Abstract
AIM
To explore how oxygen saturation in retinal blood vessels is altered in ischemic and non-ischemic branch retinal vein occlusion (BRVO).
METHODS
Fifty BRVO eyes were divided into ischemic (n=26) and non-ischemic (n=24) groups, based on fundus fluorescein angiography. Healthy individuals (n=52 and n=48, respectively) were also recruited as controls for the two groups. The mean oxygen saturations of the occluded vessels and central vessels were measured by oximetry in the BRVO and control groups.
RESULTS
In the ischemic BRVO group, the occluded arterioles oxygen saturation (SaO2-A, 106.0%±14.3%), instead of the occluded venule oxygen saturation (SaO2-V, 60.8%±9.4%), showed increases when compared with those in the same quadrant vessels (SaO2-A, 86.1%±16.5%) in the contralateral eyes (P<0.05). The oxygen saturations of the central vessels showed similar trends with those of the occluded vessels. In the non-ischemic BRVO group, the occluded and central SaO2-V and SaO2-A showed no significant changes. In both the ischemic and non-ischemic BRVOs, the central SaO2-A was significantly increased when compared to healthy individuals.
CONCLUSION
Obvious changes in the occluded and central SaO2-A were found in the ischemic BRVO group, indicating that disorders of oxygen metabolism in the arterioles may participate in the pathogenesis of ischemic BRVO.
Keywords: hypoxia, ischemia, oximetry, oxygen saturation, branch retinal vein occlusion
INTRODUCTION
Retinal vein occlusion (RVO) is the second most common retinal vascular disorder, after diabetic retinopathy, and is considered to be an important cause of visual loss[1]–[2]. Branch retinal vein occlusion (BRVO) is the most common of the RVOs, with an incidence of 0.5%-1.2%[3]–[4]. Major BRVO is comprised of a non-ischemic form and an ischemic form, detectable in one-third and two-thirds of cases, respectively[5]–[7]. At present, the definite pathogenesis of BRVO is still unclear, and researchers are mainly focusing on the compression of the veins at the arteriovenous (AV) crossings, degenerative changes within venous walls, hypercoagulability[1],[8], increased endothelin-1 produced by atherosclerotic arteries[9] and a decrease in nitric oxide [10].
The retina is the tissue that has the highest rate of oxygen consumption in the body[2]. However, oxygen is also known to be the most supply-limited metabolite, is essential and critical for retinal function, and associated with a large proportion of retinal blindness[11]. Due to mechanical compression, BRVO reduces blood flow to the part of the retina[12]–[14], which supplies the inner retina with oxygen[15]. Additionally, animal studies have demonstrated retinal hypoxia in experimental BRVO[16]–[18]. However, retinal hypoxia may contribute to the production of vascular endothelial growth factor[19], and to the development of macular edema[20] and neovascularization[21]. To some extent, the oxygen saturation can reflect the blood flow status, so it is necessary to detect the oxygenation status to estimate the retinal function indirectly.
Fluorescence fundus angiography (FFA) plays an irreplaceable role in the diagnosis, classification, guidance of treatment and evaluation of the therapeutic effects of BRVO. Although FFA is the criterion standard for the in vivo evaluation of the retinal circulation[22], it cannot reflect the oxygenation status of the retina, has risks for adverse effects, and is not readily available for all patients[23]. The retinal oximeter is a new device for measuring the oxygen saturation of the retinal vessels in vivo, noninvasively. It has been widely used in many diseases to detect the alteration of oxygen saturation in different conditions, such as glaucoma[24], diabetic retinopathy[25], retinal artery occlusion[26] and RVO[27]–[28]. Previous studies have found highly variable venule saturations and increased arteriole saturations[28], but these results were not analysed by different types of BRVOs according to the FFA. Additionally, there are still no reports about Chinese BRVO patients.
BRVO is still a controversial disease. In our study, we detected the shifts in the retinal vessel oxygen saturations in non-ischemic and ischemic BRVO patients in China, and tried to explore the possible influence factors' associations with the oximetry values. Our findings may support the classification of BRVO by FFA and explain the mechanism of different types BRVO to some extent.
SUBJECTS AND METHODS
The study protocol was reviewed and approved by the Medical Ethics Committee of Zhongshan Ophthalmic Centre, Sun Yat-sen University (No.2013MEKY028), and adhered strictly to the principles of The World Medical Association Declaration of Helsinki. All subjects signed informed consents before participation.
Subjects
The inclusion criteria for the BRVO patients were as follows: 1) Chinese (of xanthodermic origin); 2) BRVO occurred in one eye, and affected only a major branch; 3) complete case data, such as vision, fundus colour photography, FFA; and 4) no history of retinal laser treatment. The inclusion criteria for the control subjects were as follows: 1) Chinese (of xanthodermic origin); 2) best corrected visual acuity (BCVA, ETDRS charts) ≥ 65; 3) no ocular diseases; 4) no history of eye surgery.
The exclusion criteria for the BRVO patients and control subjects were as follows: 1) other ocular (diabetic retinopathy or high myopia retinopathy) and/or systematic pathologies (diabetes, severe cardiovascular or respiratory disease), which can affect the measurements of retinal oximetry; 2) a history of ocular trauma or surgery; 3) without complete case data; 4) oximetry fundus images with poor quality.
Fifty BRVO patients (50 eyes) were diagnosed with BRVO by ophthalmologists in the Zhongshan Ophthalmic Centre. The diagnosis of BRVO was based upon the biomicroscopic aspect (detection of superficial and/or deep retinal haemorrhages in a retinal quadrant, congestion and tortuosity of the corresponding venous vessels, with eventual presence of exudates, and oedema in the optic disk and retina) and the fluoroangiographic aspect, which revealed the delayed filling of the venous branch involved.
BRVO was defined as ischemic if it showed a capillary nonperfusion area >5 disk diameters (DD), retinal neovascularization or vitreous hemorrhage upon fluorescein angiography. A non-perfusion area of the retinal capillary ≤5 DD was defined as non-ischemic BRVO[5]–[7],[29], which is shown in Figure 1. The oxygen saturation was measured in the retinal blood vessels in both groups with BRVO, and compared with the eyes of age and gender-matched healthy individuals (group A, n=48; group B, n=52).
Figure 1. The classification of BRVO based on FFA and oximetry image.
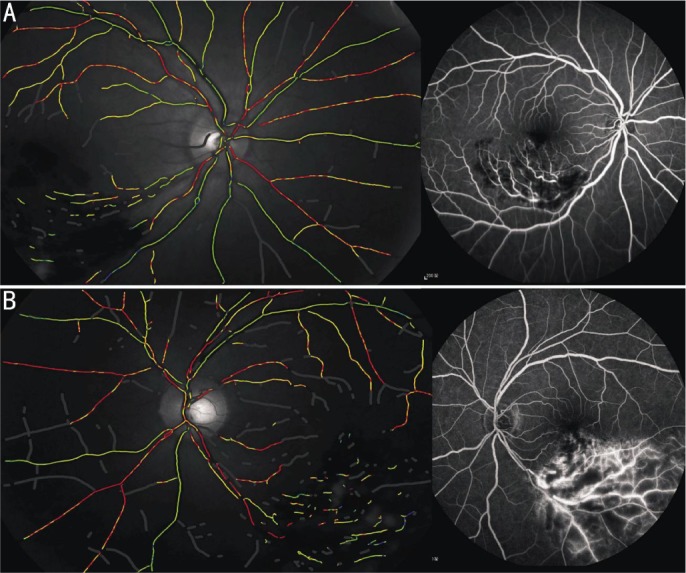
A: The left pseudocolor fundus image of non-ischemic BRVO patient, shows normal retinal oxygen saturation: SaO2-A, 92.8%, SaO2-V, 56.6%; the right FFA image shows the hemorrhage, and non-perfusion area of retinal capillary ≤5 DD; B: The left pseudocolor fundus image of ischemic BRVO patient, shows increased retinal oxygen saturation: SaO2-A, 99.2% SaO2-V, 67.1%; the right FFA image shows the hemorrhage, non-perfusion area of retinal capillary >5 DD, and retinal neovascularization.
A more detailed analysis was performed on both eyes of the BRVO patients. All measurements were performed before any treatment of the BRVO. All subjects underwent complete ophthalmic examinations that included: BCVA (ETDRS logMAR visual acuity chart), intraocular pressure (IOP) (Canon TX-20, Canon Corporation, Tokyo, Japan), slit-lamp examination (Suzhou YZ5S, Suzhou Liuliu, China), systolic blood pressure (BPsyst), diastolic blood pressure (BPdiast), heart rate (BangPu, BF-1100, Shenzhen BangPu Corporation, Shenzhen, China) and finger pulse oximetry (Biolight M70, Biolight Corporation, Zhuhai, Guangdong Province, China). The FFA and optical coherence tomography (OCT) images were obtained from the Spectralis HRA+OCT (Heidelberg Engineering, Heidelberg, Germany). The mean ocular perfusion pressure (OPPm), driving blood through the retina, was calculated by the following equation: OPPm=2/3(2/3BPdiast+1/3BPsyst)-IOP[24].
All statistics were recorded and compared, and the clinical data of the study groups is shown in Table 1.
Table 1. Clinical and demographic data for the studied groups.
| Parameters | BRVO |
Control |
||
| Ischemic | Non-ischemic | A | B | |
| No. of patients (eyes examined) | 24 (48) | 26 (52) | 48 (48) | 52 (52) |
| Gender (F/M) | 14/10 | 13/13 | 28/20 | 26/26 |
| Age (a) | 59.7±10.1 | 56.6±8.8 | 55.4±8.2 | 52.5±10.4 |
| Systolic blood pressure (mm Hg) | 142.4±18.7 | 134.5±14.3 | 120.0±10.5 | 119.8±11.0 |
| Diastolic blood pressure (mm Hg) | 91.4±12.5 | 88.0±7.7 | 79.6±9.3 | 79.6±9.8 |
| Pulse | 75.1±12.7 | 79.1±10.5 | 74.2±10.2 | 74.9±10.6 |
| Finger oximetry (%) | 97.5±0.93 | 97.0±1.4 | 97.3±1.2 | 97.3±1.2 |
| BCVA (affected/unaffected) | 35.6±24.1a/63.8±17.5 | 54.5±19.6/60.4±26.1 | 74.9±11.1 | 76.6±9.7 |
| Intraocular pressure, (affected/unaffected, mm Hg) | 13.3±3.2/13.8±2.9 | 13.5±3.0/13.5±2.6 | 13.7±2.7 | 13.6±2.6 |
| Perfusion pressure, (affected/unaffected, mm Hg) | 57.5±9.5/56.5±10.5 | 55.5±6.8/55.3±7.0 | 48.4±6.3 | 48.3±6.5 |
| Natural history (d) | 79.8±66.0 | 95.6±93.5 | ||
| No. of patients (CME) | 17 | 19 | ||
aSignificant difference in BCVA between affected eyes and unaffected eyes in ischemic BRVO group according to Kruskal-Wallis test (P<0.05). Except that, no significant difference in other aspects found between ischemic BRVO group and non-ischemic BRVO group. Corresponding control A and control B showed no significant difference in gender and age. BCVA: Best corrected visual acuity; CME: Cystoid macular edema.
x±s
Retinal Oximetry
The noninvasive retinal oximeter (Oxymap ehf., Reykjavik, Iceland) has been described previously, and is composed of two digital cameras (1600×1200 pixels), a custom-made optical adapter and a beam splitter. It can generate two fundus images with two wavelengths (570 nm and 600 nm) simultaneously, then calculate the optical density (OD) of the retinal arterioles and venules at both wavelengths. Moreover, there is an approximately linear relationship between the optical density ratio (ODR), vessel width (w) and oxygen saturation[9],[23] [SaO2=(a×ODR+b)+(c×w+d); calibration parameters: a=-1.28, b=1.24; diameter correction parameters: c=0.0097, d=-0.14]. However, the calibration constants are not perfect, and in some cases the measurements exceed 100%. Therefore, the oximetry values obtained may be used for comparison, and may differ from the absolute saturation values.
Imaging and Analysis
The pupils were dilated with 0.5% tropicamid (Shenyang Xingji Corporation, Shenyang, Liaoning Province, China). All of the fundus images were taken in a dark room, and performed by the same skilled photographers with consistent parameters. All subjects were examined twice, and all images were centred on the optic disc, with about one minute left between the two images. The best quality image was selected for analysis.
The oxygen saturation and the width of the retinal vessels can be analysed automatically using the Oxymap Analyser version 2.4 specialized software.
First Analysis
For this analysis, the retinal vessels were divided into three categories: 1) vessels affected by the occlusion; 2) vessels in the BRVO eye, which were not affected by the occlusion and 3) vessels in the fellow eye. The measured vessels not affected by the occlusion (categories 2 and 3) were chosen so that they were comparable in location to the affected vessels in the same patient. For example, if a major superotemporal venule 1) was occluded, a major inferotemporal venule 2) was chosen for comparison in the same eye, with a major superotemporal venule 3) in the fellow eye. The affected arteriole (category 1) was chosen as the arteriole that supplied the affected area to the greatest degree. The first and second degrees in each four quadrants were analysed, respectively, and shown in Figure 2. This kind analysis was only performed on the BRVO group.
Figure 2. Oxygen saturation map and fundus image of a patient with BRVO.
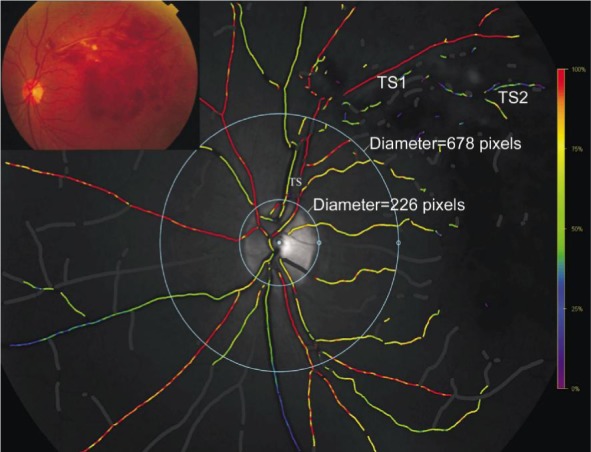
We performed two kinds of analysis. First kind, the vessel segments between the two circles (approximately central retina) were selected for analysis; Second kind, the first- and second- degree vessels in each four quadrant were selected for analysis. Occluded vessels were strictly chosen.
Second Analysis
The optic disc was first excluded by a circle, then a second circle was created with three times the optic disc radius, and the vessel segments between the two circles (stands approximately for the blood supply of the central retina) were selected for analysis. The mean oxygen saturation values of all of the selected arteries and veins were also analysed and shown in Figure 2 (diameter of the first circle was 226 pixels; diameter of the second circle was 678 pixels ). This analysis was performed on both eyes of the BRVO patients and the control group.
Statistical Analysis
All statistical analyses were made using the statistics software SPSS, version 21. Friedman's test and Dunn's post-test were used for comparison between the BRVO eye and contralateral eye. The Kruskal-Wallis test was used for the comparisons between the BRVO patients and the healthy individuals. A Spearman test was performed with the following predictor variables included: age, gender, finger pulse oxygen saturation (FO), ocular perfusion pressure (OPP), duration and arteriole/venule diameter (AD, VD). Then, the generalized linear models analysis of predictor variables associated with SaO2-V, SaO2-A and SaO2-AV in the occluded vessels was done. The data are expressed as the means ±standard deviations (SD), and a P<0.05 was considered to be statistically significant.
RESULTS
Oxygen Saturation and Diameter in Occluded Vessels
The comparisons of the mean oxygen saturation and vessel diameter for the occluded vessels and unaffected vessels, and contralateral eyes in the BRVO groups, are shown in Table 2, Figures 3 and 4.
Table 2. Comparison of oxygen saturation and vessel diameter for occluded vessels with unaffected vessels and contralateral eye in BRVO groups.
| Groups | Ischemic BRVO |
Non-ischemic BRVO |
||||||
| Affected eye |
Contralateral eye vessels | P | Affected eye |
Contralateral eye vessels | P | |||
| Occluded vessels | Unaffected vessels | Occluded vessels | Unaffected vessels | |||||
| SaO2-V (%) | 60.8±9.4 | 56.9±10.4 | 62.4±10.9 | 0.128 | 57.5±14.5 | 57.7±10.9 | 58.8±11.1 | 0.538 |
| SaO2-A (%) | 106.0±14.3 | 94.0±6.8a | 86.1±16.5c | <0.001 | 100.3±19.0 | 92.9±9.1 | 92.1±8.4 | 0.264 |
| SaO2-AV (%) | 45.2±15.8 | 37.1±11.2a | 23.7±18.8c | <0.001 | 42.8±22.9 | 35.1±9.7 | 33.3±11.1 | 0.867 |
| VD (pixels) | 14.5±1.9 | 15.5±2.3 | 14.2±2.3 | 0.368 | 14.8±2.6 | 14.7±1.9 | 15.5±1.3 | 0.467 |
| AD (pixels) | 12.3±2.1 | 13.3±1.5 | 13.2±1.1 | 0.065 | 13.4±1.8 | 13.7±1.4 | 13.3±1.3 | 0.827 |
Significantly increased SaO2-A and SaO2-AV can be seen in occluded vessels in ischemic BRVO group (P<0.05). Data were collected according first kind of analysis in method. Friedman's test was performed for comparison, P<0.05 was considered statistically significant. aSignificant difference between occluded vessels and unaffected vessels in occluded eye in ischemic BRVO group according to Dunn's post test (P<0.05); cSignificant difference between occluded vessels and vessels in corresponding quadrant of contralateral eye in ischemic BRVO group according to Dunn's post test (P<0.05). SaO2-V: Venous oxygen saturation; SaO2-A: Arterioles oxygen saturation; SaO2-AV: A-V difference oxygen saturation; VD: Venous diameter; AD: Arterioles diameter.
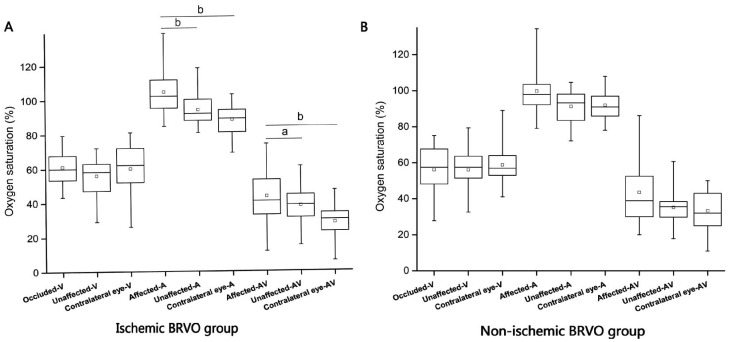
Figure 3. Comparison of oxygen saturation for occluded vessels with unaffected vessels and contralateral eye in BRVO groups.
A: Significantly increased SaO2-A and SaO2-AV can be seen in occluded vessels in ischemic BRVO group (P<0.01), SaO2-V in occluded vessels a little higher than unaffected vessels in ischemic BRVO eye (though no significant difference); B: No significant differences were found between SaO2-V, SaO2-A and SaO2-AV within non-ischemic BRVO group. aStatistically significant difference P<0.05; bStatistically significant difference P<0.01.
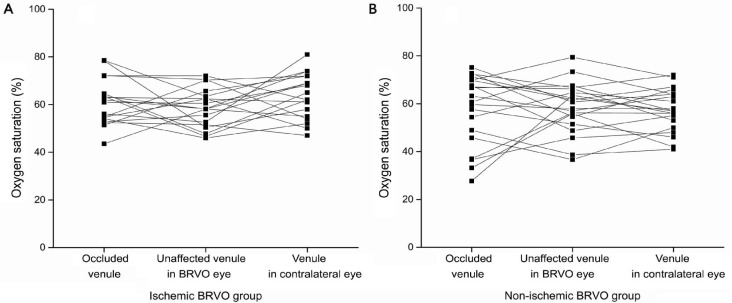
Figure 4. The venules oxygen saturation (%) in ischemic and non-ischemic BRVO group.
A: SaO2-V in occluded vessels is fluctuant (range 51.4%-71.2%), a little higher than unaffected vessels in ischemic BRVO eye (though no significant difference), also comparable to correspond quadrant in contralateral eye; B: SaO2-V in occluded vessels is rather more fluctuant (range 43%-72%) in non-ischemic BRVO eye, higher than unaffected eye, also comparable to contralateral eye. The lines connect measurements on the same patients. Measurements, which may be affected by several confounding factors, have been excluded.
In the ischemic BRVO group, the mean SaO2-V was 60.8%±9.4% in the occluded retinal venules, which was a little higher than the unaffected venules in the BRVO eye, and comparable to the contralateral eye (P>0.05) (Table 2, Figures 3 and 4). The mean SaO2-A (106.0%±14.3%) and SaO2-AV (45.2%±15.8%) in the occluded area suffered a significant increase (P<0.05). However, the VD and AD showed no significant difference within the group (P>0.05).
In the non-ischemic BRVO group, the mean SaO2-V (57.5%±14.5%), SaO2-A (100.3%±19.0%), SaO2-AV (42.8%±22.9%) VD and AD in the occluded area showed no significant difference within the groups (P>0.05). The SaO2-V in the occluded area was similar to the ischemic BRVO group, but more fluctuant (Figure 4).
Oxygen Saturation and Diameter in Central Retinal Vessels
Comparisons of the mean oxygen saturation and vessel diameter of the central and all retinal vessels in the BRVO patients, with the contralateral eye and healthy individuals, are presented in Tables 3 and 4, Figure 5.
Table 3. Comparison of oxygen saturation and vessel diameter of affected eye and contralateral eye in BRVO groups.
| Groups | Ischemic BRVO eye | Contralateral eye | P | Non-ischemic BRVO eye | Contralateral eye | P |
| Central retina | ||||||
| SaO2-V (%) | 61.1±6.3 | 61.3±7.5 | 0.851 | 59.6±8.1 | 61.6±4.6 | 0.554 |
| SaO2-A (%) | 100.7±3.8 | 96.0±6.8 | 0.011 | 98.1±4.8 | 97.5±5.3 | 0.606 |
| SaO2-AV (%) | 39.6±6.6 | 34.8±5.5 | 0.019 | 38.5±7.7 | 35.9±7.3 | 0.213 |
| VD (pixels) | 15.9±1.3 | 15.8±1.6 | 0.778 | 16.1±1.7 | 16.2±1.3 | 0.772 |
| AD (pixels) | 12.4±1.0 | 13.5±1.4 | 0.008 | 13.0±1.6 | 13.5±1.3 | 0.345 |
| Whole retina | ||||||
| SaO2-V (%) | 57.1±9.0 | 58.0±10.3 | 0.975 | 56.7±9.8 | 58.2±7.7 | 0.792 |
| SaO2-A (%) | 96.6±3.9 | 92.5±7.6 | 0.021 | 94.7±5.5 | 93.1±5.3 | 0.443 |
| SaO2-AV (%) | 39.5±9.4 | 34.5±6.0 | 0.093 | 38.0±7.8 | 34.9±7.2 | 0.155 |
| VD (pixels) | 14.8±1.0 | 14.7±1.3 | 0.452 | 15.2±1.4 | 14.7±1.0 | 0.178 |
| AD (pixels) | 12.1±0.7 | 12.7±1.0 | 0.030 | 12.8±1.3 | 12.7±1.0 | 0.970 |
Significantly increased SaO2-A and SaO2-AV can be seen in central and whole retina in ischemic BRVO group (P<0.05). AD in Ischemic BRVO eye was significantly narrower than contralateral eye (P<0.05). No significant differences were found between SaO2-V, SaO2-A and SaO2-AV within non-ischemic BRVO group. VD and AD in both groups showed no significance. No significant difference found between ischemic BRVO group and non-ischemic BRVO group (Data were not shown). Data were collected according second kind of analysis in method. Kruskal-Wallis test were performed for comparison, P<0.05 was considered statistically significant.
Table 4. Comparison of oxygen saturation and vessel diameter in BRVO groups with healthy individuals.
| Central retina | Ischemic BRVO | Control A | P | Non-ischemic BRVO | Control B | P |
| SaO2-V (%) | 62.1±6.0 | 60.3±6.0 | 0.261 | 58.3±8.9 | 60.4±6.4 | 0.465 |
| SaO2-A (%) | 101.3±5.4 | 94.7±5.5 | 0.00003 | 97.4±5.2 | 94.4±5.2 | 0.035 |
| SaO2-AV (%) | 39.1±6.4 | 34.4±5.1 | 0.003 | 39.1±8.8 | 34.1±5.1 | 0.018 |
| VD (pixels) | 16.1±1.2 | 15.9±1.5 | 0.430 | 16.2±1.6 | 16.0±1.4 | 0.958 |
| AD (pixels) | 12.3±1.1 | 13.3±1.2 | 0.001 | 12.9±1.5 | 13.5±1.2 | 0.056 |
Significantly increased SaO2-A and SaO2-AV can be seen in both ischemic and non-ischemic BRVO group (P<0.05). AD in Ischemic BRVO eye was significantly narrower than healthy eye (P<0.05). Data were collected according second kind of analysis in method. Kruskal-Wallis test were performed for comparison, P<0.05 was considered statistically significant.
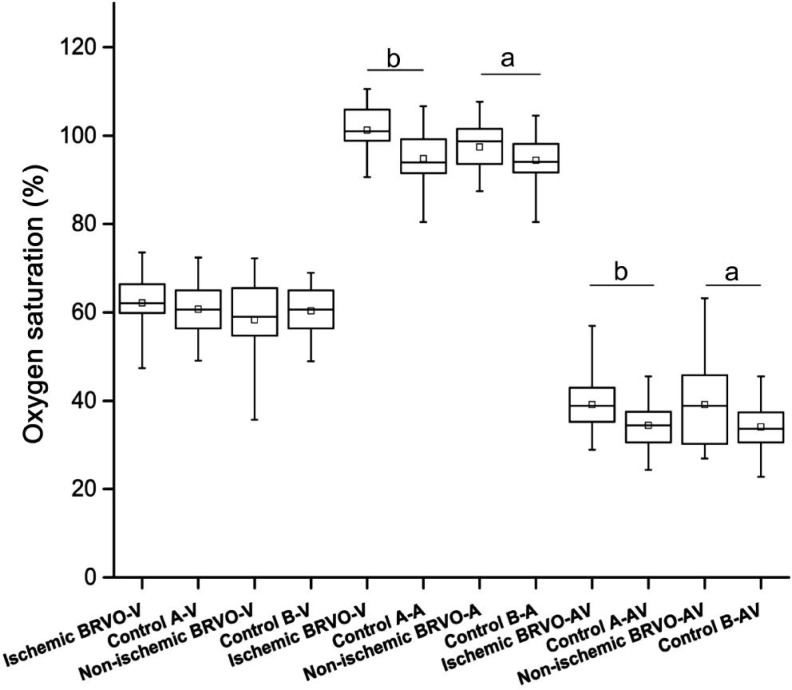
Figure 5. Comparison of oxygen saturation and vessel diameter in BRVO groups with healthy individuals in central retina.
Significantly increased SaO2-A and SaO2-AV can be seen in both ischemic and non-ischemic BRVO group compared to healthy individuals (P<0.05). No significant difference found between ischemic BRVO group and non-ischemic BRVO group (P>0.05). aStatistically significant difference P<0.05; bStatistically significant difference P<0.01.
In the ischemic BRVO group, whether comparing to the contralateral eye or healthy individuals, the mean SaO2-V in the central retina and whole retina were comparable (P>0.05). However, the mean SaO2-A and SaO2-AV significantly increased, and the AD significantly narrowed (P<0.05).
In the non-ischemic BRVO group, when compared to the contralateral eye, the SaO2-A, SaO2-V and SaO2-AV in the central retina and whole retina showed no significant changes (P>0.05). However, significantly increased SaO2-A and SaO2-AV in the central retina were found, when compared to healthy individuals. There was no difference between the VD and AD in all of the groups (P>0.05).
Multivariate Analysis
The generalized linear models analysis of the predictor variables associated with the VS in the occluded vessels showed that the VS is influenced by age (Table 5). The fitting curve of the SaO2-V to age is shown in Figure 6. The generalized linear models analysis of the predictor variables associated with SaO2-A and SaO2-AV showed no significant correlation.
Table 5. Generlized linear regression analyses of predictor variables of factors associated with SaO2-V in occluded vessels.
| Parameters | B | Std. Error | 95% Wald confidence interval |
P | |
| Lower | Upper | ||||
| Intercept | -61.80 | 139.49 | -335.21 | 211.60 | 0.658 |
| Male | 4.05 | 3.52 | -2.85 | 10.96 | 0.250 |
| Female | 0a | ||||
| Duration (d) | 0.03 | 0.02 | -0.02 | 0.07 | 0.223 |
| Age (a) | -0.43 | 0.15 | -0.72 | -0.14 | 0.003 |
| FO (%) | 1.80 | 1.41 | -0.96 | 4.56 | 0.201 |
| OPP (mm Hg) | 0.01 | 0.21 | -0.40 | 0.43 | 0.945 |
| VD (pixel) | -0.49 | 0.74 | -1.94 | 0.97 | 0.513 |
| Scale | 101.966b | 21.50 | 67.45 | 154.14 | |
Age showed negatively associated with VS in occluded vessels (P<0.05). Omnibus test: P=0.026. P<0.05 was considered statistically significant.aSet to zero because this parameter is redundant; bMaximum likelihood estimate.
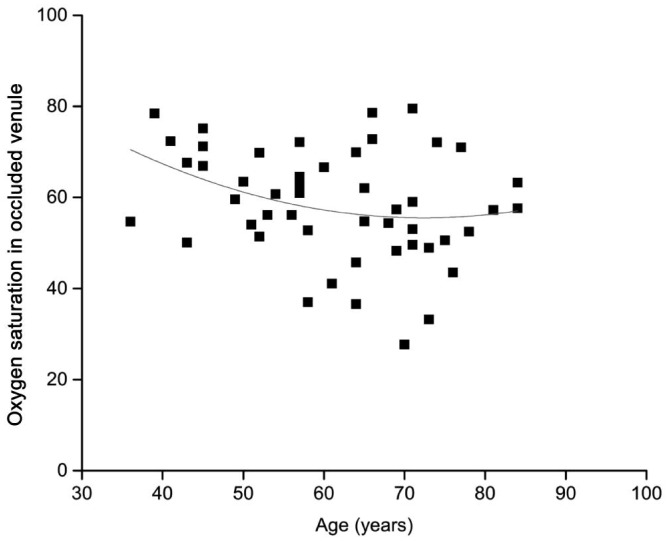
Figure 6. The scatter plots and fitted curves for SaO2-V to age in BRVO group.
The age was plotted on the X-axis, and the oximetry data (SaO2-V) are plotted on the Y-axis. The fitted curve for SaO2-V to age was shown in red (R2=0.06, y= -1.64x2+0.01x+37.47), indicating a negative correlation between SaO2-V and age.
DISCUSSION
Our study is the first to show that retinal oxygen metabolism is altered in ischemic and non-ischemic BRVO retinopathy. In the ischemic BRVO group, the occluded SaO2-A significantly increased and the occluded SaO2-V fluctuated, when compared to contralateral eyes or healthy individuals. The central SaO2-A and SaO2-V showed a similar trend with those of the occluded vessels; however, the AD was significantly narrow in the central retina. In the non-ischemic BRVO group, the SaO2-A, SaO2-V and SaO2-AV in the occluded vessels or the central retinal vessels showed no significant difference, when compared with the contralateral eye; whereas a significantly increased SaO2-A of the central retinal vessels was found when compared to healthy individuals.
In our study, an increased SaO2-A was found in the ischemic BRVO group, which was consistent with recent studies in BRVO patients[28]. In the majority of cases, arterial disease is a predominant pathogenic mechanism for BRVO. BRVO can occur at almost all AV crossings sharing an adventitial sheath with an artery[30]. An increased blood flow in the artery may increase haemodynamic stress at the AV crossing, resulting in venous endothelial injury[31]. Moreover, atherosclerotic arteries may be producing increased endothelin-1, stimulating venous vasoconstriction[9]. Therefore, the arterial pathology is critical to the occurrence of BRVO. With regard to the high SaO2-A in ischemic BRVO, we propose that there are several reasons for the results. First, since the arteries and veins interact with each other, the branch venous obstruction occurred at the AV crossings, and the increased back-pressure leads to the stagnation of the blood flow in the vein[32]. Especially in ischemic BRVO, the retinal perfusion status evaluated by the FFA showed poorly in the occluded area. Thus, the demand of oxygen consumption in the area decreased, and the carboxyhaemoglobin was higher in the artery.
Secondly, ischemic pathological changes can induce more capillary non-perfusion areas, with the production of vascular endothelial growth factor, neovascularization[21] and more severe hypoxia[16],[33]; which, in turn, also leads the arteries to locally upregulate certain vasoconstrictive messengers (ET-1)[34]–[35]. Thus, arteriolar constriction and the artery diameter are narrower, parallel to our results (Table 3). The blood flow is negatively associated with the diameters, so the blood flow is increased in the arteries in ischemic BRVO. Therefore, relatively slow oxygen consumption and relatively fast oxygen delivery may partly explain the high SaO2-A.
In non-ischemic BRVO, the SaO2-A is comparable to the contralateral eye, since there is no significant difference in the clinical and demographic data between the two types of BRVO groups. This may support that it was the retinal perfusion status and extent of hypoxia that lead to the different SaO2-A in the ischemic and non-ischemic BRVO. Since the systolic blood pressure in the ischemic and non-ischemic BRVO groups is higher than in the corresponding healthy control groups[36], it may be partly attributed to a higher SaO2-A when compared with healthy individuals.
The principle of oximetry is utilizing the different extinction coefficients of oxygenated haemoglobin and haemoglobin, and the accumulation of light sensitive metabolic substances, which may show the pseudomorphic increase of oxygen saturation[37]. These can be responsible for exceeding 100% of the measurements of the arteriole's oxygen saturation. Despite this, the data is still well supported to be suitable for comparison. Hence, SaO2-A, as an indirect evaluation of the retinal perfusion status, is also an important indicator to distinguish different types of BRVO.
The SaO2-V in the occluded vessels in ischemic and non-ischemic BRVO eyes shows a fluctuating trend (Figure 4), but the mean SaO2-V of the ischemic and non-ischemic BRVO groups was 60.8% and 57.5%. This is comparable to the contralateral eye, which is parallel to previous studies suggesting that the median oxygen saturation in the venules affected by BRVO was 59%, with no difference in the contralateral eye [28]. Additionally, there was no significant difference in the SaO2-V in the central retina of ischemic and non-ischemic BRVO, whether compared with the contralateral eye or healthy individuals. We speculate that this may be because there is a balanced mechanism between the supply and demand for oxygen in the occluded area in BRVO patients. This can be attributed to the following reasons: first, due to vein obstruction, blood stagnates in the venous system, thus increasing the pressure in the capillary system leading to a derangement in the Starling's forces. This reduces the blood flow in the retinal capillary system, leading to decreased oxygen delivery to the retina[32]. Previous studies have demonstrated hypoxia in the BRVO eye[16],[31],[38]–[39]. The hypoxic retina takes more oxygen from the per-unit volume of the blood, decreasing the amount of blood flow through the capillary bed, resulting in a lower SaO2-V.
Second, the hemodynamic impairment downstream of the AV crossings and hypoxia lead to retinal atrophy[30],[40]; thus, the oxygen consumption is reduced. Third, the blood supply may be increased by recanalization of the thrombotic occlusion [40] and/or by the maturation of the collateral circulation[41]–[42].
Fourth, the formation of the arteriovenous shunt makes the blood go into the venules directly, without oxygen extraction by the retinal tissues which have been found in the retina of healthy human eyes[40], resulting in higher SaO2-V values.
Fifth, ischemic BRVO is always coupled with complications, such as retinal neovascularization, which may lead to vitreous traction; thus, the oxygen transport through the vitreous cavity increased, which may alleviate retinal hypoxia in BRVO[18],[41]. Therefore, although the extent of vein obstruction and the perfusion status to the retina in ischemic and non-ischemic BRVO are different, there are still some different balance mechanisms between the supply and demand for oxygen in the two types of BRVO. This demonstrates the rather variable SaO2-V; in other words, the shifts in the oxygen saturation can reflect the change in the blood flow status, which requires further study.
To our knowledge, oxygen delivery from the retinal circulation to the retinal tissue can be estimated from the SaO2-AV multiplied by the blood flow. At present, the results of the SaO2-AV in the ischemic BRVO study groups were significantly increased. We speculate that this is a compensatory increase in the oxygen intake from the retinal circulation in response to a relative lack of oxygen supply to the retina, due to a reduced retinal blood flow. In the non-ischemic BRVO eye, the retinal perfusion status is not that poor, with little compensatory oxygen intake, without significant SaO2-AV change. Moreover, since the mean SaO2-A increased, and the mean SaO2-V is comparable, the mean SaO2-AV would be increased. Therefore, the comparison of the SaO2-AV before and after treatment would be more meaningful to the individuals.
In the present study, in the ischemic BRVO group, age was negatively associated with the SaO2-V, which is in conflict with a recent study of healthy young adults[43]. In that study, the age was positively associated with the venular SO2 values, supporting the well-known perspective that advancing age is a very important risk factor for BRVO[29].
There were several limitations to the present study. One weakness was the low number of patients that were examined. Future studies should increase the sample size, be divided into more detailed stages, and be grouped according to severity. The measurements of the retinal vessel oxygen saturation in patients with BRVO are demanding. The strict inclusion criteria should have included patients free of haemorrhage in the occluded area, and those not receiving blood from non-occluded venules, which would only relate to mild BRVO. This may prevent the risk of bias. Furthermore, the classification of BRVO is based mainly on the FFA, combined with the clinical features, but different severities of BRVO may result in different oxygen saturations. Additionally, the oximetry analysis of the oxygen saturation is based on reflected light, the fundus pigment, changes and the diameter of the retinal vessels in BRVO (the SaO2-A in some cases would exceed 100%), as well as cataracts, which can cause errors in the measured saturation levels. However, there is currently no consensus on the optimal method for the measurement of oxygen saturation in the retinal vasculature. Oximetry is also a relatively safe and effective way to take measurements.
In conclusion, our results first indicate that SaO2-V is fluctuant in the two different types of BRVO, compared with contralateral eyes and age and gender matched healthy individuals. This may reflect a balance between the supply and demand for oxygen in the occluded area in BRVO patients. Obvious changes in the occluded and central SaO2-A were found in the ischemic BRVO group, indicating that disorders of oxygen metabolism in arterioles may participate in the pathogenesis of ischemic BRVO, but not in non-ischemic BRVO. Therefore, the retinal oximetry technique, which reflects the oxygen metabolism in vessels, can indirectly reflect the retinal perfusion status evaluated by the FFA. It can also be used in support of the classification of BRVO, and help to explain the mechanism of different types of BRVO.
Acknowledgments
Foundations: Supported by the National Science & Technology Pillar Program of the Twelfth Five-year Plan (2012BAI08B04); Open Research Funds of the State Key Laboratory of Ophthalmology.
Conflicts of Interest: Lin LL, None; Dong YM, None; Zong Y, None; Zheng QS, None; Fu Y, None; Yuan YG, None; Huang X, None; Qian G, None; Gao QY, None.
REFERENCES
- 1.Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008;33:111–131. doi: 10.1080/02713680701851902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B. Ocular effects of changes in oxygen and carbon dioxide tension. Trans Am Ophthalmol Soc. 1968;66:423–474. [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, Kowalski JW, Nguyen H, Wong TY, International Eye Disease Consortium The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2):313–319. doi: 10.1016/j.ophtha.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laouri M, Chen E, Looman M, Gallagher M. The burden of disease of retinal vein occlusion: review of the literature. Eye (Lond) 2011;25(8):981–988. doi: 10.1038/eye.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayreh SS, Rojas P, Podhajsky P, Montague P, Woolson RF. Ocular neovascularization with retinal vascular occlusion-III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology. 1983;90(5):488–506. doi: 10.1016/s0161-6420(83)34542-5. [DOI] [PubMed] [Google Scholar]
- 6.Parodi MB, Bandello F. Branch retinal vein occlusion: classification and treatment. Ophthalmologica. 2009;223(5):298–305. doi: 10.1159/000213640. [DOI] [PubMed] [Google Scholar]
- 7.Parodi MB, DI Stefano G, Ravalico G. Grid laser treatment for exudative retinal detachment secondary to ischemic branch retinal vein occlusion. Retina. 2008;28(1):97–102. doi: 10.1097/IAE.0b013e318074bc1d. [DOI] [PubMed] [Google Scholar]
- 8.Jefferies P, Clemett R, Day T. An anatomical study of retinal arteriovenous crossings and their role in the pathogenesis of retinal branch vein occlusions. Aust N Z J Ophthalmol. 1993;21(4):213–217. doi: 10.1111/j.1442-9071.1993.tb00959.x. [DOI] [PubMed] [Google Scholar]
- 9.Fraenkl SA, Mozaffarieh M, Flammer J. Retinal vein occlusions: the potential impact of a dysregulation of the retinal veins. EPMA J. 2010;1:253–261. doi: 10.1007/s13167-010-0025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donati G, Pournaras CJ, Pizzolato GP, Tsacopoulos M. Decreased nitric oxide production accounts for secondary arteriolar constriction after retinal branch vein occlusion. Invest Ophthalmol Vis Sci. 1997;38(7):1450–1457. [PubMed] [Google Scholar]
- 11.Anderson BJ, Saltzman HA. Retinal oxygen utilization measured by hyperbaric blackout. Arch Ophthalmol. 1964;72:792–795. doi: 10.1001/archopht.1964.00970020794009. [DOI] [PubMed] [Google Scholar]
- 12.Avila CP, Bartsch DU, Bitner DG, Cheng L, Mueller AJ, Karavellas MP, Freeman WR. Retinal blood flow measurements in branch retinal vein occlusion using scanning laser Doppler flowmetry. Am J Ophthalmol. 1998;126(5):683–690. doi: 10.1016/s0002-9394(98)00114-7. [DOI] [PubMed] [Google Scholar]
- 13.Fujio N, Feke GT, Ogasawara H, Goger DG, Yoshida A, McMeel JW. Quantitative circulatory measurements in branch retinal vessel occlusion. Eye (Lond) 1994;8(Pt 3):324–328. doi: 10.1038/eye.1994.66. [DOI] [PubMed] [Google Scholar]
- 14.Horio N, Horiguchi M. Retinal blood flow analysis using intraoperative video fluorescein angiography combined with optical fiber-free intravitreal surgery system. Am J Ophthalmol. 2004;138(6):1082–1083. doi: 10.1016/j.ajo.2004.06.079. [DOI] [PubMed] [Google Scholar]
- 15.Linsenmeier RA. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol. 1986;88(4):521–542. doi: 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pournaras CJ, Tsacopoulos M, Strommer K, Gilodi N, Leuenberger PM. Experimental retinal branch vein occlusion in miniature pigs induces local tissue hypoxia and vasoproliferative microangiopathy. Ophthalmology. 1990;97(10):1321–1328. doi: 10.1016/s0161-6420(90)32415-6. [DOI] [PubMed] [Google Scholar]
- 17.Pournaras CJ, Miller JW, Gragoudas ES, Husain D, Munoz JL, Tolentino MJ, Kuroki M, Adamis AP. Systemic hyperoxia decreases vascular endothelial growth factor gene expression in ischemic primate retina. Arch Ophthalmol. 1997;115(12):1553–1558. doi: 10.1001/archopht.1997.01100160723009. [DOI] [PubMed] [Google Scholar]
- 18.Noergaard MH, Bach-Holm D, Scherfig E, Bang K, Jensen PK, Kiilgaard JF, Stefánsson E, la Cour M. Dorzolamide increases retinal oxygen tension after branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 2008;49(3):1136–1141. doi: 10.1167/iovs.07-0508. [DOI] [PubMed] [Google Scholar]
- 19.Funk M, Kriechbaum K, Prager F, Benesch T, Georgopoulos M, Zlabinger GJ, Schmidt-Erfurth U. Intraocular concentrations of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumab. Invest Ophthalmol Vis Sci. 2009;50(3):1025–1032. doi: 10.1167/iovs.08-2510. [DOI] [PubMed] [Google Scholar]
- 20.Recupero SM, Perdicchi A, Scuderi GL, Amodeo S, Medori EM, Leonardi A. Visual acuity in central and branch vein retinal occlusion in the presence of macular edema: 1 year of follow-up. Ann Ophthalmol (Skokie) 2006;38(2):107–110. doi: 10.1385/ao:38:2:107. [DOI] [PubMed] [Google Scholar]
- 21.Shilling JS, Kohner EM. New vessel formation in retinal branch vein occlusion. Br J Ophthalmol. 1976;60(12):810–815. doi: 10.1136/bjo.60.12.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novotny HR, Alvis DL. A method of photographing fluorescence in circulating blood in the human retina. Circulation. 1961;24:82–86. doi: 10.1161/01.cir.24.1.82. [DOI] [PubMed] [Google Scholar]
- 23.Spaide RF, Klancnik JM, Cooney MJ. Retinal Vascular Layers Imaged by Fluorescein Angiography and Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2015;133(1):45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]
- 24.Vandewalle E, Abegão Pinto L, Olafsdottir OB, De Clerck E, Stalmans P, Van Calster J, Zeyen T, Stefánsson E, Stalmans I. Oximetry in glaucoma: correlation of metabolic change with structural and functional damage. Acta Ophthalmol. 2014;92(2):105–110. doi: 10.1111/aos.12011. [DOI] [PubMed] [Google Scholar]
- 25.Jorgensen C, Bek T. Increasing oxygen saturation in larger retinal vessels after photocoagulation for diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014;55(8):5365–5369. doi: 10.1167/iovs.14-14811. [DOI] [PubMed] [Google Scholar]
- 26.Hardarson SH, Elfarsson A, Agnarsson BA, Stefánsson E. Retinal oximetry in central retinal artery occlusion. Acta Ophthalmol. 2013;91(2):189–190. doi: 10.1111/j.1755-3768.2012.02393.x. [DOI] [PubMed] [Google Scholar]
- 27.Hardarson SH, Stefánsson E. Oxygen saturation in central retinal vein occlusion. Am J Ophthalmol. 2010;150(6):871–875. doi: 10.1016/j.ajo.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Hardarson SH, Stefánsson E. Oxygen saturation in branch retinal vein occlusion. Acta Ophthalmol. 2012;90(5):466–470. doi: 10.1111/j.1755-3768.2011.02109.x. [DOI] [PubMed] [Google Scholar]
- 29.Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina. 2013;33(5):901–910. doi: 10.1097/IAE.0b013e3182870c15. [DOI] [PubMed] [Google Scholar]
- 30.Schweitzer D, Hammer M, Kraft J, Thamm E, Königsdörffer E, Strobel J. In vivo measurement of the oxygen saturation of retinal vessels in healthy volunteers. IEEE Trans Biomed Eng. 1999;46(12):1454–1465. doi: 10.1109/10.804573. [DOI] [PubMed] [Google Scholar]
- 31.Shui YB, Holekamp NM, Kramer BC, Crowley JR, Wilkins MA, Chu F, Malone PE, Mangers SJ, Hou JH, Siegfried CJ, Beebe DC. The gel state of the vitreous and ascorbate-dependent oxygen consumption: relationship to the etiology of nuclear cataracts. Arch Ophthalmol. 2009;127(4):475–482. doi: 10.1001/archophthalmol.2008.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurram MM. Effect of posterior sub-tenon triamcinolone in macular edema due to non-ischemic vein occlusions. J Clin Diagn Res. 2013;7(12):2821–2824. doi: 10.7860/JCDR/2013/6473.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behzadian MA, Wang XL, Al-Shabrawey M, Shabrawey M, Caldwell RB. Effects of hypoxia on glial cell expression of angiogenesis-regulating factors VEGF and TGF-beta. Glia. 1998;24(2):216–225. [PubMed] [Google Scholar]
- 34.Muraoka Y, Tsujikawa A, Murakami T, Ogino K, Kumagai K, Miyamoto K, Uji A, Yoshimura N. Morphologic and functional changes in retinal vessels associated with branch retinal vein occlusion. Ophthalmology. 2013;120(1):91–99. doi: 10.1016/j.ophtha.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 35.Singer M, Tan CS, Bell D, Sadda SR. Area of peripheral retinal nonperfusion and treatment response in branch and central retinal vein occlusion. Retina. 2014;34(9):1736–1742. doi: 10.1097/IAE.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 36.Mikhailova MA, Sizova MV, Shelankova AV. Pathogenesis of retinal vein occlusions. Vestn Oftalmol. 2014;130(2):88–92. [PubMed] [Google Scholar]
- 37.Blondal R, Sturludottir MK, Hardarson SH, Halldorsson GH, Stefánsson E. Reliability of vessel diameter measurements with a retinal oximeter. Graefes Arch Clin Exp Ophthalmol. 2011;249(9):1311–1317. doi: 10.1007/s00417-011-1680-2. [DOI] [PubMed] [Google Scholar]
- 38.Zhao J, Sastry SM, Sperduto RD, Chew EY, Remaley NA. Arteriovenous crossing patterns in branch retinal vein occlusion. The Eye Disease Case-Control Study Group. Ophthalmology. 1993;100(3):423–428. doi: 10.1016/s0161-6420(93)31633-7. [DOI] [PubMed] [Google Scholar]
- 39.Christoffersen N L, Larsen M. Pathophysiology and hemodynamics of branch retinal vein occlusion. Ophthalmology. 1999;106(11):2054–2062. doi: 10.1016/S0161-6420(99)90483-9. [DOI] [PubMed] [Google Scholar]
- 40.Frangieh GT, Green WR, Barraquer-Somers E, Finkelstein D. Histopathologic study of nine branch retinal vein occlusions. Arch Ophthalmol. 1982;100(7):1132–1140. doi: 10.1001/archopht.1982.01030040110020. [DOI] [PubMed] [Google Scholar]
- 41.Genevois O, Paques M, Simonutti M, Sercombe R, Seylaz J, Gaudric A, Brouland JP, Sahel J, Vicaut E. Microvascular remodeling after occlusion-recanalization of a branch retinal vein in rats. Invest Ophthalmol Vis Sci. 2004;45(2):594–600. doi: 10.1167/iovs.03-0764. [DOI] [PubMed] [Google Scholar]
- 42.Muqit MM, Saidkasimova S, Keating D, Murdoch JR. Long-term study of vascular perfusion effects following arteriovenous sheathotomy for branch retinal vein occlusion. Acta Ophthalmol. 2010;88(3):e57–e65. doi: 10.1111/j.1755-3768.2010.01877.x. [DOI] [PubMed] [Google Scholar]
- 43.Man RE, Sasongko MB, Kawasaki R, Noonan JE, Lo TC, Luu CD, Lamoureux EL, Wang JJ. Associations of retinal oximetry in healthy young adults. Invest Ophthalmol Vis Sci. 2014;55(3):1763–1769. doi: 10.1167/iovs.13-13320. [DOI] [PubMed] [Google Scholar]