Abstract
Introduction
TB meningitis (TBM) diagnosis is difficult and novel diagnostic methods are needed. The World Health Organization recommends Xpert MTB/RIF (Xpert) as the initial TBM diagnostic test, based on two studies reporting suboptimal sensitivity (~50–60%).
Objective
To study the effect of cerebrospinal fluid (CSF) centrifugation on Xpert performance for TBM detection.
Design
107 predominantly HIV-infected adults with suspected meningitis were screened prospectively in Kampala, Uganda. CSF was tested by 1) microscopy for acid-fast bacilli; 2) Mycobacteria growth indicator tube culture; 3) Xpert of 2mL of unprocessed CSF; 4) Xpert of centrifuged CSF. Diagnostic performance was measured against an a priori composite reference standard of any positive CSF tuberculosis test.
Results
17% (18/107) of participants had definite TBM. When CSF was centrifuged, Xpert had better sensitivity (72%, 13/18) than when using 2mL of unprocessed CSF (28%, 5/18; P=0.008). The median centrifuged CSF volume was 6mL (IQR 4–10mL). Mycobacterial culture yielded 71% (12/17) sensitivity at a median delay of 27 days. Only 39% were positive both by culture and centrifuged Xpert, with additional cases detected by Xpert and culture.
Conclusions
Centrifuging of CSF optimizes Xpert diagnostic performance for detection of TBM. A combination of culture and Xpert detected the largest number of cases.
Keywords: Extrapulmonary Tuberculosis, Tuberculosis Meningitis, Human Immunodeficiency Virus, Laboratory Diagnostics, Xpert MTB/Rif
Introduction
Tuberculosis meningitis (TBM) is notoriously difficult to diagnose. Cerebrospinal fluid (CSF) smear microscopy for acid fast bacilli (AFB) has abysmal sensitivity (~15%), and mycobacterial culture is too slow to be clinically meaningful.1 Due to the HIV epidemic, TBM has emerged as the second most common cause of adult meningitis in Africa.2–7 The Xpert MTB/RIF (Xpert, Cepheid, Sunnyvale, CA) test, a fully automated, cartridge-based PCR assay is now available worldwide, with FDA approval for pulmonary TB diagnosis. The World Health Organization (WHO) has endorsed Xpert as the preferred initial test to investigate TBM.8,9
Data on Xpert performance in CSF in adults are limited to reports of small numbers of meningitis patients in extra-pulmonary TB cohorts10–18, multiple meta-analyses9,19,20, and two well-conducted TBM cohort studies.21,22 In South Africa, Patel et al found 67% sensitivity with Xpert in microbiologically proven TBM.22 This study initially used small volumes (1mL) of unprocessed CSF in combination with the Xpert sample reagent (mucolytic and anti-infective compounds designed for sputum). A later subset of 27 TBM cases using 3mL of centrifuged CSF exhibited increased sensitivity of 82% (22/27) although without direct comparison with un-centrifuged CSF.22 When these samples were analyzed against clinical criteria as in the Nhu cohort,21,23 the overall sensitivity was only 36%.22 In Vietnam, Nhu et al found that Xpert exhibited 59% sensitivity and 99% specificity using consensus clinical criteria as the reference standard.21 Nhu and colleagues routinely centrifuged CSF and then divided the pellet apportioning 20% for microscopy, 20% for culture, 40% for Xpert testing, and 20% for storage. Thus, the volume actually used for Xpert testing was only 40% of the total volume obtained. In addition, 17% of specimens were low volume (<2mL), 61% moderate volume (2.1–5mL), and 22% larger volume (>5mL) prior to centrifugation, without a difference in Xpert sensitivity by CSF volume (P=.34).21 Based on the specimen volumes, although Nhu and colleagues centrifuged CSF, 78% of specimens had an effective CSF input volume of <2mL for Xpert testing when taking into account the beginning specimen volume and the proportion of the pellet actually used for testing.
We conducted a prospective cohort study to systematically analyze the effect of CSF centrifugation on the diagnostic performance of Xpert MTB/Rif as compared to non-centrifuged CSF, mycobacterial culture, or AFB microscopy for TBM diagnosis.
Study Population and Methods
CSF samples were obtained during screening for the Adjunctive Sertraline for the Treatment of Cryptococcal Meningitis trial (clinicaltrials.gov NCT01802385). Persons with suspected meningitis were screened at Mulago National Referral Hospital, Kampala Uganda from October 2013 until October 2014. All participants or their surrogate provided written informed consent. Eligible persons were ≥18 years of age. Institutional review board approvals occurred. Diagnostic information was made available to clinicians in real-time.
Bedside Testing
After lumbar puncture, bedside testing was performed for cryptococcal antigen (CrAg LFA, IMMY, Norman, OK) and glucose. Determine™ TB LAM antigen (Alere Inc, Waltham MA, USA) was tested on the CSF in the first ~100 participants, was universally negative, and stopped thereafter. Participants without cryptococcal meningitis then underwent additional testing for TBM and bacterial meningitis. Participants with cryptococcal meningitis who had concern of TBM co-infection were also included at physician discretion.
Tuberculosis CSF Diagnostic Testing
Tuberculosis testing is fully described in Figure 1. After bedside testing, ~1mL of CSF was removed for routine microbiology testing, 2mL of unprocessed CSF removed for Xpert (un-centrifuged CSF); and the remaining volume centrifuged at 3000g for 15 minutes. All supernatant except for 4mL was removed. The remaining 4mL was re-suspended via vortex. Subsequently 2mL was removed for centrifuged Xpert testing, and 1.5 mL for TB culture (mycobacteria growth indicator tube 0.5mL and Lowenstein-Jensen 1mL), 0.1mL AFB smear, and 0.4mL for storage. Xpert testing was conducted without sample reagent unless the CSF was grossly bloody. No CSF was grossly bloody. Cultures were performed according to manufacturer instructions at the Uganda national TB reference laboratory.
Figure 1. Schematic of CSF processing after collection.
The figure shows the process by which CSF was handled after collection in order to accomplish GeneXpert (Centrifuged/Un-centrifuged), microbiologic testing, and CSF culture (LJ and MGIT). Volumes are approximate.
Consensus Clinical Criteria
Data were collected in order utilize the uniform case definition for use in clinical research as described by Marais and colleagues.23 Points were given based on the criteria as specified in the uniform case definitions, and participants placed in categories of definite, probable, possible and not TBM for the purposes of this study. Among this population with meningitis, definite TBM was defined in accordance with the consensus TBM definition as any CSF test positivity by microscopy, culture, or PCR (including Xpert).23
Statistical Analysis
Statistical analysis was performed using SPSS version 22 (IBM Inc., Armonk, NY). Mean baseline clinical characteristics and demographic data were compared by diagnosis via independent t-test. Performance between Xpert protocols was evaluated with McNemar’s test for concordance. Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were calculated using a composite reference standard of any CSF Xpert, CSF AFB, or CSF culture positivity. As per the Marais et al. uniform TBM case definition,23 a composite reference standard was chosen for defining “definite TBM” as culture is an imperfect TBM reference standard,23 and the likelihood of TB detection in the CSF of an HIV-infected person with lymphocytic meningitis representing a false positive result is low.9
Results
Of 257 participants with suspected meningitis, 165 (64%) had cryptococcal meningitis, and 107 (n=13 with cryptococcal meningitis) underwent further TB testing with Xpert on un-centrifuged CSF and AFB smear. Due to limited volume, 89% (95/107) participants had Xpert testing using concentrated CSF, and 75% (80/107) participants had mycobacterial cultures performed. HIV-infection was present in 98% (105/107) of participants. Definite TBM was diagnosed in 18 HIV-infected participants testing positive by TBM per the pre-specified composite reference standard of any CSF test positivity (n=5 Xpert un-centrifuged CSF; n=13 Xpert centrifuged CSF; n=12 culture; n=4 AFB microscopy) (Figure 2 and Figure 3). Non-TBM diagnoses (n=98) included: cryptococcal meningitis (n=13), bacterial meningitis (n=1), lymphocytic aseptic meningitis of presumed viral etiology (n=55), normal CSF profile (n=20). There were no significant baseline differences between those with and without TBM aside from CSF leukocyte count, which was higher in those with TBM (Table 1).
Figure 2. Venn diagram of overlap in TB meningitis diagnostics.
The Venn diagram displays 18 participants diagnosed by each diagnostic test and the overlap. One participant who was composite (Xpert on centrifuged CSF) positive had invalid culture results. Although the diagnostic sensitivity was similar between centrifuged CSF with Xpert and culture, only 39% (7/18) were positive by both culture and centrifuged Xpert.
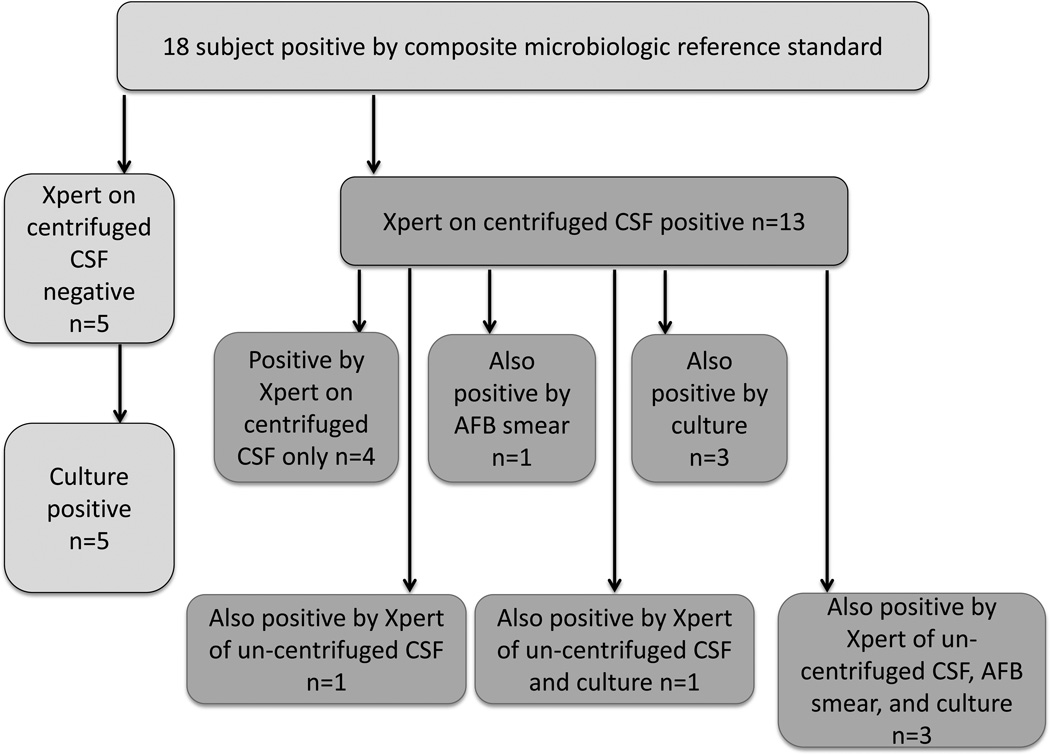
Figure 3. Flow chart of methods by which each TBM case was detected microbiologically.
18 subjects were microbiologically confirmed to have TBM. 13 cases were positive by Xpert of centrifuged CSF (right), among those cases the other methods by which M tuberculosis was detected are noted below. 5 cases were not detected by Xpert testing of centrifuged CSF (left) but were detected by culture.
Table 1.
Patient Characteristics
| TB Meningitis N=18 |
Other Meningitis N=89 |
P value | |
|---|---|---|---|
| Age, years | 40 (30–45) | 36 (30–44) | 0.95 |
| Male, percent | 66% (12/18) | 61% (54/89) | 0.79 |
| Headache duration, days | 14 (14–21) | 14 (7–21) | 0.84 |
| CD4 count, cells/µL | 50 (15–175) | 45 (10–125) | 0.82 |
| CSF white cells/μL | 92 (≤4–409) | ≤4 (≤4–83) | 0.04 |
| CSF lymphocytes, % | 100% (100-100) | 100% (100-100) | 0.48 |
| CSF total protein, mg/dL | 74 (28–272) | 46 (20–133) | 0.97 |
| CSF glucose, mg/dL | 64 (19–95) | 66 (48–82) | 0.42 |
| Prior TB diagnosis | 22% (4/18) | 25% (22/89) | 0.80 |
| Receiving TB treatment | 11% (2/18) | 14% (12/89) | 0.77 |
| Alive at discharge | 60% (9/15) | 48% (31/65) | 0.68 |
Values given as median (IQR) or percent (n). Receiving TB treatment refers to TB treatment prior to diagnostic lumbar puncture. Among 89 participants with other meningitis, etiologies included Cryptococcus neoformans var grubii (n=13), Streptococcus pneumoniae (n=1), lymphocytic aseptic meningitis of unknown etiology (n=55), and suspected meningitis with normal CSF profile of <5 leukocytes/µL and protein <40mg/dL (n=20).
Xpert MTB/Rif Diagnostic Performance
Table 2 compares diagnostic performance for microbiologically proven definite TBM among CSF: AFB smear, culture, and both methods of Xpert testing. Xpert on un-centrifuged CSF produced a sensitivity of 28% (5/18). Centrifugation of the CSF improved performance of Xpert to 72% (13/18) sensitivity and 95% NPV. No samples returned error results on Xpert testing. Mycobacterial culture yielded 71% (12/17) sensitivity and 93% NPV. The median time to notification of positive culture was 27 (IQR, 16–39) days. Microscopy with AFB smear on centrifuged CSF yielded only 22% sensitivity (4/18).
Table 2.
CSF Diagnostic Performance for TB Meningitis of Xpert MTB/Rif, Culture, and AFB Smear Microscopy
| CSF Test | N | Sensitivity | Specificity | Positive Predictive Value |
Negative Predictive Value |
|---|---|---|---|---|---|
|
Xpert 2mL un- centrifuged CSF |
107 | 28% (5/18) |
100% (89/89) |
100% (5/5) |
87% (89/102) |
|
Xpert centrifuged CSF |
95 | 72% (13/18) |
100% (77/77) |
100% (13/13) |
94% (77/82) |
| Culture | 80 | 71% (12/17) |
100% (63/63) |
100% (12/12) |
93% (63/68) |
|
AFB Smear by Microscopy |
107 | 22% (4/18) |
100% (89/89) |
100% (4/4) |
86% (89/103) |
Culture performed by Bactec Mycobacterial growth indicator tube (MGIT) with 12 of 17 positive, and Löwenstein-Jensen media with 7 of 14 positive.
Centrifuged CSF testing by Xpert and culture was not fully concordant (n=7 positive by both, n=6 centrifuged Xpert only, n=5 culture only). Similarly, of 93 CSF specimens tested by both Xpert protocols (un-centrifuged vs. centrifuged), 5 were positive by both methods and 8 were positive by centrifuged Xpert only (P=0.008). When only participants who had all of AFB smear, culture, and Xpert on centrifuged and un-centrifuged CSF performed (n=80 total, 17 TBM) were analyzed, and sensitivities were 24% (4/17) for AFB microscopy, 29% (5/17) for Xpert on un-centrifuged CSF, 71% (12/17) for Xpert on centrifuged CSF, and 71% (12/17) by culture. Two participants diagnosed with TBM were receiving antecedent TB induction therapy for pulmonary TB (<30 days) at the time of meningitis diagnosis. Of these, one was positive only by Xpert (un-centrifuged and centrifuged CSF), and the other was positive by culture and Xpert of centrifuged CSF only.
Rifampin resistance by Xpert testing was detected in two persons, not detected in seven persons, and indeterminate in three persons. Additionally, one rifampin resistance result was discordant being “detected” in 2mL of non-centrifuged CSF and not-detected in 8mL of centrifuged CSF in a person who had received two days of TB therapy and in whom CSF culture was sterile.
CSF Volume
The median volume of CSF centrifuged was 6mL (IQR 4–10mL). The median minimal volume required for any positive Xpert test (either un-centrifuged or centrifuged CSF not counting volume used for other testing, n=13) was 3.5mL (IQR 2–7.5mL), with 5 participants requiring only 2 mL of un-centrifuged CSF. Of those 8 participants with positive Xpert results only on centrifuged CSF, the median volume used for Xpert testing of centrifuged CSF was 6mL (IQR 3.75–8.5mL). Of the five participants TB culture positive but Xpert negative, the median centrifuged CSF volume used for Xpert testing was less (median 3.5mL; IQR 1.75–7mL).
TBM Classification Based on Consensus Uniform Clinical Case Definition
As noted above, participants were classified based on the consensus uniform case definition.23 Definite TBM was present in 18 participants as defined above with positive PCR, culture, and/or AFB smear of CSF. Two participants had probable TBM (i.e. ≥10 points on the diagnostic criteria scale with at least two points coming from CSF criteria). Possible TBM was present in 53 persons defined by a score of 6–9 points or 6–11 points if head imaging was performed, again with 2 points coming from CSF-based criteria. Fourteen participants did not have TBM as defined by a known alternative diagnosis of cryptococcal meningitis (n=13) or Streptococcus pneumoniae (n=1) and no convincing evidence of TBM. Twenty participants did not fit in any of the above categories (e.g. score <6 points, no alternative diagnosis, and normal CSF profiles).
When Xpert testing of centrifuged CSF was compared to the uniform case definition criteria including probable or definite TBM as a reference standard we found 65% (13/20) sensitivity, 100% (75/75) specificity, 100% (13/13) PPV, and 91% (75/82) NPV. When possible TBM was included, the sensitivity of Xpert testing of centrifuged CSF dropped to 20% (13/64) sensitivity. If scored by criteria alone, of the 18 microbiologically proven cases of TBM, 1 would have been classified as probable TBM, 12 as possible TBM, and 5 would have not fit in any diagnostic category (e.g. score < 6 and no known alternative diagnosis). Further, three subjects with microbiologically proven TBM had CSF glucose, WBC counts, and protein values that were entirely within normal limits. These findings demonstrate the necessity of microbiologic investigations in TBM diagnosis.
Discussion
In this prospective study, we demonstrate that centrifugation of CSF prior to Xpert MTB/Rif automated testing significantly improves detection of Mycobacterium tuberculosis. Using Xpert, centrifuged CSF had 72% sensitivity compared with only 28% sensitivity when 2mL of un-centrifuged CSF was tested. Second, we performed testing without using the Xpert Sample Reagent, which is a mucolytic and decontaminant, designed for sputum. The necessity of centrifugation of a large volume of CSF to improve Xpert sensitivity is vital, life-saving knowledge now that the WHO has recommended Xpert as the initial diagnostic TBM test.8,9
Two prior studies examined Xpert rigorously for TBM; however, the effect of centrifugation was not systematically studied in direct comparison to un-centrifuged CSF.21,22 The study by Patel and colleagues initially used ~1mL of CSF but later noted a non-statistical trend of increased sensitivity when centrifuging 3mL of CSF.22 The study by Nhu et al routinely centrifuged CSF however volumes varied, yet 78% of specimens tested had an original volume of ≤5mL sent for TB testing, of which 40% was used for Xpert testing, equating to <2mL of CSF actually tested by Xpert.21 Neither study directly compared the performance of Xpert on centrifuged and un-centrifuged CSF.21,22
Despite improved diagnostic accuracy using centrifuged CSF for Xpert compared with un-centrifuged CSF, the ideal CSF volume to collect is unknown. Yet, had the centrifugation step not been taken, 44% of persons would not have been diagnosed by Xpert testing with TBM (median volume centrifuged 8mL), and 22% would not have been diagnosed with TBM at all. The current Xpert is not a panacea for the challenges of TBM diagnostics, as 28% persons were culture positive without positive Xpert. Quite surprising, the overlap of both culture-positive and Xpert-positive TBM persons was only 39%.
Fundamentally, the pauci-bacillary nature of TBM is a primary reason that microbiologic confirmation remains challenging.24 Xpert has an analytical sensitivity detection threshold of approximately ~100 CFU/mL M. tuberculosis organisms.22,25 CSF centrifugation can increase the number of TB bacilli present in the given input volume inserted into the Xpert MTB/Rif cartridge (~2mL), thus making detection possible if the necessary threshold is reached. Interestingly, though MGIT culture has an analytical sensitivity threshold of ≤10 bacilli,26 we found equivalent sensitivity (71%) compared to Xpert. It is possible that unviable bacilli detectable by PCR but not culture - with ~10–15% of participants already receiving TB treatment at screening account for cases detected by Xpert but not culture. This is a plausible, real world limitation of culture. On the other hand, 28% of cases positive by culture were not by Xpert. This is likely due to not being able to reach the limit of detection threshold, even with centrifugation. Yet TB culture, even where available, takes too long to be clinically useful for TBM, where mortality is rapid.27 Thus, centrifugation of CSF for use with Xpert is a step that improves sensitivity in an already rapid test – allowing for clinically relevant results. For persons initially negative by Xpert in whom TBM is suspected, repeat lumbar punctures with larger CSF volumes sent for Xpert testing may improve the diagnostic yield.
Rifampin resistance testing by Xpert in this study produced mixed results, and the paucibacillary nature of TBM creates possible technical challenges. In our population, rifampin resistance testing was indeterminate in three subjects and resistance detected in two subjects. However, among one person with resistance detected, there were discordant results having resistance detected in the 2mL non-centrifuged sample, but resistance not detected in the 7mL centrifuged sample. As the CSF was collect two days after TB therapy was started, such discordant results could reflect either hetero-resistance or more likely a paucity of DNA present to amplify. The Xpert assay can occasionally produce false positive rifampin resistance results28. In expected paucibacillary scenarios (e.g. cerebrospinal, pleural, or peritoneal fluid), when rifampin resistance is unexpectedly detected by Xpert, repeat Xpert testing, or mycobacterial culture and drug susceptibility testing would be ideal, if available. However for immediate clinical guidance, before launching second line TB therapy, one might consider repeat GeneXpert testing with a large volume sample collection with centrifugation. Clinicians should be aware that discordant rifampin resistance can be observed in paucibacillary specimens
Uniform TBM case definitions developed for use in clinical research were utilized in this study.23 This composite reference standard creates the potential for an incorporation bias, whereby any positive TB test in CSF, including a positive Xpert test, confirms the definite TBM. We thought this was reasonable based on the published experience with Xpert,21,22 WHO recommends use of Xpert as the initial preferred test for TBM,8,9 and the high pretest probability of a positive automated PCR test on CSF in a HIV-infected population with AIDS and a median CD4 count of 50 cells/µL representing true TBM. As a commercial molecular assay, a positive Xpert would be defined as a definite TBM via the Marais et al. uniform clinical case definition.23
Our study was conducted in a majority (98%) HIV-infected population, thus these data are difficult to generalize outside of this population. However, the concept of concentrating clinical samples to increase the number of bacilli present in the input specimen for Xpert testing is generalizable. This concept is generalizable beyond CSF to any other bodily fluid tested (e.g. urine, stool, gastric, pleural, or peritoneal fluids). Future studies on concentration of other specimen types should be pursued. Although the relatively small overall number of TBM patients included in this prospective study is a limitation for the precision of the sensitivity estimate; the rigorous study design allows confidence that our primary research question was answered. We sought to answer whether centrifugation of CSF would increase diagnostic yield with GeneXpert. A larger sample size would be unlikely to change this principle finding (P=0.008).
In summary, we have shown that centrifugation of CSF for Xpert MTB/Rif testing improves diagnostic yield over unprocessed CSF, with similar sensitivity as CSF culture. The WHO has recommended Xpert as the preferred initial diagnostic test for TBM,9 and our study suggests collection of 6–10mL of CSF with centrifugation will improve the TB diagnostic yield of Xpert. Additional point-of-care TB diagnostics are needed.
Acknowledgments
Acknowledgements and Funding:
The authors wish to thank Drs. Paul Bohjanen, Yukari Manabe, and Bryan Rock, for support and input. We thank Andrew Akampurira, Tonny Luggya, Francis Kakooza, Olive Mbabazi, Grace Turyasingura, and the Infectious Disease Institute’s translational laboratory staff, Dr. Moses Joloba and his mycobacterial laboratory staff at Makerere University. We thank Drs. Henry Nabeta and Abdu Musubire for clinical care in Kampala. We also thank Danielle Amisano for input regarding the nature of the Xpert MTB/Rif assay.
This work was supported by the National Institute of Neurologic Disorders and Stroke, the National Institute of Allergy and Infectious Disease, and the Fogarty International Center at the National Institutes of Health (R01NS086312, T32AI055433, R25TW009345). None of the funders had a role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
All authors contributed significantly to the work fulfilling the criteria for authorship and have no conflicts of interest.
References
- 1.Bahr NC, Boulware DR. Methods of rapid diagnosis for the etiology of meningitis in adults. Biomark Med. 2014;8(9):1085–1103. doi: 10.2217/bmm.14.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marais S, Pepper DJ, Schutz C, Wilkinson RJ, Meintjes G. Presentation and outcome of tuberculous meningitis in a high HIV prevalence setting. PLoS One. 2011;6(5): e20077. doi: 10.1371/journal.pone.0020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durski KN, Kuntz KM, Yasukawa K, Virnig BA, Meya DB, Boulware DR. Cost-effective diagnostic checklists for meningitis in resource-limited settings. J Acquir Immune Defic Syndr. 2013;63(3):e101–e108. doi: 10.1097/QAI.0b013e31828e1e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajasingham R, Rhein J, Klammer K, et al. Epidemiology of meningitis in an HIV-infected Ugandan cohort. Am J Trop Med Hyg. 2015;92(2):274–279. doi: 10.4269/ajtmh.14-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis. 2010;10:67. doi: 10.1186/1471-2334-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berenguer J, Moreno S, Laguna F, et al. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N Engl J Med. 1992;326(10):668–672. doi: 10.1056/NEJM199203053261004. [DOI] [PubMed] [Google Scholar]
- 7.Woldeamanuel YW, Girma B. A 43-year systematic review and meta-analysis: case-fatality and risk of death among adults with tuberculous meningitis in Africa. J Neurol. 2014;261(5):851–865. doi: 10.1007/s00415-013-7060-6. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Global Tuberculosis Report- 2014. 2014
- 9.World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children: Policy Update. 2013 [PubMed]
- 10.Al-Ateah SM, Al-Dowaidi MM, El-Khizzi NA. Evaluation of direct detection of Mycobacterium tuberculosis complex in respiratory and non-respiratory clinical specimens using the Cepheid Gene Xpert(R) system. Saudi Med J. 2012;33(10):1100–1105. [PubMed] [Google Scholar]
- 11.Hillemann D, Rusch-Gerdes S, Boehme C, Richter E. Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. J Clin Microbiol. 2011;49(4):1202–1205. doi: 10.1128/JCM.02268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tortoli E, Russo C, Piersimoni C, et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur Respir J. 2012;40(2):442–447. doi: 10.1183/09031936.00176311. [DOI] [PubMed] [Google Scholar]
- 13.Vadwai V, Boehme C, Nabeta P, Shetty A, Alland D, Rodrigues C. Xpert MTB/RIF: a new pillar in diagnosis of extrapulmonary tuberculosis? J Clin Microbiol. 2011;49(7):2540–2545. doi: 10.1128/JCM.02319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunsow E, Ruiz-Serrano MJ, Lopez Roa P, Kestler M, Viedma DG, Bouza E. Evaluation of GeneXpert MTB/RIF for the detection of Mycobacterium tuberculosis and resistance to rifampin in clinical specimens. J Infect. 2014;68(4):338–343. doi: 10.1016/j.jinf.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Zeka AN, Tasbakan S, Cavusoglu C. Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J Clin Microbiol. 2011;49(12):4138–4141. doi: 10.1128/JCM.05434-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanif SN, Eldeen HS, Ahmad S, Mokaddas E. GeneXpert(R) MTB/RIF for rapid detection of Mycobacterium tuberculosis in pulmonary and extra-pulmonary samples. Int J Tuberc Lung Dis. 2011;15(9):1274–1275. doi: 10.5588/ijtld.11.0394. [DOI] [PubMed] [Google Scholar]
- 17.Moure R, Munoz L, Torres M, Santin M, Martin R, Alcaide F. Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J Clin Microbiol. 2011;49(3):1137–1139. doi: 10.1128/JCM.01831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YW, Kwak N, Seong MW, et al. Accuracy of the Xpert(R) MTB/RIF assay for the diagnosis of extra-pulmonary tuberculosis in South Korea. Int J Tuberc Lung Dis. 2015;19(1):81–86. doi: 10.5588/ijtld.14.0500. [DOI] [PubMed] [Google Scholar]
- 19.Solomons RS, van Elsland SL, Visser DH, et al. Commercial nucleic acid amplification tests in tuberculous meningitis--a meta-analysis. Diagn Microbiol Infect Dis. 2014;78(4):398–403. doi: 10.1016/j.diagmicrobio.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Penz E, Boffa J, Roberts DJ, et al. Diagnostic accuracy of the Xpert((R)) MTB/RIF assay for extra-pulmonary tuberculosis: a meta-analysis. Int J Tuberc Lung Dis. 2015;19(3):278–284. doi: 10.5588/ijtld.14.0262. [DOI] [PubMed] [Google Scholar]
- 21.Nhu NT, Heemskerk D, Thu do DA, et al. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J Clin Microbiol. 2014;52(1):226–233. doi: 10.1128/JCM.01834-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel VB, Theron G, Lenders L, et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: a prospective study. PLoS Med. 2013;10(10):e1001536. doi: 10.1371/journal.pmed.1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803–812. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 24.Boulware DR. Utility of the Xpert MTB/RIF assay for diagnosis of tuberculous meningitis. PLoS Med. 2013;10(10):e1001537. doi: 10.1371/journal.pmed.1001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helb D, Jones M, Story E, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48(1):229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Zyl-Smit RN, Binder A, Meldau R, et al. Comparison of quantitative techniques including Xpert MTB/RIF to evaluate mycobacterial burden. PLoS One. 2011;6(12):e28815. doi: 10.1371/journal.pone.0028815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torok ME, Yen NT, Chau TT, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)--associated tuberculous meningitis. Clin Infect Dis. 2011;52(11):1374–1383. doi: 10.1093/cid/cir230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson DA, Basu I, Bower J, Freeman JT, Henderson G, Roberts SA. An evaluation of the Xpert MTB/RIF assay and detection of false-positive rifampicin resistance in Mycobacterium tuberculosis. Diagn Microbiol Infect Dis. 2012;74(2):207–209. doi: 10.1016/j.diagmicrobio.2012.06.013. [DOI] [PubMed] [Google Scholar]