Useful: 40% Not Very Useful: 5% Not Useful At All: 1% Don’t Know: 3% |
Extremely Useful: 60% Somewhat Useful: 31% Not Very Useful: 3% Not Useful At All: 2% Don’t Know: 4% |
p=0.42 |
| If it was provided to you via smart phone or iPad, would you use an on-line decision support tool for antibiotic selection in your ED practice? | Definitely: 41% Probably: 42% Probably Not: 16% Definitely Not: 1% |
Definitely: 70% Probably: 24% Probably Not: 0% Definitely Not: 1% |
p=0.001 |
| If antibiotic recommendations were embedded in the electronic medical record, would you use an on-line decision support tool for antibiotic selection in your ED practice? | Definitely: 49% Probably: 46% Probably Not: 4% Definitely Not: 1% |
Definitely: 52% Probably: 41% Probably Not: 7% Definitely Not: 0% |
p=0.95 |
| Opinion on Antibiotic Use | |||
| Antibiotics are overused in the ED | Strongly Agree: 31% Agree: 56% Neutral: 9% Disagree: 4% Strongly Disagree: 0% |
Strongly Agree: 13% Agree: 44% Neutral: 32% Disagree: 11% Strongly Disagree: 0% |
p<.0001 |
| Antibiotic resistance does not present a significant problem in the ED at my institution | Strongly Agree: 1% Agree: 2% Neutral: 12% Disagree: 50% Strongly Disagree: 35% |
Strongly Agree: 0% Agree: 7% Neutral: 7% Disagree: 63% Strongly Disagree: 22% |
p=0.21 |
| Antibiotics are overused in non-ED settings at my institution | Strongly Agree: 34% Agree: 40% Neutral: 21% Disagree: 5% Strongly Disagree: 0% |
Strongly Agree: 19% Agree: 41% Neutral: 30% Disagree: 7% Strongly Disagree: 3% |
p=0.02 |
In-Depth Interviews
100% of recruited participants agreed to be interviewed. Analysis was guided by constructivist theory; using both inductive and deductive methods, the research team condensed 42 codes and concepts into five broad themes: (1) resource and environmental factors that affect care; (2) access to and quality of care received outside the ED consult; (3) patient-provider relationships; (4) clinical inertia; and (5) local knowledge production. A detailed description of these overarching themes is provided in Table 3 and described below. There was no link between provider level of confidence in prescribing (from the quantitative survey) and the major themes identified during their interviews when we compared the answers prescribers gave on the survey with their interview.
Table 3.
Codes and Themes
| Themes | Definition and Codes | Exemplar Quotes |
|---|---|---|
| 1. Impact of Resource and Environmental Factors | How accessibility and availability of material and immaterial sources shape the ED provider’s antibiotic decision-making process.
|
A. “However, as we get busier I can’t say I never prescribed an antibiotic that I didn’t need to, because you’re busy and you need to get through patients.” – Attending, Female, 2yrs. |
| B. “I would consider needing that rapid [diagnostic] test [for drug resistance]. To be able to have the luxury of honing in my diagnosis or being able to choose a narrow spectrum, or even an appropriate spectrum.” – Attending, Male, 8 yrs. | ||
| C. “We see a very broad spectrum of disease, so being able to keep up with all the antibiotic regimens and recommendations from all of the specialties I think is difficult.” – Attending, Male, 12yrs. | ||
| 2. Access and Quality of Care Received outside the ED Consult | Medical assistance patients seek or acquire before or after their medical encounter in the ED and how this care may or may not impact the trajectory of their treatment in the ED.
|
A. “Whether or not I treat with antibiotics depends on whether or not there’s evidence, but I often will set a little threshold, especially if I’m concerned about the patient’s ability to follow up, which is often an issue.” –Attending, Female, 10 yrs. |
| B. “If it’s an outpatient, usually I use the broad-spectrum antibiotics because we don’t have the luxury usually of following the patients and seeing if it’s working.” – Resident, Male, 4 yrs. | ||
| C. “If they don’t have a car or it takes them two hours on the bus. Then you might just say screw it, just go ahead, and we’ll give the antibiotic. That plays a huge role. That’s just emergency medicine.” – Did not provide demographics | ||
| 3. Patient-Provider Relationship | How the social dynamic between a healthcare consumer and their attending physician or resident shape rapport and affect healthcare decision making.
|
A. “We want to give the patient an explanation for their vague abdominal pain that’s not appendicitis. And so we call it a UTI and treat ‘em with antibiotics.” – Attending, Male, 4yrs. |
| B. “We’ve created an expectation in the population. They come asking for antibiotics, ‘cause the last three times they came, they were told they needed them. So they come time number four and say, ‘I’m here for my antibiotic.’ And we think they’re crazy. When in reality, it’s like no, we trained them to come back and get an antibiotic.” – Attending, Male, 11 yrs. | ||
| C. “It’s almost like opiates. When the physician is just so beaten down that they don’t want to argue anymore with the patient. We all want to be that person who has that hard discussion and educates the patient, but sometimes it’s really hard to do that.” –Attending, Female, 6 yrs. | ||
| 4. Clinical Inertia | Methods of practice or modes of thought that are acquired through the continual adaptation to a specific environment, so much so that the behavior becomes normalized and often unconscious. In some cases the methods of practice or modes of thought will continue even if they are irrational because they are so deeply embedded into the social landscape that they are perceived as routine or “right.”
|
A. “So I think it’s usually this sort of drive to make a diagnosis even when it’s something that’s probably early, probably viral, and just needs a little more time to declare itself.” – Attending, Male, 10 yrs. |
| B. “I think we try to reduce our cognitive load sometimes in the ED environment and sometimes antibiotics is a tick box of management for certain things – someone’s getting admitted for asthma you give them antibiotic.” – Attending, Male, 22 yrs. | ||
| C. “...’it may not be just simple this, or that,’ you know? You have to think more broadly because they decided today was the day they were coming to the emergency department.” – Attending, Male, 4 yrs. | ||
| 5. Local Knowledge Production | When administrative protocol or the expertise of ED players (providers, specialists, colleagues, researchers) through quality control measures, personal opinions or experiences generate information about antibiotic prescribing that influence clinical decision-making.
|
A. “[Having infectious disease experts in the ED makes you] more cognizant of your decision to use antibiotics or not – if they’re absolutely necessary. I think it helps the entire ED, not only to having them their on shift, but also in conferences you can have better discussions about what antibiotics to use, in actual terms.” – Resident, Female, 2 years. |
| B. “My thinking has really gone away from prescribing antibiotics for abscesses based on [our study]. These patients come back every couple days for rechecks, and I know that half of them are getting placebo. They all get better just with drainage, even though they had a ton of cellulitis before, so I’m pretty convinced.” – Attending, Male, 14 yrs. | ||
| C. “I think [ID specialists] are very important especially when there are key opinion leaders locally. They came up with algorithms and had specific recommendations for antibiotics that they came and spoke to the ED faculty and gave us the protocols and basically everyone, or I personally, followed those protocols.” – Attending, Male, 11 yrs. |
All quotes provided in this chart originate from 15 unique providers
Theme 1: Resource and Environmental Factors that Affect Care
ED providers expressed they must navigate a patchwork system of insufficient resources under time constraints, impeding antibiotic stewardship. The most frequently identified constraints were time, inadequate diagnostic testing capabilities, and perceived inappropriate or vague guidelines. While several resources were identified that could improve stewardship (e.g. patient telephone follow up, antibiograms), these were noted as not being easily accessible.
Theme 2: Access and Quality of Care Received Outside the ED Consult
Providers acknowledged treating more ‘aggressively’ when patient follow-up was uncertain, prescribing antibiotics more readily in the absence of clinical indicators and selecting broader spectrum agents.
Theme 3: Patient-Provider Relationship
The majority of ED providers said they were influenced by perceived or real patient expectations. Patient education (including level of health education) and how well the provider felt they were able to communicate with the patient were important factors influencing their decision to prescribe antibiotics even in the absence of clinical indicators.
Theme 4: Clinical Inertia
Many providers revealed they perfunctorily follow order-sets or lapse into patterns of prescription, in accordance with their colleagues. However, the drive to make a diagnosis was often a deliberate conscious habit, with lack of certainty in the diagnosis leading to provider discomfort. Multiple providers spoke at length about diagnostic uncertainty playing a role in unjustified antimicrobial prescribing.
Theme 5: Local Knowledge Production
Local knowledge, including lectures, faculty meetings, conferences, conversations between colleagues, and trainee education were identified as important factors that facilitate antimicrobial stewardship. Providers emphasized local feedback on antimicrobial prescribing should not be punitive.
Patient-Provider Observational Study
Our sample of ten patient-provider interactions, involving three ED attendings in one ED, revealed insights regarding how patients perceive their provider’s treatment decisions and their general knowledge surrounding antibiotics. Most patients simply wanted an explanation for their symptoms. No patient explicitly stated the desire for an antibiotic, nor requested the provider prescribe one. Encounters generally involved a brief set of questions and physical examination. Providers relied heavily on diagnostics; every patient received testing, with most receiving multiple tests. Patients had limited understanding and demonstrated poor knowledge of antibiotic use, side effects, or the difference between viral and bacterial infection. Many mentioned if antibiotics were overused resistance in the body would build; however, none mentioned resistance at a community level. There was extremely limited communication between patients and providers. Of the only three interactions where the provider indicated whether the infection was viral or bacterial, only two of the patients were given an explanation by their provider of why antibiotics are not as effective for viral infections.
Discussion
Our study revealed that reasons for antibiotic overprescribing in the ED are complex and shaped by numerous factors, both internal and external to providers. Data triangulation between the three components of our study maximized the ability to interpret our findings. Our findings are consistent with previous research showing barriers to implementing new guidelines are numerous and likely vary by setting and site.18 Similar to our findings, studies of European healthcare providers found that environmental (i.e., time and resources) and patient related factors (i.e., patient preference) were primary barriers to antibiotic guideline adherence,19 with peer group opinion a strong predictor of antimicrobial prescribing.20
Our in-depth interviews revealed that the ED providers’ ability to foster antibiotic stewardship is hindered by external health system factors. The ED, as a safety net, disproportionately provides care to low-income and uninsured patients. As a result, ED providers reported that they must not only account for the clinical scenario, but also consider the patient’s ability to obtain follow up care. For example, providers for whom patients had better access to follow up care were more likely to use a wait-and-see approach to antimicrobial prescribing for upper respiratory infections. Nearly every provider emphasized the fast-paced environment of the ED encourages unnecessary antibiotic use. Providers stated they often forgo diagnostic testing due to lengthy turn-around-time, in favor of prescribing.
Inappropriate antibiotic use is an important patient safety issue. An estimated 142,500 annual ED visits are for adverse events associated with systemic antibiotics.21 Our observations of ED visits suggest providers may be prescribing antibiotics based on perceived rather than actual patient expectations, consistent with non-ED literature22,23,24, highlighting inadequate communication between patients and providers. Several expressed a need to “do something” for patients, including using antibiotics as a “placebo”. Given the recent focus on patient satisfaction (e.g., Press Ganey scores) as an indicator of quality of care, there will likely be increasing focus on patient satisfaction in the ED by hospital administration and regulatory bodies, despite lack of evidence for improved outcomes with increased satisfaction.25
The ED environment socializes providers to acquire specific behaviors and beliefs. Many participants attributed antibiotic overuse to “knee-jerk reactions” or “the culture of the ED.”, or the concept of “mindlines,” where clinicians demonstrate shared rationales constructed from different spheres of influence such as specialty training, peer influence, and the pressure to “conform with perceived patient preferences” rather than follow clinical guidelines.26 Moreover, providers prescribed antibiotics even when they were not confident in their diagnoses, perceiving the risk of a poor outcome to be greater than individual patient risk of an unnecessary antibiotic. Providers articulated that azithromycin prescriptions for upper respiratory prescription are perceived to be “like water,” a “safe, cheap and effective” choice and thus, given out “like candy.”
While there is a great desire for a simple solution to antibiotic prescribing in a chaotic environment, the results from this study demonstrate the complex behavioral and environmental factors that interplay. Providers identified several potential facilitators to antimicrobial stewardship in the ED (Table 4), including local resources, partnering with patients to use a “wait and see” approach, call-back of patients for whom microbial cultures have been ordered, patient and provider education, improved diagnostic testing, provider feedback mechanisms, clinical decision support, and more tailored guidelines. Most providers referenced pocket antibiotic guides or local or national guidelines to make prescribing decisions; however, they had a difficult time keeping abreast with evolving recommendations and frequently turned to the Internet to obtain current evidence-based guidance.
Table 4.
Barriers and Facilitators to Antimicrobial Stewardship
| Antibiotic Stewardship Intervention | Barrier | Facilitator |
|---|---|---|
| Antibiograms | “They’re really difficult to read. And if you don’t have the knowledge on what you might be covering in the first place they’re a bit pointless.” – Attending, 6 yrs. | They actually had inpatient and outpatient specific biograms, and that was actually useful, and it was also sobering. – Attending, 10 yrs. |
| Wait and See Prescriptions | “If you had someone who seemed very reliable and could actually verbalize to you the plan and had a working phone that would be a person I would be willing to try it with. But, in our system, often we end up not meeting all those standards, so we just give them the antibiotic.” – Attending, 14 yrs. | “Somebody with a borderline infection. I’m not sure whether I think it’s truly bacterial infection or whether needs treatment, but the convenience of having to come back, they might have to wait 10 hours to be re-evaluated. So I let them re-evaluate themselves.” – Attending, 11 yrs. |
| Culture Callbacks | “It creates a level of comfort where physicians feel like they can order more cultures than necessary, but on the back end, the physicians or the nurse practitioners have to follow up, I feel like it probably creates a lot more extra work than necessary.” – Resident, 3 yrs. | “We’re lucky here, the nurses keep track of all the cultures that we order, blood and urine cultures, and if we have not prescribed the appropriate antibiotic or didn’t prescribe antibiotics then they let the night doc know.” – Attending, 2 yrs. |
| Patient Education | “I’ve had a lot of patients come in with an agenda and because they’ve already researched the symptoms themselves they think they have something and that they’ve figured out for themselves online.” – Attending, 27 yrs. | If you have a chance to actually talk to the patient about why you are not giving the antibiotics, it makes them understand. – Resident, 4 yrs. |
| Provider Education | Right now, a lot of the continuing education is the exact opposite. It’s pharmaceutical industry based, trying to get you to prescribe more antibiotics in a, typically, very broad-spectrum antibiotics. And so, if there were education to counter that, that might be useful. – Attending, 11 yrs. | “The fact that I work in an academic facility with residents, fellows, faculty that are always going to ask why did you use that? Why couldn’t you have just used this? That is always staying in the back of my mind that I need to be able to clearly defend my decision to use an antibiotic in a given situation.” – Attending, 8 yrs. |
| Diagnostic Testing | “It’s easier to just kind of churn through the patients than sit and wait for a rapid strep.” – Resident, 2 yrs. | “I think a completely normal urine dipstick makes a UTI less likely. It helps you pursue other diagnoses.” – Attending, 22 yrs. |
| Clinical Decision-Making Support | “I worry about it through the electronic health record because you definitely get pop-up fatigue, where you just don’t want to see anymore pop-ups and you’re like please let me discharge this patient. Just click through all of them, you know?” – Attending, 6 yrs. | “A centralized location of information, an actual website where you go to and say, this is the antibiotic and this is the condition it treats and to actually have it be free.” – Resident, 3 yrs. |
| Performance Feedback | “If people bounced back to the emergency department, it got flagged, and people reviewed that case. That was a good improvement measure you could assess.” – Attending, 4 yrs. | “I always have to make sure the patient is 100 percent satisfied with their visit by the time that they leave. Or else I’ll hear about it in a bad way.” – Attending, 4 yrs. |
| Guidelines | “The problem with guidelines in general, is there is unique patient populations. And if they’re not addressed in the guidelines, then you kinda just have to default to what you think is best.” – Attending, 10 yrs. | “We love guidelines. I mean they make it easy for us and also gives us ammunition when we’re talking to the patient. We have specific guidelines that say to do this. We have specific guidelines that say to prescribe this.” – Attending, 5 yrs. |
Particularly unexpected was that local knowledge sources, especially colleagues’ opinions, were perceived as more effective in modifying prescribing behavior than national guidelines. In fact, many providers cited specific individuals and explained how their research or opinions directly influenced their antibiotic prescription practices.
Our findings must be considered in the context of our study limitations, namely the use of a convenience sample of mainly academic EDs in two geographic regions, our small sample size and low survey response rate, and the observation of patient-provider interactions in a single ED with likely underreporting of many of themes in the fast paced ED environment. Selection bias is likely given the convenience sampling and low response rate; however, these response rates are not atypical based on prior research involving residents.27 Participation in the interview may also have led to a Hawthorne effect in our observational study. Finally, we did not collect socioeconomic or demographic data on patients; however, patient responses may depend on these indicators.
Despite these limitations, we feel our study results are an important step in better understanding antibiotic prescribing in the ED, providing critical information to designing effective ED-based antimicrobial stewardship interventions, namely the importance of local knowledge generation rather than a “one size fits all” approach. Potential interventions to address barriers to change in the ED include educational outreach, feedback to the clinical care team, and process change.28 While providers are amenable to the use of novel and easily accessible resources, formal audit mechanisms may not be easily accepted or effective in an ED environment. Best practices solutions may be multifaceted, incorporating shared decision making with patients,29,30,31 although the burden of appropriate antibiotic prescribing falls largely on the provider. Finally, any solution to improving antimicrobial prescribing in the ED will need to take into account the patient-provider relationship and local healthcare system support in order to be successful. A multidisciplinary approach, incorporating behavioral sciences, may reduce barriers to behavior change in the prescribing process and aid in guiding effective interventions for antimicrobial stewardship in the ED.12
Acknowledgments
Funding and Acknowledgements: This project was supported by Award Numbers UL1TR000075 and KL2TR000076 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. We would like to acknowledge Kristin Breslin for her assistance with data collection.
Footnotes
Prior presentation: Results from the quantitative survey were presented at IDWeek 2013 in October 2013 in San Francisco, California
Transparency Declarations: The authors have no financial conflicts of interest to declare.
Contributor Information
Larissa May, Email: larissa.may@gmail.com, 2120 L Street, NW, Suite 450, Washington, DC 20037, Phone: 202-741-2923, Fax: 202-741-2921.
Glencora Gudger, Email: coraglen@gmail.com, 2120 L Street, NW, Suite 450, Washington, DC 20037, Phone: 202-741-2920, Fax: 202-741-2921.
Paige Armstrong, Email: parmstro@email.gwu.edu, 2120 L Street, NW, Suite 450, Washington, DC 20037, Phone: 202-741-2920, Fax: 202-741-2921.
Gillian Brooks, Email: brooksg6@gmail.com, 2120 L Street, NW, Suite 450, Washington, DC 20037, Phone: 202-741-2920, Fax: 202-741-2921.
Pamela Hinds, Email: pshinds@childrensnational.org, Children’s National Health System, Department of Nursing Research and Quality Outcomes, Center for Translational Research, 111 Michigan Avenue, NW, 6th Floor Research, Washington, DC 20010. Professor, Department of Pediatrics, The George Washington University, Phone: 202-476-4432, Fax: 202-476-3425.
Rahul Bhat, Email: rgbhat77@gmail.com, Georgetown University, Department of Emergency Medicine, 110 Irving Street, NW, Washington, DC 20010, Phone: 202-877-8080, Fax: 202-877-7633.
Gregory J. Moran, Email: gmoran@ucla.edu, Department of Emergency Medicine and Division of Infectious Diseases, Olive View-UCLA Medical Center, 14445 Olive View Drive, Sylmar, CA 91342, Phone: (818) 364-3110, Fax: (818) 364-3268.
Lisa Schwartz, Email: lschwartz@gwu.edu, Department of Clinical Research and Leadership, The George Washington University School of Medicine and Health Sciences, 2100-W Pennsylvania Ave, NW, Washington, DC 20037, Phone: 202-994-7792, Fax: (202) 994-0870.
Sara E. Cosgrove, Email: scosgro1@jhmi.edu, Department of Medicine, Division of Infectious Diseases, Johns Hopkins Medical Institutions Osler 425, 600 N. Wolfe St., Baltimore, MD 21287, Phone: 443-287-4570, Fax: 410-614-0888.
Eili Y. Klein, Email: eklein@jhu.edu, Center for Advanced Modeling, Department of Emergency Medicine, Johns Hopkins University, 5801 Smith Avenue, Davis Building Suite 3220, Baltimore MD, 21209, Phone: 410-735-7559, Fax: 410-735-6440.
Richard E. Rothman, Email: rrothman@jhmi.edu, Department of Emergency Medicine, Johns Hopkins Medical Institutions, 5801 Smith Avenue, Davis Building Suite 3220, Baltimore MD, 21209, 410-735-6428 T, 410-735-6440 F.
Cynthia Rand, Email: crand@jhmi.edu, Division of Pulmonary and Critical Care Medicine, The Johns Hopkins Institutions, 5501 Hopkins Bayview Circle, Baltimore, MD 21224, Telephone: 410-550-0545, Fax: 410-550-2612.
References
- 1.Lieberman JM. Appropriate antibiotic use and why it is important: the challenges of bacterial resistance. Pediatr Infect Dis J. 2003;22:1143–51. doi: 10.1097/01.inf.0000101851.57263.63. [DOI] [PubMed] [Google Scholar]
- 2.Shlaes DM, Gerding DN, John JF, Jr, et al. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America joint committee on the prevention of antimicrobial resistance: Guidelines for the prevention of antimicrobial resistance in hospitals. Clin Infect Dis. 1997;25(3):584–99. doi: 10.1086/513766. [DOI] [PubMed] [Google Scholar]
- 3.Karras D. Antibiotic misuse in the emergency department. Acad Emerg Med. 2006;13(3):331–3. doi: 10.1197/j.aem.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 4.Samore MH, Tonnerre C, Hannah EL, Stoddard GJ, Borotkanics RJ, Haddadin B, Harbarth S. Impact of outpatient antibiotic use on carriage of ampicillin-resistant Escherichia coli. Antimicrob Agents Chemother. 2011;55(3):1135–41. doi: 10.1128/AAC.01708-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 6.Hicks LA, Chien YW, Taylor TH, Jr, Haber M, Klugman KP Active Bacterial Core Surveillance (ABCs) Team. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53(7):631–9. doi: 10.1093/cid/cir443. [DOI] [PubMed] [Google Scholar]
- 7.Roumie CL, Halasa NB, Grijalva CG, et al. Trends in antibiotic prescribing for adults in the United States—1995 to 2002. J Gen Intern Med. 2005;20:697–702. doi: 10.1111/j.1525-1497.2005.0148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kane BG, Degutis LC, Sayward HK, D’Onofrio G. Compliance with the Centers for Disease Control and Prevention recommendations for the diagnosis and treatment of sexually transmitted diseases. Acad Emerg Med. 2004;11:371–377. doi: 10.1197/j.aem.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Schouten JA, Hulscher ME, Kullberg BJ, Cox A, Gyssens IC, van der Meer JW, Grol RP. Understanding variation in quality of antibiotic use for community-acquired pneumonia: effect of patient, professional and hospital factors. J Antimicrob Chemother. 2005;56:575–82. doi: 10.1093/jac/dki275. [DOI] [PubMed] [Google Scholar]
- 10.May L, Harter K, Yadav K, Strauss R, Abualenain J, Keim A, Schmitz G. Practice patterns and management strategies for purulent skin and soft-tissue infections in an urban academic ED. Am J Emerg Med. 2012;30(2):302–10. doi: 10.1016/j.ajem.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 11.Grover ML, Bracamonte JD, Kanodia AK, Bryan MJ, Donahue SP, Warner AM, Edwards FD, Weaver AL. Assessing adherence to evidence-based guidelines for the diagnosis and management of uncomplicated urinary tract infection. Mayo Clin Proc. 2007 Feb;82(2):181–5. doi: 10.4065/82.2.181. [DOI] [PubMed] [Google Scholar]
- 12.Jick TD. Mixing qualitative and quantitative methods: Triangulation in action. Administrative science quarterly. 1979:602–611. [Google Scholar]
- 13.Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004 Jul 12;164(13):1451–6. doi: 10.1001/archinte.164.13.1451. [DOI] [PubMed] [Google Scholar]
- 14.Abbo L, Sinkowitz-Cochran R, Smith L, Ariza-Heredia E, Gómez-Marín O, Srinivasan A, Hooton TM. Faculty and resident physicians’ attitudes, perceptions, and knowledge about antimicrobial use and resistance. Infect Control Hosp Epidemiol. 2011;32(7):714–8. doi: 10.1086/660761. [DOI] [PubMed] [Google Scholar]
- 15.Mills J, Bonner A, Francis K. Adopting a constructivist approach to grounded theory: Implications for research design. International Journal of Nursing Practice. 2006;12(1):8–13. doi: 10.1111/j.1440-172X.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 16.Tversky A, Kahneman D. Judgment under Uncertainty: Heuristics and Biases. Science. 1974;185(4157):1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- 17.Bettinghaus EP. Health promotion and the knowledge-attitude-behavior continuum. Prev Med. 1986;15(5):475–91. doi: 10.1016/0091-7435(86)90025-3. [DOI] [PubMed] [Google Scholar]
- 18.Cabana M, Rand C, Powe N, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 19.Charani E, Edwards R, Sevdalis N, et al. Behavior Change Strategies to Influence Antimicrobial Prescribing in Acute Care: A Systematic Review. Clinical Infectious Diseases. 2011;53:651–62. doi: 10.1093/cid/cir445. [DOI] [PubMed] [Google Scholar]
- 20.De Souza V, MacFarlane A, Murphy AW, Hanahoe B, Barber A, Cormicam M. A qualitative study of factors influencing antimicrobial prescribing by non-consultant hospital doctors. Journal of Antimicrobial Chemotherapy. 2006;58:840–843. doi: 10.1093/jac/dkl323. [DOI] [PubMed] [Google Scholar]
- 21.Shehab N, Patel PR, Srinivisan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clinical Infectious Diseases. 2008;47:735–43. doi: 10.1086/591126. [DOI] [PubMed] [Google Scholar]
- 22.Ong S, Nakase J, Moran GJ, Karras DJ, Kuehnert MJ, et al. Antibiotic use for emergency department patients with upper respiratory infections: prescribing practices, patient expectations, and patient satisfaction. Annals of emergency medicine. 2007;50(3):213–220. doi: 10.1016/j.annemergmed.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro E. Injudicious antibiotic use: An unforeseen consequence of the emphasis on patient satisfaction? Clinical Therapeutics. 2002;24(1):197–204. doi: 10.1016/s0149-2918(02)85015-9. [DOI] [PubMed] [Google Scholar]
- 24.Cockburn J, Pit S. Prescribing behaviour in clinical practice: patients’ expectations and doctors’ perceptions of patients’ expectations—a questionnaire study. BMJ. 1997;315(7107):520–523. doi: 10.1136/bmj.315.7107.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenton JJ, Jerant AF, Bertakis KD, Franks P. The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–411. doi: 10.1001/archinternmed.2011.1662. [DOI] [PubMed] [Google Scholar]
- 26.Chandler CIR, Jones C, Boniface G, Kaseem J, Reyburn H, Whitty CJM. Guidelines andmindlines: why do clinical staff over-diagnose malaria in Tanzania? A qualitative study Malaria Journal. 2008;7:53. doi: 10.1186/1475-2875-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.May L, Katz R, Johnston L, Sanza M, Petinaux B. Assessing physicians’ in training attitudes and behaviors during the 2009 H1N1 influenza season: a cross-sectional survey of medical students and residents in an urban academic setting. Influenza Other Respir Viruses. 2010;4(5):267–75. doi: 10.1111/j.1750-2659.2010.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, Grilli R, Harvey E, Oxman A, O’Brien MA. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001 Aug;39(8 Suppl 2):II2–45. [PubMed] [Google Scholar]
- 29.Leblanc A, Légaré F, Labrecque M, Godin G, Thivierge R, Laurier C, Côté L, O’Connor AM, Rousseau M. Feasibility of a randomised trial of a continuing medical education program in shared decision-making on the use of antibiotics for acute respiratory infections in primary care: the DECISION+ pilot trial. Implement Sci. 2011 Jan 18;6:5. doi: 10.1186/1748-5908-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wanderer JP, Sandberg WS, Ehrenfeld JM. Real-time alerts and reminders using information systems. Anesthesiol Clin. 2011 Sep;29(3):389–96. doi: 10.1016/j.anclin.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waldron N, Dey I, Nagree Y, Xiao J, Flicker L. A multifaceted intervention to implement guideline care and improve quality of care for older people who present to the emergency department with falls. BMC Geriatr. 2011;11:6. doi: 10.1186/1471-2318-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]